Abstract
Study Objectives:
To evaluate whether paradoxical sleep deprivation-induced memory impairments are due to release of glucocorticoids, by means of corticosterone inhibition with metyrapone.
Design:
The design was a 2 (Groups [control, paradoxical sleep-deprived]) × 2 (Treatments [vehicle, metyrapone]) study, performed in 2 experiments: Acute treatment (single injection given immediately after 96 hours of sleep deprivation) and chronic treatment (8 injections, twice per day, throughout the sleep-deprivation period). Animals were either paradoxical sleep-deprived or remained in their home cages for 96 hours before training in contextual fear conditioning and received intraperitoneal injections of a corticosterone synthesis inhibitor, metyrapone. Memory performance was tested 24 hours after training.
Subjects:
Three-month old Wistar male rats.
Measurements:
Freezing behavior was considered as the conditioning index, and adrenocorticotropic hormone and corticosterone plasma levels were determined from trunk blood of animals sacrificed in different time points. Animals were weighed before and after the paradoxical sleep-deprivation period.
Results:
Acute metyrapone treatment impaired memory in control animals and did not prevent paradoxical sleep deprivation-induced memory impairment. Likewise, in the chronic treatment, paradoxical sleep-deprived animals did not differ from control rats in their corticosterone or adrenocorticotropic hormone response to training, but still did not learn as well, and did not show any stress responses to the testing. Chronic metyrapone was, however, effective in preventing the weight loss typically observed in paradoxical sleep-deprived animals.
Conclusions:
Our results suggest that glucocorticoids do not mediate memory impairments but might be responsible for the weight loss induced by paradoxical sleep deprivation.
Citation:
Tiba PA; de Menezes Oliveira MG; Rossi VC; Tufik S; Suchecki D. Glucocorticoids are not responsible for paradoxical sleep deprivation-induced memory impairments. SLEEP 2008;31(4):505-515.
Keywords: paradoxical sleep deprivation, learning, memory, metyrapone, contextual fear conditioning, corticosterone, weight loss, rat
A LARGE NUMBER OF ANIMAL STUDIES SUGGEST A RELATIONSHIP BETWEEN SLEEP AND MEMORY. MOST OF THEM USE STRATEGIES SUCH AS EXPLORING common events occurring during learning and subsequent sleep, comparing the performance of individuals before and after a period of sleep, and examining the outcomes of partial or total sleep deprivation on the performance in memory tasks.1–3
Employment of the sleep deprivation procedure frequently demonstrates that this manipulation has deleterious effects on memory performance in both animals and humans.4,5 Studies from our laboratory have shown that 96 hours of paradoxical sleep deprivation (PSD) before training impairs the performance of rats in memory tasks, such as inhibitory avoidance and contextual fear conditioning.6–10 PSD also disrupts the performance in other tasks, including the spatial version of the Morris Water Maze (MWM),11 8-arm/box maze,12,13 and appetitive discrimination task.14
The studies supporting a relationship between paradoxical sleep and memory that have used PSD have been strongly criticized because of the confounding nonspecific effects of the method, e.g., increased locomotor activity and activity of the hypothalamic-pituitary-adrenal (HPA) axis, which could be responsible for producing the alterations in performance observed.15–17 The key components of the HPA axis include the corticotropin-releasing hormone (CRH) neurons of the paraventricular nucleus, which stimulate the secretion of adrenocorticotropic hormone (ACTH) from the anterior pituitary. Circulating ACTH acts on the adrenal cortex, where it stimulates the release of glucocorticoids (cortisol in humans and corticosterone in rodents), which then feed back to the brain and pituitary to shut off the stress response. In addition to the neuroendocrine limb, the stress also involves activation of the sympathetic adrenomedullary system. The procedures commonly used to induce sleep deprivation result in hypertrophy of the adrenal glands and increased ACTH and corticosterone levels, indicating its stressful characteristics.18,19 Therefore, it is difficult to distinguish between the outcomes of stress and sleep loss on memory performance.
It is well known that the effects of glucocorticoids on cognition are dependent on their circulating levels. Very high or very low levels of this hormone are prejudicial to memory consolidation, a relationship known as an inverted U-shape.20,21 Conrad and colleagues22 tested the effects of corticosterone or its agonists (aldosterone and RU362) and antagonists (RU318 and RU555) on a Y-maze task and reported that both blockade of glucocorticoid receptors and high levels of corticosterone or its agonists impair memory performance, suggesting that altered occupancy by corticosterone or its agonists of different receptors (glucocorticoid/ mineralocorticoid) could underlie the bimodal action of corticosterone on memory. A recent study shows that chronic stress-induced memory impairment is due to hypersecretion of corticosterone at the time of training, since treatment with metyrapone (an inhibitor of the corticosterone synthesizing enzyme 11-β-hydroxylase) immediately before training in a Y-maze prevents the deleterious effect of chronic stress, indicating that this effect is mediated by HPA axis dysregulation, such as reduced hippocampal glucocorticoid receptor expression, rather than by structural changes in the hippocampus.23 Removal of the adrenal glands (adrenalectomy or systemic administration of metyrapone, impairs the performance of rats on the MWM task.24,25 Glucocorticoid receptor antagonists induce similar deficits in this task,26 whereas posttraining administration of dexamethasone, a glucocorticoid receptor agonist, enhances the performance of animals in tasks such as aversive and appetitive contextual fear conditioning.27 This impairment observed after adrenal removal is reversed by corticosterone administration, suggesting that glucocorticoids, rather than other adrenal hormones, are directly responsible for adrenalectomy-induced memory impairments.28
Given that sleep deprivation impairs learning in the rat, and that it also results in augmented secretion of glucocorticoids, which can interfere with memory consolidation processes, a recent paper addressed this issue by eliminating the adrenal stress response by means of adrenalectomy. Stable physiologic levels of corticosterone were maintained in adrenalectomized rats with an implanted pellet. Adrenalectomy plus corticosterone replacement does not modify the effect of 72 hours of sleep deprivation on acquisition in the MWM, demonstrating that deficits in spatial task acquisition are not affected by the adrenal stress response.29 It is important to bear in mind that adrenalectomy also eliminates other adrenal hormones, such as epinephrine, which interacts with corticosterone in the modulation of memory consolidation30–32; moreover, adrenalectomy performed before training also inhibits corticosterone release during all phases of memory: acquisition, consolidation, and retrieval. Using a more refined approach, the present study addressed the question of whether the effects of PSD-induced memory impairments would be mediated by corticosterone, by administrating metyrapone acutely or chronically during the sleep-deprivation period and, therefore, eliminating the interference produced by increased glucocorticoids levels.
METHODS
Subjects
Male Wistar rats, aged 3 months, were obtained from the Department of Psychobiology breeding colony. The animals were kept in groups of 4 in plastic cages filled with sawdust bedding in a room under controlled temperature (23°C ± 2°C) and light/dark cycle (lights on from 07:00 to 19:00), with food and water ad libitum. Animal studies were approved by the Animal Care and Use Committee of UNIFESP and were in accordance with National Institutes of Health guidelines on animal care (CEP # 0071/05).
PSD Procedure
Rats were sleep deprived by the modified multiple platform method33 for 96 hours. Sleep deprivation was conducted by placing 8 rats in a large water tank (145 × 30 × 41 cm) containing 12 narrow platforms (6 cm in diameter). This procedure completely abolishes paradoxical sleep but also decreases slow-wave sleep by approximately 35%.34 The presence of multiple platforms avoids the lack of movement and isolation associated with earlier techniques of sleep deprivation. Animals in the control group remained in their home cages in the same room where the sleep deprivation procedure took place and were placed daily in the water tank for 1 hour, between 12:00 and 13:00.
Apparatus
The contextual fear conditioning (CFC) test apparatus consisted of an acrylic box, measuring 30 × 21 × 30 cm. The apparatus had black walls with white visual patterns (2 squares measuring 5.5 × 5.5 cm and 3 measuring 4.0 × 4.0 cm made of white cardboard). The top was covered with transparent acrylic. The floor consisted of a metal grid (0.4-cm diameter rods placed 1.2 cm apart) connected to a shock generator and control module (AVS—Projetos Especiais, Sao Paulo, Brazil), through which foot shocks could be delivered.
Contextual Fear Conditioning
The task was carried out during 2 consecutive days. On the first day (training), the animals were individually placed in the black box, where they remained for 2 minutes. The behavior of each animal was recorded continuously by measuring the seconds it remained in freezing (defined as complete immobility and absence of vibrissae movements) minute by minute. After this period, rats received 5 foot shocks (0.8 mA, 1-s duration) at 30-second intervals and were removed from the apparatus 1 minute after the last foot shock. Behavioral reaction to foot shock, measured by paw flinch and vocalization, was recorded as a sensitivity parameter. CFC tests were performed on the second day, 24 hours after training. All rats were placed in the same training context, and no foot shock was delivered. The time in freezing was recorded minute by minute for 5 minutes. The freezing/minute ratio was taken as a measure of contextual conditioning.
Drug preparation and Administration
The dose of 100 mg/kg (in a volume of 2.0 mL/kg) of 11-β-hydroxylase inhibitor metyrapone [2-methyl-l,2-di-3-pyridyl-1-propanone (Sigma)] was chosen for being effective to inhibit corticosterone secretion in a pilot study. The drug was dissolved in polyethylene glycol and diluted with a 0.9% saline solution to reach the appropriate concentration. The final concentration of polyethylene glycol was 40%. The vehicle solution contained the same polyethylene glycol concentration. The drug and vehicle solution were administered intraperitoneally.
Experimental Procedure
Rats were randomly distributed into 1 of 4 groups: paradoxical sleep-deprived injected with metyrapone (PSD MET) or vehicle (PSD VEH) and control treated with metyrapone (CTL MET) or vehicle (CTL VEH). PSD began in the morning (07:00). In Experiment 1, a single metyrapone (or vehicle) injection was administered at 07:00 of the last day of sleep deprivation, e.g., at the end of 96 hours of sleep deprivation, and, 90 minutes later, animals were either sacrificed or submitted to CFC training and sacrificed 15 minutes after the test. In Experiment 2, animals received chronic administration of metyrapone or vehicle, and the first injection was given at the onset of the sleep-deprivation period (07:00). Rats received 2 injections per day (07:00 and 19:00) throughout the 4 days of sleep deprivation, for a total of 8 injections. The half-life of metyrapone is very short (about 1.9 ± 0.7 hours), but, apparently, metyrapol, an active metabolite, shows very similar effects to metyrapone and, therefore, may be involved in the pharmacologic activity of metyrapone in vivo.35 In this way, this experimental design allowed us to cease administration on the night before training, thus preventing the memory impairment induced by acute metyrapone administration. PSD and control animals were sacrificed 90 minutes after the last injection (08:30) or after the sleep-deprivation period (07:00). The remaining animals were submitted to training in CFC. Half of the animals were sacrificed 15 minutes after training (posttraining) and the other half were tested (posttest) 24 hours later. Animals were then sacrificed 15 minutes after testing (Figure 1). Plasma was collected for determination of ACTH and corticosterone levels (n = 8/group per time point). All animals were weighed at the beginning of the experiment (initial) and before (final weight) the sacrifice.
Figure 1.
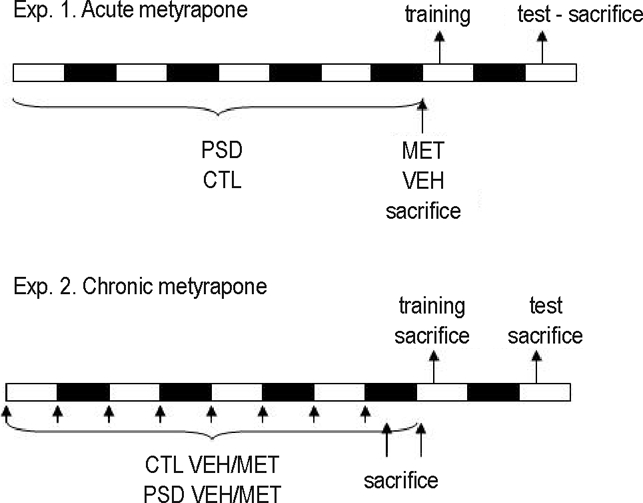
Experimental procedure of experiment 1 (acute metyrapone [MET]) and experiment 2 (chronic MET). White rectangles represent light phase and black rectangles represent dark phase of the light-dark cycle. In both experiments, animals were distributed in 4 groups: control (CTL) receiving vehicle (VEH) or MET and paradoxical sleep deprivation (PSD) receiving VEH or MET. In the acute treatment, animals received 1 single injection after 96 hours of sleep deprivation and were sacrificed 90 minutes later or were submitted to contextual fear conditioning (CFC) training and test. The trained animals were sacrificed 15 minutes after test, 24 hours after the training. In the chronic experiment, animals received 2 injections per day (07:00 and 19:00) during 4 days of sleep deprivation (or at the equivalent time point for the CTL group). Animals were sacrificed either 90 minutes after the last injection (21:00); immediately after sleep deprivation (07:00); 15 minutes after training or 15 minutes after the test in CFC.
Blood Sampling and Hormone Plasma Levels
Trunk blood was collected in precooled plastic tubes containing 0.1 mL of ethylenediaminetetraacetic acid (60 mg/mL). Blood was centrifuged at 2300 revolutions per minute for 20 minutes at 4°C. Plasma was collected in polycarbonate tubes and frozen at −20°C.
Corticosterone levels were determined, in duplicate, by a double antibody radioimmunoassay method, specific for rats and mice, using a commercial kit (ICN Biomedicals, Costa Mesa, CA). The sensitivity of the assay is 3.125 ng/mL, and intraassay and interassay variations are, respectively, 10.3% and 7.1%. ACTH was assayed by a sequential chemiluminescence immunometric method using a monoclonal murine antibody specific for ACTH (DPC Immulite, Los Angeles, CA). The sensitivity of the assay is 9 pg/mL and intraassay and interassay variations are 9.6% and 9.4%, respectively.
Statistical Analysis
Data concerning the behavior of rats in the CFC were analyzed by a 3-way analysis of variance (ANOVA) for repeated measures, with Group (PSD × control), Treatment (metyrapone × vehicle), and Minute (repeated measure = before and after shock for training and each of the 5 minutes for test) as main factors.
ACTH and corticosterone in Experiment 1 (acute treatment) were analyzed by a 2-way ANOVA, with Group and Treatment as main factors. Hormone data from Experiment 2 were analyzed separately for each time-point (post-last injection; post-PSD; posttraining and posttest) by a factorial two-way ANOVA with Group (PSD x control) and Treatment (metyrapone x vehicle) as main factors.
For body weight analysis, we used an index (final weight × 100/ initial weight), and statistical analysis was conducted using a 2-way ANOVA, with Group (PSD × control) and Treatment (metyrapone × vehicle) as factors.
A Pearson correlation test was carried out between hormone parameters (ACTH and corticosterone levels) and total freezing time during the test session.
When necessary, a posthoc analysis was performed by the Newman-Keuls test, with a P value ≤ 0.05 being considered statistically significant.
RESULTS
Experiment 1. Acute Metyrapone Treatment
Hormone Parameters
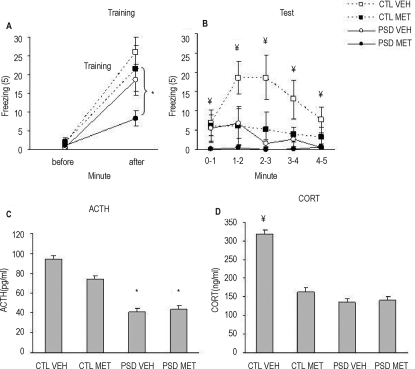
Figure 2 presents ACTH (A) and corticosterone (B) levels of control and PSD animals, which received metyrapone or vehicle 90 minutes before the sacrifice. Two-way ANOVA revealed group (F1, 27 = 5.39; P = 0.028) and treatment effects (F1, 27 = 22.07; P < 0.001), in which PSD animals presented greater ACTH release than control animals and metyrapone-treated animals released more ACTH than vehicle-treated ones. For corticosterone levels, ANOVA revealed a treatment effect (F1, 27 = 7.01; P = 0.01), in which metyrapone-treated animals released less corticosterone than did vehicle-treated ones.
Figure 2.
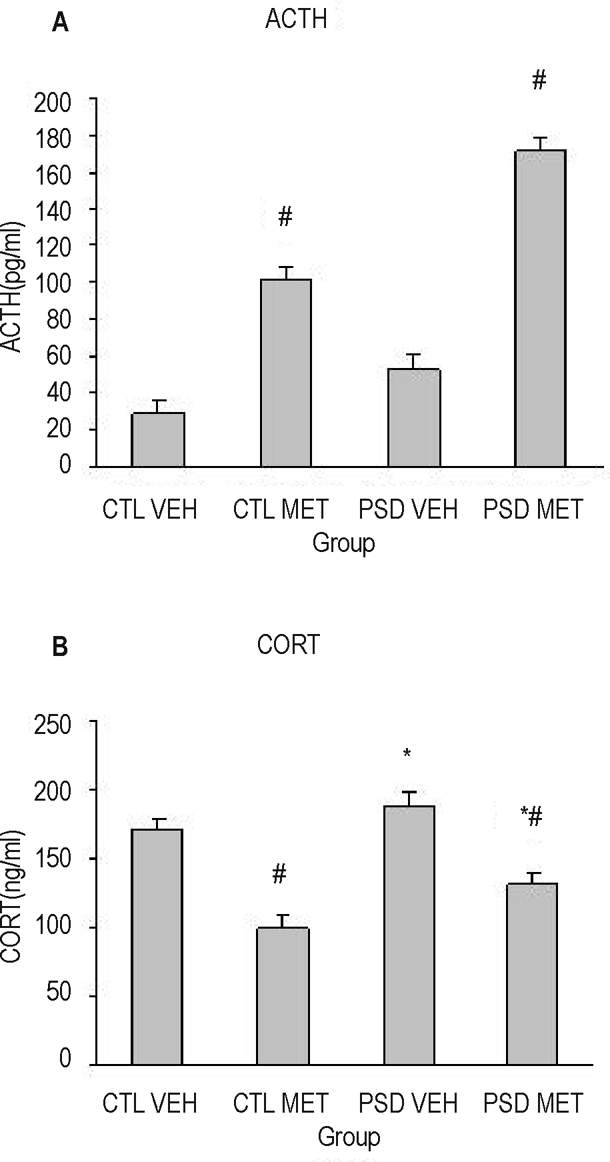
Adrenocorticotropic hormone (ACTH) (A) and corticosterone (CORT) (B) plasma levels, assessed 90 minutes after metyrapone (MET) or vehicle (VEH) acute injection in paradoxical sleep deprivation (PSD) or control (CTL) groups. *Different from CTL group (analysis of variance group effect). #Different from VEH group (ANOVA treatment effect). n = 8/group.
Contextual Fear Conditioning
Figure 3A shows the behavioral results of acute metyrapone treatment on CFC training. ANOVA showed a Group (F1, 28 = 6.09; P = 0.02), Minute (F1, 28 = 62.9; P = 0.00001), and Group × Minute interaction (F1, 28 = 4.61; P = 0.04). Newman-Keuls posthoc analysis indicated that foot shock induced freezing response, but, in PSD animals, this response was lower than in control animals. No treatment effect was found. During the test session (Figure 3B), ANOVA revealed a Group (F1, 28 = 8.3; P = 0.008) and Treatment (F1, 28 = 5.27; P = 0.04) effect, in which PSD animals displayed less freezing time than control animals (P = 0.008), and metyrapone-treated rats froze less than vehicle-treated animals (P = 0.03). Figure 3C presents ACTH levels 15 minutes after the test session. ANOVA detected a Group effect (F1, 28 = 19.9; P = 0.0002), in which PSD animals showed lower ACTH levels than control animals. Corticosterone levels are shown in Figure 3D, and ANOVA revealed a Group (F1, 28 = 13.08; P = 0.001), Treatment (F1, 28 = 6.78; P = 0.01), and Group × Treatment interaction (F1, 28 = 7.9; P = 0.009). Posthoc analysis indicated that all groups displayed less corticosterone levels than control-vehicle animals (P = 0.0005). No difference was found for paw flinch or vocalization indices among groups.
Figure 3.
Freezing response (mean/min) before and after foot shock during contextual fear conditioning (CFC) training (A) and during 5 minutes (mean/min) of CFC test (B). Adrenocorticotropic hormone (ACTH) (C) and corticosterone (CORT) (D) plasma levels were obtained 15 minutes after CFC test session of paradoxical sleep deprivation (PSD) (96 hours of PSD - circles, full line) and CTL (control - squares, dotted line) animals, which received an acute injection of MET (metyrapone - dark squares/circles) or VEH (vehicle - open squares/circles) 90 minutes before training. *Different from CTL group (analysis of variance [ANOVA] group effect). ¥Different from the other groups (analysis of variance group × treatment interaction effect). n = 8/group.
Correlations
A significant correlation was found for total freezing during test session and ACTH (r = 0.49, n = 8) and corticosterone levels (r = 0.56, n = 8). When considering only the control group, a correlation was still found for corticosterone levels (r = 0.53, n = 8).
Experiment 2. Chronic Metyrapone Treatment
Weight Variation
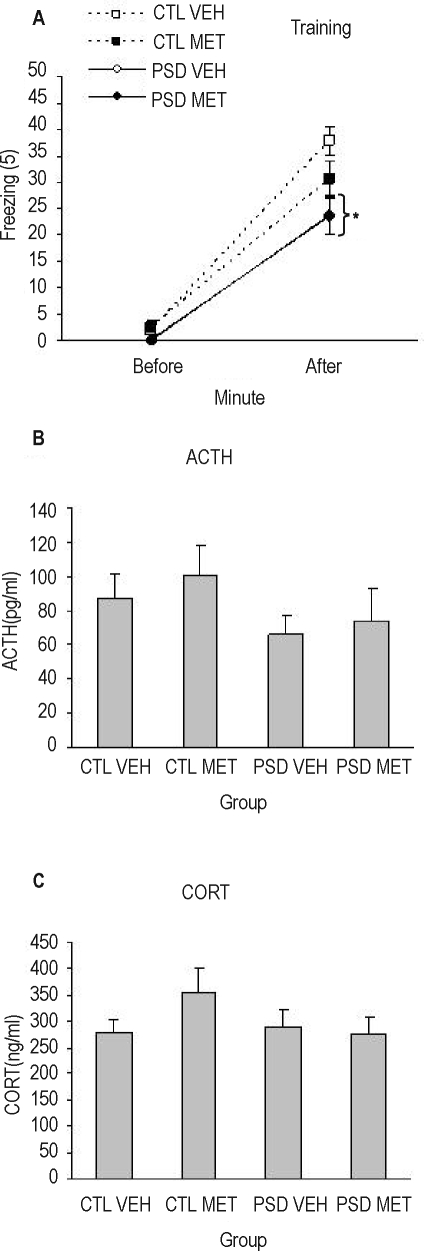
Main effects of Group (F1, 88 = 4.07; P = 0.05), Treatment (F1, 88 = 10.12; P = 0.002), and a Group × Treatment interaction effect (F1, 88 = 8.69; P = 0.004) were shown. Posthoc analysis pointed out that PSD-vehicle animals lost weight during the 4-day period, being different from the other groups (P < 0.002).
Hormone Parameters
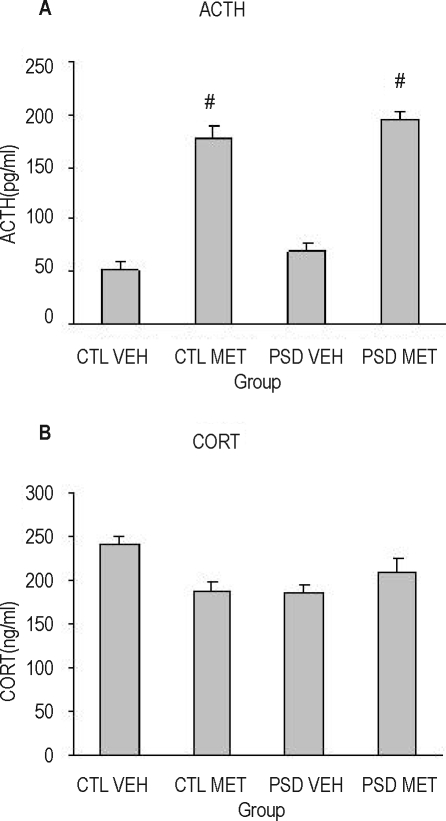
Figure 5 shows the results of ACTH (A) and corticosterone (B) from animals sacrificed 90 minutes after the last injection of metyrapone or vehicle (e.g., during the dark phase, at 21:00). Two-way ANOVA revealed a treatment effect for ACTH (F1,27 = 31.45; P < 0.0001), in which metyrapone-treated animals presented higher ACTH plasma levels than vehicle-treated ones. For corticosterone, no effect was found.
Figure 5.
Adrenocorticotropic hormone (ACTH) (A) and corticosterone (CORT) (B) plasma levels of animals sacrificed 90 minutes (20:30) after receiving the last (8th) metyrapone (MET) or vehicle (VEH) injection. PSD refers to paradoxical sleep deprivation; CTL, control. #Different from VEH group (analysis of variance treatment effect). N = 7–8/group.
Figure 4.
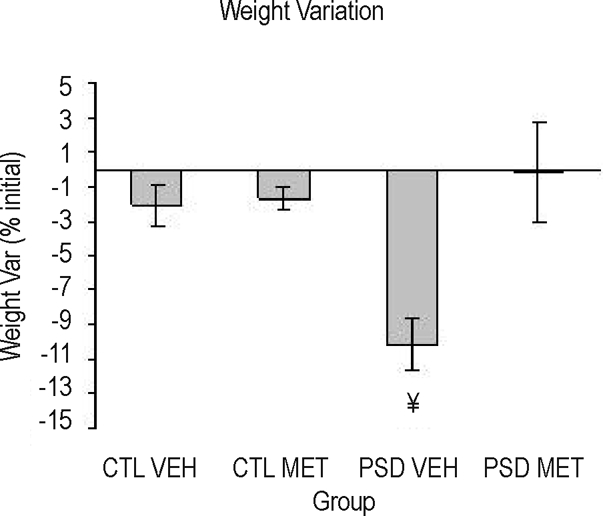
Weight variation (final × 100/initial) of sleep deprived (PSD) and control (CTL) animals injected chronically with metyrapone (MET) or vehicle (VEH). ¥Different from other the groups (analysis of variance group × treatment interaction effect). n = 20–25/group.
When the sacrifice was performed immediately after the PSD period (during the light phase, at 07:00 h), a group effect for ACTH (F1,28 = 10.33; P = 0.003) and corticosterone (F1,28 = 6.03; P = 0.02) was detected, in which PSD animals secreted more ACTH and corticosterone levels than did control animals (Figure 6 A and 6B).
Figure 6.
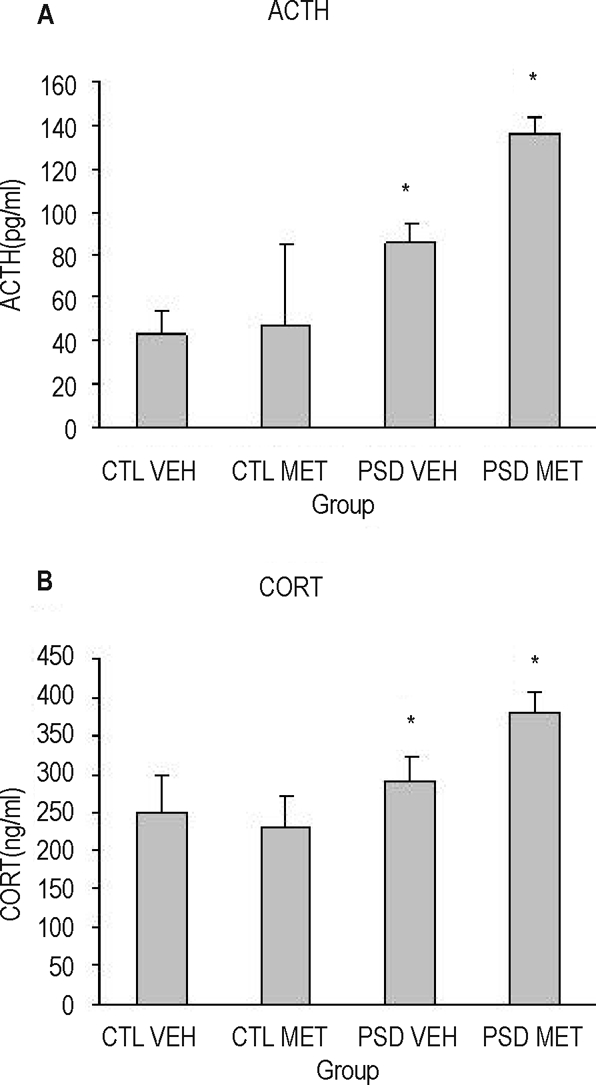
Adrenocorticotropic hormone (ACTH) (A) and corticosterone (CORT) (B) plasma levels of animals submitted to paradoxical sleep deprivation (PSD) or control (CTL) condition, chronically treated with metyrapone (MET) or vehicle (VEH). Animals were sacrificed in the morning (07:00), 12 hours after the last injection. *Different from CTL group (analysis of variance group effect). n = 7–8/group.
Contextual Fear Conditioning
A Group (F1,57 = 10.85; P=0.002), Minute (F1,57 = 253,5; P < 0.00001), and a Group × Minute interaction was found in the training session (F1,57 = 5,89; P = 0.018), indicating that all animals exhibited more freezing time after foot shock than before and that control group froze more during the last minute than PSD group (P < 0.0002). No statistical differences were found for ACTH and corticosterone levels for animals sacrificed after training (Figures 7A–C).
Figure 7.
(A) Freezing response (mean/min) before and after foot shock during training in the contextual fear conditioning (CFC). Adrenocorticotropic hormone (ACTH) (B) and corticosterone (C) plasma levels in chronically treated animals sacrificed 15 minutes after training. PSD refers to 96 hours of paradoxical sleep deprivation; CTL, control; MET, metyrapone; VEH, vehicle. * Indicates that both PSD groups, regardless of the treatment, are different from their respective CTL groups. n = 14–16 for behavioral results and 7–8 for hormone results.
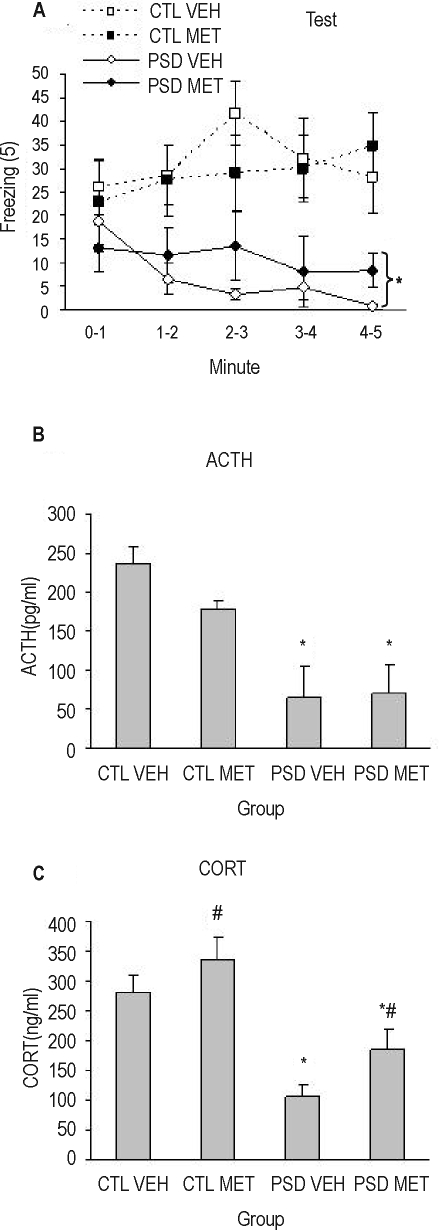
Again, during the test session, a Group (F1,27 = 15.08; P = 0.0006) and a Group × Minute interaction was found (F4,108 = 5,22; P = 0.0007). Regardless of the treatment, PSD animals showed less freezing time throughout the entire test session (P ≤ 0.01). A Group effect was found in 2-way ANOVA for ACTH levels (F1,26 = 21.88; P < 0.0001), in which PSD animals displayed less ACTH secretion than control animals after the test session. For corticosterone, a Group (F1,26 = 29.15; P < 0.0001) and Treatment effect (F1,26 = 4.74; P = 0.04) was detected, in which PSD animals exhibited less corticosterone secretion than control ones and metyrapone-treated animals displayed more corticosterone secretion than vehicle-treated ones (Figures 8A–C). No difference was found for paw flinch or vocalization indices among groups.
Figure 8.
(A) Freezing response (mean/min) during 5 minutes (mean/min) of contextual fear conditioning (CFC) test. ACTH (B) and corticosterone (C) plasma levels in animals after 96 hours of paradoxical sleep deprivation (PSD). CTL refers to control; MET, animals that received chronic administration of metyrapone; VEH, vehicle chronically treated animals. Rats were sacrificed 15 minutes after the CFC test. *Indicates that both PSD groups, regardless of the treatment, are different from their respective CTL groups. #Different from VEH group (analysis of variance treatment effect). n = 7–8/group.
Correlations
A significant correlation was found for total freezing during test session and ACTH (r = 0.50, n = 8) and corticosterone levels (r = 0.75, n = 8). When considering only the control group, a correlation was still found for corticosterone levels (r = 0.66, n = 8).
DISCUSSION
The main finding of the present study was that acute or chronic inhibition of corticosterone release immediately after or during PSD, respectively, did not prevent the impairment of memory induced by this adverse situation. Chronic treatment, however, was effective in preventing the weight loss frequently observed in paradoxical sleep-deprived animals.33,36 To the best of our knowledge, this is the first study showing that metyrapone abolishes the loss in body weight so typically found in sleep-deprived animals. Repeated stress,37 as well as PSD,33,36,38,39 has been shown to decrease body weight, although the issue regarding increased food intake is under scrutiny. Recently, the alleged PSD-induced hyperphagia was questioned by Martins and coworkers,39 who observed intense food waste that had been interpreted as food intake. Nonetheless, with the appropriate corrections of assessment, it is still possible to observe increased food intake by PSD rats, especially on the last days of sleep deprivation.36,39 It was expected that sleep deprivation would act as a stressor, either by itself or because of the methodology applied. Since chronic metyrapone treatment prevented the weight loss, our findings strongly suggest that the effect of PSD on weight loss is mediated by elevated corticosterone levels, whereas memory impairment may result from other factors.
Our results with acute metyrapone treatment are in agreement with others showing that administration of this drug before training impairs acquisition in the water maze task.23,40 Recently, a study showed that acute administration of metyrapone prevented chronic stress-induced impairments on spatial memory. In the present study, acute treatment did not inhibit PSD-induced memory impairment; on the contrary, it also impaired the performance of control rats. This effect might have been due to the dose used (100 mg/kg), which was higher than those used in Wright's study (35 and 75 mg/kg).23
In the present study, corticosterone secretion was attenuated during sleep deprivation only when metyrapone was administered chronically, e.g., the drug was not present at training or test moments. A similar treatment for control rats did not interfere with their performance during both training and test sessions. In addition, all rats exhibited a similar hormone response to training, which is expected, since corticosterone is important for memory consolidation.28 These results are in line with those reported by Ruskin and coworkers,29 who showed that adrenalectomy does not modify the effect of sleep deprivation on the MWM acquisition. Collectively, these results demonstrate that the adrenal stress response is not the source of deficits in spatial task acquisition induced by pretraining sleep deprivation. The choice of using chronic metyrapone treatment instead of adrenalectomy was done because the former induces a temporary, reversible, and dose-dependent inhibition of glucocorticoid synthesis. When using surgical removal of the adrenal gland, corticosterone release is inhibited during all phases of memory, and the purpose of the present study was to prevent elevated corticosterone levels only during sleep deprivation but not during memory acquisition, consolidation, or retrieval. Another advantage of the metyrapone treatment over adrenalectomy is that metyrapone does not disrupt synthesis and release of the adrenomedullary hormone epinephrine, which is also important for memory consolidation.30
The fact that even chronic metyrapone treatment failed to prevent the memory deficit indicates that PSD-induced memory impairment is not due to elevated corticosterone levels. Because acute treatment with metyrapone induced memory impairment, we used 8 administrations, the last 1 taking place on the night before training. Perhaps, for this reason, we did not observe the inhibiting effect of metyrapone on corticosterone secretion when the animals were sacrificed in the following morning, e.g., before training. We refrained from using the ninth administration because in Experiment 1, we showed the acute effect of the substance on memory. In a pilot study, we observed that 9 injections effectively eliminated the corticosterone response in paradoxical sleep-deprived animals (data not shown). From this result, added to the fact that ACTH levels were elevated and that chronic treatment prevented weight loss, we presumed that metyrapone was acting during the PSD period. One might speculate that the elevated levels of corticosterone at 19:00 (last injection) and immediately after PSD (07:00, before training) might be responsible for the memory impairment observed in sleep-deprived animals; however results from our lab have shown that 24 hours of pretraining sleep deprivation does not affect inhibitory avoidance task.6
Interestingly, we found no differences in corticosterone levels between PSD and control animals sacrificed at night (after the eighth injection). But it should be kept in mind that, in most of the studies carried out in our laboratory, animals were sacrificed during the day (in which PSD animals always present elevated corticosterone levels).19,33,36 Since baseline corticosterone peak occurs during the activity period (dark phase for rodents), generally, responses to stress applied at night are smaller in magnitude than those seen following stress applied at the beginning of the light cycle.41 Therefore, during the night, corticosterone levels are so high that they could mask the sleep deprivation affect. Nevertheless, it was possible to indirectly observe the treatment effect because ACTH levels were elevated in metyrapone-treated animals.
In Experiment 2, all animals displayed similar ACTH and corticosterone levels in the posttraining period, as well as similar behavioral reaction to foot shock (e.g., paw flinch and vocalization), supporting the idea of a comparable reaction to the shock delivered during training and that the effect of the metyrapone had worn off during training. The similar hormone release after training also supports the idea that memory acquisition or consolidation is not impaired by a distinct corticosterone availability, precluding the idea that differences in training-induced corticosterone secretion could be responsible for the memory impairment resulting from PSD. Sleep-deprived animals presented lower ACTH and corticosterone levels during the test, which indicates that the environment was less aversive to these rats, which ultimately reflects the decreased learning during the training, despite the fact that the hormone response of PSD rats at the time of training was similar to that of control animals. After the test session, a clear correlation between performance and hormone response was observed when analyzing all groups together. In both acute and chronic experiments, the animals that displayed high freezing behavior were those that secreted more ACTH and corticosterone in response to the exposure to the aversive context. The correlation was also valid when considering only the control group. Because deprived animals did not remember the aversive environment (Experiment 2), a floor effect might have flattened the hormone release, and no correlation between corticosterone or ACTH levels and freezing time was found. These results are in agreement with others showing the close relationship between release of stress hormones and memory performance.42 Animals chronically treated with metyrapone showed an increase in corticosterone release after the test session. This effect could be due to a supersensitivity of the adrenal gland to its tropic hormone, ACTH, insofar as chronic inhibition of corticosterone release diminished the negative feedback on the hypothalamus and pituitary, increasing ACTH secretion, which could result in upregulation of its own receptor in the adrenal gland. Thus, when the treatment ceased, even smaller doses of ACTH (e.g., reduced release in deprived animals) would induce greater corticosterone release. Another explanation for this effect involves a likely rebound effect of the 11-β-hydroxylase enzyme after cessation of the treatment, with increase activity of the enzyme leading to enhanced corticosterone production and, possibly, release in chronically treated animals.
Several studies have shown the relationship between sleep and memory, although sleep deprivation studies frequently are criticized for their use of stressful procedures. Even though a perfect control group is difficult to achieve, it is always a concern in sleep deprivation procedures. In the case of the water tank method, as was used in this study, large platform controls are commonly used. This group is submitted to the same environment, but the size of the platform is big enough for the animals to sleep. We have shown previously that large platform controls do not present memory impairment,6 although they are also sleep-deprived, to a lesser extent.34 The running treadmill method of sleep deprivation involves a certain amount of exercise and, as does the platform method, induces immobilization. Exercise groups, as well as immobilization stress control groups, present corticosterone levels similar to those of sleep-deprived animals, but they do not present with memory impairment.43,44 A sleep deprivation protocol planned to be less stressful involves the presentation of novel objects as a stimulus to keep the animal awake for 6 hours. This procedure also impairs spatial memory in the MWM task.45 Interestingly, novel-object presentation or environmental enrichment has been shown to result in enhanced performance in several memory tasks,46,47 even though rats kept in this situation present with higher levels of baseline plasma corticosterone levels.48 Therefore, it is possible to conclude that memory deficits observed with this type of sleep deprivation protocol are not caused by the stresses of the procedure per se, since both acute (novel object presentation) and chronic (environmental enrichment) exposures to novel stimuli have beneficial effects on memory.
The work from Rotllant's group49,50 suggests that metyrapone could act as a stressor, since elevated levels of ACTH and glucose, as well as c-fos activation in several brain areas, can be observed after acute administration. This possibility can not be excluded, although most of the effects observed were induced by a higher dose (200 mg/kg). The dose of 100 mg/kg used in the present study was chosen based on a pilot study (unpublished data) in which lower doses were not enough to inhibit corticosterone release in sleep-deprived animals. Therefore, other HPA axis hormones could also account for the memory impairment induced by sleep deprivation, since we observed elevated ACTH levels after metyrapone treatment, which indicates reduced corticosterone negative feedback at the pituitary level, but also at the hypothalamic level, from where CRH is released. ACTH would be an unlikely mediator of this memory impairment, since it has been shown to be an antiamnestic agent51,52. However, the same argument may not hold true for CRH, given the controversy in regard to its effect on memory. On one hand, bilateral infusions of a CRH receptor antagonist into the basolateral amygdala immediately after inhibitory avoidance training produces dose-dependent impairment of performance.53 On the other hand, continuous administration of CRH-R1 antagonist by means of a minipump improves acquisition and performance on the MWM, reduces CRH mRNA in the paraventricular nucleus of the hypothalamus, and increases mRNA of glucocorticoid receptors in the hippocampus54 Therefore, metyrapone treatment might have induced elevated CRH release, which could account for the memory impairment.
Our results suggest that glucocorticoids per se are not involved in PSD-induced memory deficit, although there is the likelihood that increased CRH resulting from blockade of corticosterone synthesis affects memory performance. Which alterations provoked by sleep deprivation other than stress could lead directly to memory deficits still remain uncertain, but one possible candidate mechanism is that sleep deprivation induces changes in cholinergic neurotransmission, with reductions in acetylcholine release, acetylcholinesterase activity, and receptor sensitivity.55–58 The cholinergic system is closely related to cognitive function,59 and previous results from our laboratory have shown that sleep deprivation-induced memory impairment can be blocked by treatment with the cholinergic agonist pilocarpine during the sleep deprivation period.7 It is possible, therefore, that sleep deprivation alters cholinergic neurotransmission, leading, in turn, to memory impairment.
ACKNOWLEDGMENTS
The authors are in debt to Ricardo Borges Machado and Marcos Vinicius Buncheidt for assistance in the behavioral testing, injections and for the animal care and to Adriana Fernandes Faria for helping with ACTH hormone determinations. This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP - CEPID# 98/14303-3) and Associação Fundo de Incentivo à Psicofarmacologia (AFIP). Paula Ayako Tiba is the recipient of a Ph.D. fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). Vanessa Contatto Rossi is the recipient of an Undergraduate Research Fellowship from FAPESP. Deborah Suchecki, Maria Gabriela Menezes de Oliveira and Sergio Tufik are the recipients of research fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no conflicts of interest.
REFERENCES
- 1.Peigneux P, Laureys S, Delbeuck X, Maquet P. Sleeping brain, learning brain. The role of sleep for memory systems. Neuroreport. 2001;12:A111–24. doi: 10.1097/00001756-200112210-00001. [DOI] [PubMed] [Google Scholar]
- 2.Smith C. Sleep states and memory processes. Behav Brain Res. 1995;69:137–45. doi: 10.1016/0166-4328(95)00024-n. [DOI] [PubMed] [Google Scholar]
- 3.Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44:121–33. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 4.Smith C. Sleep states, memory processes and synaptic plasticity. Behav Brain Res. 1996;78:49–56. doi: 10.1016/0166-4328(95)00218-9. [DOI] [PubMed] [Google Scholar]
- 5.Smith C. Sleep states and memory processes in humans: procedural versus declarative memory systems. Sleep Med Rev. 2001;5:491–506. doi: 10.1053/smrv.2001.0164. [DOI] [PubMed] [Google Scholar]
- 6.Bueno OF, Lobo LL, Oliveira MG, Gugliano EB, Pomarico AC, Tufik S. Dissociated paradoxical sleep deprivation effects on inhibitory avoidance and conditioned fear. Physiol Behav. 1994;56(4):775–9. doi: 10.1016/0031-9384(94)90241-0. [DOI] [PubMed] [Google Scholar]
- 7.Bueno OF, Oliveira GM, Lobo LL, Morais PR, Melo FH, Tufik S. Cholinergic modulation of inhibitory avoidance impairment induced by paradoxical sleep deprivation. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:595–606. doi: 10.1016/s0278-5846(00)00095-6. [DOI] [PubMed] [Google Scholar]
- 8.Dametto M, Suchecki D, Bueno OF, Moreira KM, Tufik S, Oliveira MG. Social stress does not interact with paradoxical sleep deprivation-induced memory impairment. Behav Brain Res. 2002;129:171–8. doi: 10.1016/s0166-4328(01)00345-x. [DOI] [PubMed] [Google Scholar]
- 9.Moreira KM, Hipolide DC, Nobrega JN, Bueno OF, Tufik S, Oliveira MG. Deficits in avoidance responding after paradoxical sleep deprivation are not associated with altered [3H]pirenzepine binding to M1 muscarinic receptors in rat brain. Brain Res. 2003;977:31–7. doi: 10.1016/s0006-8993(03)02688-x. [DOI] [PubMed] [Google Scholar]
- 10.Dubiela FP, de Oliveira MG, Moreira KD, Nobrega JN, Tufik S, Hipolide DC. Learning deficits induced by sleep deprivation and recovery are not associated with altered [(3)H]muscimol and [(3)H]flunitrazepam binding. Brain Res. 2005;1037:157–63. doi: 10.1016/j.brainres.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Youngblood BD, Zhou J, Smagin GN, Ryan DH, Harris RB. Sleep deprivation by the “flower pot” technique and spatial reference memory. Physiol Behav. 1997;61:249–56. doi: 10.1016/s0031-9384(96)00363-0. [DOI] [PubMed] [Google Scholar]
- 12.Smith CT, Conway JM, Rose GM. Brief paradoxical sleep deprivation impairs reference, but not working, memory in the radial arm maze task. Neurobiol Learn Mem. 1998;69:211–7. doi: 10.1006/nlme.1997.3809. [DOI] [PubMed] [Google Scholar]
- 13.Bjorness TE, Riley BT, Tysor MK, Poe GR. REM restriction persistently alters strategy used to solve a spatial task. Learn Mem. 2005;12:352–9. doi: 10.1101/lm.84805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stern WC. Acquisition impairments following rapid eye movement sleep deprivation in rats. Physiol Behav. 1971;7:345–52. doi: 10.1016/0031-9384(71)90312-x. [DOI] [PubMed] [Google Scholar]
- 15.Horne JA, McGrath MJ. The consolidation hypothesis for REM sleep function: stress and other confounding factors--a review. Biol Psychol. 1984;18:165–84. doi: 10.1016/0301-0511(84)90001-2. [DOI] [PubMed] [Google Scholar]
- 16.Vertes RP, Eastman KE. The case against memory consolidation in REM sleep. Behav Brain Sci. 2000;23:867–76. doi: 10.1017/s0140525x00004003. [DOI] [PubMed] [Google Scholar]
- 17.Siegel JM. The REM sleep-memory consolidation hypothesis. Science. 2001;294:1058–63. doi: 10.1126/science.1063049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coenen AM, van Luijtelaar EL. Stress induced by three procedures of deprivation of paradoxical sleep. Physiol Behav. 1985;35:501–4. doi: 10.1016/0031-9384(85)90130-1. [DOI] [PubMed] [Google Scholar]
- 19.Suchecki D, Lobo LL, Hipolide DC, Tufik S. Increased ACTH and corticosterone secretion induced by different methods of paradoxical sleep deprivation. J Sleep Res. 1998;7:276–81. doi: 10.1046/j.1365-2869.1998.00122.x. [DOI] [PubMed] [Google Scholar]
- 20.Korte SM. Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci Biobehav Rev. 2001;25:117–42. doi: 10.1016/s0149-7634(01)00002-1. [DOI] [PubMed] [Google Scholar]
- 21.Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci U S A. 2004;101:853–8. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conrad CD, Lupien SJ, McEwen BS. Support for a bimodal role for type II adrenal steroid receptors in spatial memory. Neurobiol Learn Mem. 1999;72:39–46. doi: 10.1006/nlme.1998.3898. [DOI] [PubMed] [Google Scholar]
- 23.Wright RL, Lightner EN, Harman JS, Meijer OC, Conrad CD. Attenuating corticosterone levels on the day of memory assessment prevents chronic stress-induced impairments in spatial memory. Eur J Neurosci. 2006;24:595–605. doi: 10.1111/j.1460-9568.2006.04948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roozendaal B, Bohus B, McGaugh JL. Dose-dependent suppression of adrenocortical activity with metyrapone: effects on emotion and memory. Psychoneuroendocrinology. 1996;21:681–93. doi: 10.1016/s0306-4530(96)00028-5. [DOI] [PubMed] [Google Scholar]
- 25.McCormick CM, McNamara M, Mukhopadhyay S, Kelsey JE. Acute corticosterone replacement reinstates performance on spatial and nonspatial memory tasks 3 months after adrenalectomy despite degeneration in the dentate gyrus. Behav Neurosci. 1997;111:518–31. doi: 10.1037//0735-7044.111.3.518. [DOI] [PubMed] [Google Scholar]
- 26.Oitzl MS, de Kloet ER. Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behav Neurosci. 1992;106:62–71. doi: 10.1037//0735-7044.106.1.62. [DOI] [PubMed] [Google Scholar]
- 27.Zorawski M, Killcross S. Posttraining glucocorticoid receptor agonist enhances memory in appetitive and aversive Pavlovian discrete-cue conditioning paradigms. Neurobiol Learn Mem. 2002;78:458–64. doi: 10.1006/nlme.2002.4075. [DOI] [PubMed] [Google Scholar]
- 28.Pugh CR, Tremblay D, Fleshner M, Rudy JW. A selective role for corticosterone in contextual-fear conditioning. Behav Neurosci. 1997;111:503–11. [PubMed] [Google Scholar]
- 29.Ruskin DN, Dunn KE, Billiot I, Bazan NG, LaHoste GJ. Eliminating the adrenal stress response does not affect sleep deprivation-induced acquisition deficits in the water maze. Life Sci. 2006;78:2833–8. doi: 10.1016/j.lfs.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Borrell J, De Kloet ER, Versteeg DH, Bohus B. Inhibitory avoidance deficit following short-term adrenalectomy in the rat: the role of adrenal catecholamines. Behav Neural Biol. 1983;39:241–58. doi: 10.1016/s0163-1047(83)90910-x. [DOI] [PubMed] [Google Scholar]
- 31.Borrell J, de Kloet ER, Bohus B. Corticosterone decreases the efficacy of adrenaline to affect passive avoidance retention of adrenalectomized rats. Life Sci. 1984;34:99–104. doi: 10.1016/0024-3205(84)90336-9. [DOI] [PubMed] [Google Scholar]
- 32.Roozendaal B, Carmi O, McGaugh JL. Adrenocortical suppression blocks the memory-enhancing effects of amphetamine and epinephrine. Proc Natl Acad Sci U S A. 1996;93:1429–33. doi: 10.1073/pnas.93.4.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suchecki D, Tufik S. Social stability attenuates the stress in the modified multiple platform method for paradoxical sleep deprivation in the rat. Physiol Behav. 2000;68:309–16. doi: 10.1016/s0031-9384(99)00181-x. [DOI] [PubMed] [Google Scholar]
- 34.Machado RB, Hipolide DC, Benedito-Silva AA, Tufik S. Sleep deprivation induced by the modified multiple platform technique: quantification of sleep loss and recovery. Brain Res. 2004;1004:45–51. doi: 10.1016/j.brainres.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 35.Nagamine S, Horisaka E, Fukuyama Y, et al. Stereoselective reductive metabolism of metyrapone and inhibitory activity of metyrapone metabolites, metyrapol enantiomers, on steroid 11 beta-hydroxylase in the rat. Biol Pharm Bull. 1997;20:188–92. doi: 10.1248/bpb.20.188. [DOI] [PubMed] [Google Scholar]
- 36.Hipólide DC, Suchecki D, Pimentel de Carvalho Pinto A, Chiconelli Faria E, Tufik S, Luz J. Paradoxical sleep deprivation and sleep recovery: effects on the hypothalamic-pituitary-adrenal axis activity, energy balance and body composition of rats. J Neuroendocrinol. 2006;18:231–8. doi: 10.1111/j.1365-2826.2006.01412.x. [DOI] [PubMed] [Google Scholar]
- 37.Bhatnagar S, Vining C, Iyer V, Kinni V. Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. J Neuroendocrinol. 2006;18:13–24. doi: 10.1111/j.1365-2826.2005.01375.x. [DOI] [PubMed] [Google Scholar]
- 38.Koban M, Le WW, Hoffman GE. Changes in hypothalamic corticotropin-releasing hormone, neuropeptide Y, and proopiomelanocortin gene expression during chronic rapid eye movement sleep deprivation of rats. Endocrinology. 2006;147:421–31. doi: 10.1210/en.2005-0695. [DOI] [PubMed] [Google Scholar]
- 39.Martins PJ, D'Almeida V, Nobrega JN, Tufik S. A reassessment of the hyperphagia/weight-loss paradox during sleep deprivation. Sleep. 2006;29:1233–8. doi: 10.1093/sleep/29.9.1233. [DOI] [PubMed] [Google Scholar]
- 40.Akirav I, Kozenicky M, Tal D, Sandi C, Venero C, Richter-Levin G. A facilitative role for corticosterone in the acquisition of a spatial task under moderate stress. Learn Mem. 2004;11:188–95. doi: 10.1101/lm.61704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kant GJ, Mougey EH, Meyerhoff JL. Diurnal variation in neuroendocrine response to stress in rats: plasma ACTH, beta-endorphin, beta-LPH, corticosterone, prolactin and pituitary cyclic AMP responses. Neuroendocrinology. 1986;43:383–90. doi: 10.1159/000124553. [DOI] [PubMed] [Google Scholar]
- 42.Gisquet-Verrier P, Botreau F, Venero C, Sandi C. Exposure to retrieval cues improves retention performance and induces changes in ACTH and corticosterone release. Psychoneuroendocrinology. 2004;29:529–56. doi: 10.1016/s0306-4530(03)00085-4. [DOI] [PubMed] [Google Scholar]
- 43.McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci. 2003;23:9687–95. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tartar JL, Ward CP, McKenna JT, et al. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci. 2006;23:2739–48. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guan Z, Peng X, Fang J. Sleep deprivation impairs spatial memory and decreases extracellular signal-regulated kinase phosphorylation in the hippocampus. Brain Res. 2004;1018:38–47. doi: 10.1016/j.brainres.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 46.Izquierdo LA, Barros DM, Medina JH, Izquierdo I. Novelty enhances retrieval of one-trial avoidance learning in rats 1 or 31 days after training unless the hippocampus is inactivated by different receptor antagonists and enzyme inhibitors. Behav Brain Res. 2000;117:215–20. doi: 10.1016/s0166-4328(00)00286-2. [DOI] [PubMed] [Google Scholar]
- 47.Izquierdo LA, Viola H, Barros DM, et al. Novelty enhances retrieval: molecular mechanisms involved in rat hippocampus. Eur J Neurosci. 2001;13:1464–7. doi: 10.1046/j.0953-816x.2001.01530.x. [DOI] [PubMed] [Google Scholar]
- 48.Moncek F, Duncko R, Johansson BB, Jezova D. Effect of environmental enrichment on stress related systems in rats. J Neuroendocrinol. 2004;16:423–31. doi: 10.1111/j.1365-2826.2004.01173.x. [DOI] [PubMed] [Google Scholar]
- 49.Rotllant D, Ons S, Carrasco J, Armario A. Evidence that metyrapone can act as a stressor: effect on pituitary-adrenal hormones, plasma glucose and brain c-fos induction. Eur J Neurosci. 2002;6:693–700. doi: 10.1046/j.1460-9568.2002.02120.x. [DOI] [PubMed] [Google Scholar]
- 50.Rotllant D, Armario A. A single dose of metyrapone caused long-term dysregulation of the hypothalamic-pituitary-adrenal axis in the rat. Neuroscience. 2005;130:427–34. doi: 10.1016/j.neuroscience.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 51.Rigter H, Van Riezen H. Pituitary hormones and amnesia. Curr Dev Psychopharmacol. 1979;5:67–124. [PubMed] [Google Scholar]
- 52.Dias RD, Izquierdo I. Memory modulation by the administration of ACTH, adrenaline or beta-endorphin after training or prior to testing in an inhibitory avoidance task in rats. Braz J Med Biol Res. 1983;16:333–7. [PubMed] [Google Scholar]
- 53.Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc Natl Acad Sci U S A. 2002;99:13908–13. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fenoglio KA, Brunson KL, Avishai-Eliner S, Stone BA, Kapadia BJ, Baram TZ. Enduring, handling-evoked enhancement of hippocampal memory function and glucocorticoid receptor expression involves activation of the corticotropin-releasing factor type 1 receptor. Endocrinology. 2005;146:4090–6. doi: 10.1210/en.2004-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bowers MB, Jr, Hartmann EL, Freedman DX. Sleep deprivation and brain acetylcholine. Science. 1966;153:1416–7. doi: 10.1126/science.153.3742.1416. [DOI] [PubMed] [Google Scholar]
- 56.Tsuchiya K, Toru M, Kobayashi T. Sleep deprivation: changes of monoamines and acetylcholine in rat brain. Life Sci. 1969;8:867–73. [PubMed] [Google Scholar]
- 57.Tufik S, Troncone LR, Braz S, Silva-Filho AR, Neumann BG. Does REM sleep deprivation induce subsensitivity of presynaptic dopamine or postsynaptic acetylcholine receptors in the rat brain? Eur J Pharmacol. 1987;140:215–9. doi: 10.1016/0014-2999(87)90808-9. [DOI] [PubMed] [Google Scholar]
- 58.Camarini R, Benedito MA. Rapid eye movement (REM) sleep deprivation reduces rat frontal cortex acetylcholinesterase (EC 3.1.1.7) activity. Braz J Med Biol Res. 1997;30:641–7. doi: 10.1590/s0100-879x1997000500012. [DOI] [PubMed] [Google Scholar]
- 59.Jerusalinsky D, Kornisiuk E, Izquierdo I. Cholinergic neurotransmission and synaptic plasticity concerning memory processing. Neurochem Res. 1997;22:507–15. doi: 10.1023/a:1027376230898. [DOI] [PubMed] [Google Scholar]