Abstract
Study Objective:
Insomnia impacts the course of major depressive disorder (MDD), hinders response to treatment, and increases risk for depressive relapse. This study is an initial evaluation of adding cognitive behavioral therapy for insomnia (CBTI) to the antidepressant medication escitalopram (EsCIT) in individuals with both disorders.
Design and setting:
A randomized, controlled, pilot study in a single academic medical center.
Participants:
30 individuals (61% female, mean age 35±18) with MDD and insomnia.
Interventions:
EsCIT and 7 individual therapy sessions of CBTI or CTRL (quasi-desensitization).
Measurements and results:
Depression was assessed with the HRSD17 and the depression portion of the SCID, administered by raters masked to treatment assignment, at baseline and after 2, 4, 6, 8, and 12 weeks of treatment. The primary outcome was remission of MDD at study exit, which required both an HRSD17 score ≤ 7 and absence of the 2 core symptoms of MDD. Sleep was assessed with the insomnia severity index (ISI), daily sleep diaries, and actigraphy.
EsCIT + CBTI resulted in a higher rate of remission of depression (61.5%) than EsCIT + CTRL (33.3%). EsCIT + CBTI was also associated with a greater remission from insomnia (50.0%) than EsCIT + CTRL (7.7%) and larger improvement in all diary and actigraphy measures of sleep, except for total sleep time.
Conclusion:
This pilot study provides evidence that augmenting an antidepressant medication with a brief, symptom focused, cognitive-behavioral therapy for insomnia is promising for individuals with MDD and comorbid insomnia in terms of alleviating both depression and insomnia.
Citation:
Manber R; Edinger JD; Gress JL; San Pedro-Salcedo MG; Kuo TF; Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. SLEEP 2008;31(4):489-495.
Keywords: Major depressive disorder, Insomnia, Cognitive behavioral therapy, Remission
DIFFICULTY INITIATING AND/OR MAINTAINING SLEEP IS COMMON IN MAJOR DEPRESSIVE DISORDER (MDD) BUT IS OFTEN INADEQUATELY ADDRESSED. Subjective and objective (electroencephalographic) sleep disturbances are associated with slower and lower rates of remission from depression.1–3 Depressed patients with abnormal sleep profiles have significantly poorer clinical outcomes with respect to symptom ratings, attrition and remission rates, and the stability of response to treatment than those with more normal sleep profiles.2,4 Patients with MDD who experience sleep continuity disturbance and early morning awakening are also more likely to have suicidal ideation than those without such disturbances.5 Collectively, these findings indicate that insomnia symptoms hinder response to antidepressant treatment.
Sleep disturbance does not always resolve with antidepressant treatment. Sleep difficulties are also common residual symptoms in individuals who have responded to depression treatment.6–10 Continued insomnia following the acute phase of antidepressant therapy poses a significant risk for relapse. For example, two-thirds of patients with persistent insomnia at the end of treatment with nortriptyline and interpersonal psychotherapy relapsed within one year after switching to pill placebo. In contrast, 90% of patients with good sleep at the end of the acute treatment remained well during the first year after discontinuing antidepressants.11 Additionally, there are indications that insomnia may be a first-occurring prodromal symptom in previously depression-remitted persons.12 Thus, insomnia is often more than merely a correlate or symptom of the depressive illness; it also affects the course of the illness, response to treatment, and when unresolved, it is a risk factor for relapse.
The prevailing model for the development of insomnia is based on the diathesis-stress model whereby a “stressor” precipitates insomnia in predisposed individuals. This model posits that, with time, conditioned insomnia develops and persists even after the stressor is removed. Specifically, as anxiety about not being able to sleep grows, it can lead to cognitive and/or somatic arousal that further interferes with sleep and perpetuates the sleep problem.13 When these sleep difficulties become associated with significant distress or impairment of function in significant domains, all criteria for a diagnosis of insomnia are met and the individual experiences comorbid MDD and insomnia. Thus, insomnia is no longer simply a symptom of depression, but has become an independent disease process and a comorbid disorder that can subsequently hinder antidepressant response.
Cognitive-behavioral therapy for insomnia (CBTI) is a skill-based, nonpharmacological intervention with many attributes that make it appealing for addressing insomnia in the context of MDD. Extensive research summarized in several meta-analyses14–17 has shown that CBTI produces improvements in primary insomnia equivalent to those achieved during acute treatment with hypnotic medications18,19 in terms of reducing nocturnal wakefulness, increasing sleep efficiency, and improving subjective sleep quality.14,20 There is also some evidence that CBTI is effective for insomnia that is comorbid with depression.21–25 Most important, sleep improvements achieved during CBTI endure up to 2 years after the course of CBTI is completed.26 This attribute of CBTI is particularly important in the context of depression, as patients who remain insomnia free are likely to remain depression free for longer periods of time than those whose insomnia recurs.12,27
The aim of the present randomized controlled pilot study was to evaluate the feasibility, acceptability, and indications of efficacy of combining an antidepressant medication (escitalopram) with CBTI in people with MDD and insomnia. The main outcome measure was remission from MDD, which is considered the ultimate goal of depression treatment.28
METHOD
Participants
Participants were recruited through newspaper advertisement, electronic bulletin boards, community postings, and brochures in clinics. The advertisements invited participation in a study on depression and insomnia and did not disclose the study hypothesis. The advertising materials stated that “participants will receive psychotherapy for insomnia and medication for depression.” Recruitment took place between June 2004 and August 2006. The study protocol was approved by the human subjects committee at Stanford University School of Medicine, and all participants provided a written consent for participation.
To be included participants had to: (1) be between the ages of 18 and 75, (2) meet the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR)29 criteria for MDD (determined by a Structured Clinical Interview for the DSM-IV (SCID)30; (3) score at least 14 on the 17-item Hamilton Rating Scale for Depression (HRSD17)31; (4) meet DSM-IV-TR criteria for insomnia based on the Duke Structured Interview for Sleep Disorders (available from coauthor JE); (5) meet the following two quantitative criteria for insomnia, based on 2 weeks of daily sleep diaries: a) sleep onset latency > 30 min and/or wake after sleep onset > 30 min per night at least 3 nights per week; b) total sleep time ≤ 6.5 h at least 3 times per week; (6) be free of any psychotropic or hypnotic medication for at least 14 days (45 days for fluoxetine) prior to the screening visit.
Participants were excluded for the following reasons: (1) current active suicidal potential, psychotic features, or having received ECT or vagal nerve stimulation treatment during the last year. (These presentations are not suitable to an experimental protocol unless it is specifically designed to address such severe presentations.); (2) seasonal pattern of MDD (to avoid confounding the result with spontaneous remission due to seasonal variations in mood); (3) history of treatment with the study medication escitalopram or failing at least two SSRIs; (4) conditions incompatible with the study medication escitalopram (e.g., pregnancy or lactation, not using a reliable birth control method, history of seizure disorder, presence of diseases, and conditions that produce altered metabolism or hemodynamic responses, and hepatic or renal dysfunction); (5) current ongoing psychotherapy, pharmacotherapy, alternative therapy, or any other treatment of claimed efficacy for depression or insomnia including any over-the-counter medications or herbs (e.g., melatonin, valerian, kava, hop extract, St John wort, SAMe); (6) Ten or more arousals per hour of sleep related to respiratory events (apneas and hypopneas); (7) ten or more periodic limb movement events per hour during sleep; (8) meeting The International Classification Of Sleep Disorders, Second edition (ICSD-2)32 criteria for circadian rhythm disorder, parasomnia, narcolepsy, or other primary sleep disorder; (9) uncontrolled medical conditions; (10) comorbid psychiatric conditions other than MDD (based on a SCID interview); (11) abnormal thyroid function (based on laboratory testing) or abnormal urine drug screen; (12) inadequate English language fluency.
Treatments
Participants received 12 weeks of EsCIT, open label and concomitantly 7 individual sessions of CBTI or CTRL therapy. The frequency of CBTI and CTRL treatment was identical (5 weekly sessions followed by 2 biweekly sessions). EsCIT was selected as a representative of the most commonly prescribed class of antidepressant medications, the selective serotonin inhibitors (SSRIs). It has the added advantage that the proportion of patients reporting daytime somnolence (6%) and insomnia (9%) as side effects is equivalent (product insert: http://www.frx.com/products/lexapro.aspx). The initial dose of EsCIT was 5 mg. It was increased to 10 mg during the second week, with additional increases up to 20 mg based on clinical response and tolerability. Medication management followed a standardized protocol33 and included biweekly visits for the first 2 months and a final study visit at the end of treatment (end of week 12).
CBTI included the following components: (a) education about normal sleep, sleep in depression, circadian rhythms, and impact of substances (Session 1); (b) sleep restriction34 and stimulus control instructions35 (introduced during Session 2 and adjusted during subsequent sessions); (c) management of stress and of cognitive and somatic arousals (Session 3); (d) cognitive restructuring36 (provided throughout the intervention); and (e) instructions for continued schedule adjustment and relapse prevention (Session 7).
The CTRL intervention consisted of a quasi-desensitization procedure that has been used successfully as a control therapy in a seminal insomnia outcome study.37 The CTRL treatment also included education about sleep and sleep hygiene (to increase the credibility of the intervention to both therapists and patients) but it did not include any other active components of CBTI. The most recent practice parameters for the psychological and behavioral treatments of insomnia concluded that there is “insufficient evidence” for sleep hygiene education to be an option as a single therapy.38
Both CBTI and CTRL therapies focused only on sleep; neither addressed mood, anhedonia, or other symptoms of depression. All participants were instructed to limit caffeine intake to the equivalent of no more than 3 cups of coffee per day and avoid all caffeine consumption in the late afternoon and evening hours. They were also advised not to consume alcohol too close to bedtime, to avoid eating large meals late at night, and to make the bedroom environment adequately dark, temperate, and quiet.
Two licensed clinical psychologist provided both CBTI and CTRL therapy, with equal case loads for each therapy. The therapists, both naïve to CBTI, were trained in the delivery of CBTI through reviewing the treatment manuals and role-playing. Therapists received supervision initially weekly and later biweekly. Training in CTRL followed the same procedures. To increase the likelihood that the therapists will perceive the 2 therapies as equally credible and expect them to be equally effective, the therapists were told that although CBTI is the standard treatment for insomnia, it has not been tested for insomnia comorbid with depression and that issues of motivation might hinder compliance with treatment recommendations. It was further stated that the CTRL therapy, which is based on desensitization, might be particularly relevant to treating insomnia in depressed patients as depressed individuals are prone to rumination. Therapists were told that the aim of the study was to compare 2 forms of psychotherapy for the treatment of insomnia in depression.
Measures
The main depression measures were the HRSD17 and the depression portion of the SCID, both administered at each study visit (at baseline and after 2, 4, 6, 8, and 12 weeks of treatment) by trained raters masked to participants' treatment assignments. The intraclass correlation based on 22 HRSD interviews was 0.96. When examined symptom-by-symptom, the median intraclass correlation was 0.85. The main outcome measure was remission of MDD at study exit, which required both an HRSD17 -score ≤ 7 and absence of the 2 core symptoms of MDD.
Changes in sleep variables were measured with daily sleep diaries and actigraphs. The daily sleep diary provides data on bedtime, rise time, minutes to sleep onset latency, minutes awake after sleep onset, minutes awake before planned (early morning awakening), and subjective sleep quality. Actigraphs provide objective minute-by-minute data on sleep-wake states. An actigraph is a watch-size motion-recording apparatus that contains an acceleration sensor, a processor, and memory. The processor records physical motion and translates it to numerical digital data. Actigraphy provides an acceptable objective longitudinal measure of sleep continuity in natural settings.39 Each participant wore an Actiwatch™ by Minimitter daily on the nondominant wrist for 2 weeks at baseline, between weeks 6 and 8 (midpoint of treatment), and between weeks 14 and 16 (end of treatment). To increase the accuracy of scoring, participants noted in the diary the times that the event marker for onset and offset of the time in bed were pressed, and documented when the unit was not worn. The following measures, recorded and derived from the diary and actigraphy data, were analyzed: total wake time (TWT; summation of sleep onset latency, wake time after sleep onset, and early morning awakening), total sleep time (TST), sleep efficiency (SE; the ratio between TST and time in bed); and subjective sleep quality (diary only).
The clinical significance of the results was evaluated with the Insomnia Severity Index (ISI).36 This measure provides an index of the global severity of insomnia, including perceived daytime consequences and distress. It has good psychometric properties.40 The score range is between 0 and 28. Scores in the range 0 to 7 represent “no clinically significant insomnia”; scores in the range of 8 to 14 represent “sub-threshold insomnia”; scores in the range of 15 to 21 represent “clinical insomnia (moderate severity)”; and scores in the range 22 to 28 represent “clinical insomnia (severe).” The ISI was administered at baseline, treatment week 5, and at the end of treatment. Remission of insomnia was defined by an ISI score ≤ 7.
Expectations of benefit from treatment were completed independently by the therapists and patients after the second session, when the 2 therapies diverged. Patients' expectations were measured with 4 items taken from the California Psychotherapy Alliance Scales (CALPAS)41,42 and the Session Evaluation Form (SEF).43 Since the items were not on the same scale, the average z-scores of the 4 items constituted the patient expectation score. Therapists' expectations of benefit for their client were computed as the average of the following 2 items, both rated on a 5-point Likert scale: “Predict the likelihood that this patient will benefit from the particular treatment you are offering.” (1 = not very likely and 5 = very likely) and “How much do you expect this patient to improve by the end of the treatment?” (1 = not at all and 5 = very much).
To assess compliance with the CBTI recommendations, therapists rated each patient on a 5-point Likert scale as to how much each instruction was followed. The Likert scale was anchored as follows: 0 = poor/no compliance; 1 = marginal; 2 = fair; 3 = good; 4 = very good; 5 = excellent.
The instructions that were rated were as follows: (1) go to bed only when sleepy; (2) get out of bed when unable to sleep initially; (3) get out of bed when unable to sleep in the middle of the night; (4) wake up at prescribed time; (5) get out of bed shortly after waking up; (6) use bed and bedroom only for sleep. An overall compliance score was computed as the mean score of all items across all time points.
Procedures
Participants who expressed initial interest in the study (N=763) were screened as follows: 1) a 15-min telephone screening interview was used to ensure basic study criteria were met; 2) in person interviews using the SCID, HRSD17, and the Duke Structured Interview for Sleep Disorders; 3) in-home polysomnography to determine RDI and PLMS index; 4) laboratory screens (thyroid and urine drug screen); 5) 2 weeks of sleep diaries to verify that research criteria for insomnia were met; and 6) a meeting with the study physician to ensure that there were no contra-indications for administering escitalopram. Qualified participants (N = 30) entered a 2 week baseline period, during which they completed sleep diaries and wore actigraphs. Participants were then randomized to EsCIT+CBTI (N = 15) or EsCIT + CTRL (N = 15). Randomization was performed in blocks of 2 and separate randomization tables were created for individuals with HRSD17 scores above and below 20 to ensure equal distribution of depression severity in the two conditions. Treatment allocation was concealed from the principal investigator and all other investigators and personnel, except for the study coordinator, who assigned participants to their groups and indicated the assignment in the randomization table, and the psychotherapists. The participants, the psychiatrist managing the medications, and the clinical raters were masked to treatment conditions. Seasonal variations in light were unlikely to confound the results as CBTI and CTRL were both administered equally at the different times of the year.
RESULTS
Baseline Characteristics
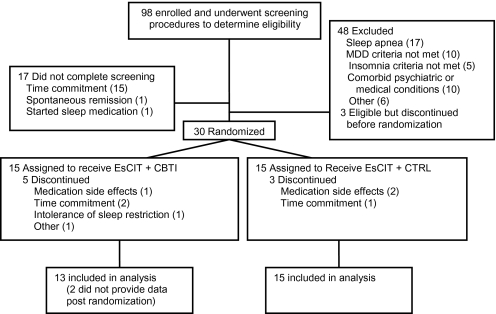
Participant flow from enrollment until the end of treatment is depicted in Figure 1. The analyzable sample consisted of all randomized participants who attended at least one post randomization assessment visit (13 in EsCIT + CBTI and 15 in EsCIT + CTRL). Demographic and baseline characteristics of the sample with respect to depression and insomnia appear in Table 1. There were no group differences in any of the baseline variables depicted in Table 1.
Figure 1.
Patient flow in the study
Table 1.
Baseline Characteristics
| CBTI Mean (SD) | CTRL Mean (SD) | Total Mean (SD) | |
|---|---|---|---|
| Age (years) | 49.5 (13.6) | 47.8 (13.4) | 48.6 (13.3) |
| Female | 54% | 67% | 61% |
| Hispanic | 8% | 7% | 7% |
| Caucasian non-Hispanic | 77% | 67% | 71% |
| Age of depression onset (years) | 37 (16.6) | 33 (19) | 35 (18) |
| Duration of current MDE (months) | 20.4 (22.9) | 16.6 (15.7) | 18.3 (19) |
| Number of past depressive episodes | 2.3 (1.6) | 2.9 (2.5) | 2.6 (2.1) |
| Double depression (Dysthymia – MDD) | 46% | 47% | 46% |
| Age of insomnia onset (years) | 38 (15) | 35 (14) | 36 (14) |
| Duration of current insomnia episode (months) | 27.9 (20.7) | 32.6 (35.6) | 30.4 (28.7) |
| BMI (Kg/m2) | 26.3 (5.4) | 25.5 (4.8) | 25.9 (5.0) |
| PLM (events per hour) | 0.4 (1.3) | 0.2 (0.8) | .03 (1.0) |
| Arousal RDI (events per hour) | 2.5 (2.0) | 2.1 (2.2) | 2.3 (2.1) |
Arousal RDI – average number of respiratory events associated with arousal per hour of sleep.
All P-values were ≥ 0.4.
Indication of Efficacy
EsCIT + CBTI resulted in a higher rate of remission from depression (61.5%) than EsCIT + CTRL (33.3%), but this large difference was not statistically significant (P = 0.13 for a one-tailed Fisher exact test). The mean baseline and endpoint HRSD17 scores and HRSD17 scores after removing the 3 sleep items, are depicted in Table 2 as are the Cohen's-d effect sizes for the differential change in HRSD scores.
Table 2.
Change in Depression Symptom Severity (Mean (SD))
| CBTI (n=14) |
CTRL (n=14) |
Cohen's-d | |||
|---|---|---|---|---|---|
| Baseline | Endpoint | Baseline | Endpoint | ES | |
| HRSD17 | 19.9 (3.8) | 7.9 (6.6) | 20.7 (5.8) | 11.0 (8.7) | 0.27 |
| HRSD17 minus sleep items | 15.5 (3.8) | 5.8 (5.6) | 16.7 (5.2) | 8.8 (7.3) | 0.24 |
HRSD17 = 17-item Hamilton Rating Scale for Depression; HRSD17 minus sleep items = HRSD17 scores after removing the three sleep items. Endpoint values are based on the last available observation. ES – effect size.
Credibility
Participants' expectations of sleep benefit, measured at the end of session 2 as the mean of the item z-scores, were −0.45 (0.64) for EsCIT + CBTI and −0.68 (0.70) for EsCIT + CTRL. The difference did not reach statistical significance (t = 0.88; P = 0.39; Cohen's-d = 0.34). To test the impact of patients' expectations on the main outcome we tested a logistic regression model with remission status as the dependent variable. The predictors included treatment group, patient expectations, both centered, and their interaction. The overall model fit was significant (χ2 = 9.4, P = 0.024). Of the variables in the model only the interaction term was significant (Wald = 4.5, P = 0.033). Follow up examination of the 4 subgroups based on split median of all patients' expectations (CBTI-Low expectation, CBTI-High expectation, CTRL-Low expectation, CTRL-High Expectation) revealed that expectations played a significant role in the EsCIT + CTRL group, with 0% (0/9) of those with low expectation attaining remission status and 83% (5/6) of those with high expectations attaining remission status. In contrast, in the EsCIT + CBTI group, 80% (4/5) of those with low expectation remitted and only 43% (3/7) of those with high expectation remitted. Therapists' expectations of benefit were significantly higher for participants in the EsCIT + CBTI group, 4.5 (0.49), than for those in the EsCIT + CTRL group, 3.5 (0.84) for CTRL (t = 3.5; P = 0.002; Cohen's-d = 1.31) and were significantly correlated with patients' expectations (r = 0.40, P < 0.05).
Impact on Sleep
Table 3 summarizes the pre- and post-treatment values for the ISI, sleep diary and actigraphy derived sleep parameters using the last available data. Participants in the CBTI group had larger improvement than those in CTRL group in all measures, except for TST. The largest effect sizes were observed for the improvement in diary based sleep efficiency (SE) and the insomnia severity index (ISI). The proportion of participants who achieved insomnia remission (ISI score ≤ 7) at the last available observation was 50.0% in the EsCIT + CBTI group and 7.7% in the EsCIT + CTRL group (χ2 = 5.7, P = 0.05). The correlation between change in ISI scores and patients' expectation of sleep benefits was significant (r = 0.49, P = 0.015). Higher expectations of benefit were associated greater reductions in ISI scores. Although there is not sufficient power to conduct a meaningful mediation analysis to determine if improvement in sleep mediated differential remission rates between the two insomnia therapies, we describe the relationship between remission form depression and remission from insomnia in the whole sample (combining both groups). Among those whose insomnia remitted 83% also experienced remission of depression, whereas among those whose insomnia did not remit only 39% experienced remission of depression (χ2 = 3.6, P = 0.08).
Table 3.
Sleep Measures Pre- and Post-Treatment (Mean [SD])
| EsCIT + CBTI |
EsCIT + CTRL |
Cohen's-d ES | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | |||
| ISI | 23.1 (2.4) | 9.5 (6.3) | 21.5 (5.0) | 14.3 (5.1) | 1.03 | |
| DIARY and ACTIGRAPHY | ||||||
| TWT (Min) | Diary | 150.5 (71.0) | 72.0 (78.8) | 149.0 (78.3) | 90.7 (72.0) | 0.31 |
| Actigraph | 88.2 (32.3) | 72.0 (33.6) | 79.2 (51.4) | 82.2 (54.6) | 1.1 | |
| TST (Min) | Diary | 329 (74) | 371 (55) | 330 (70) | 349 (85) | 0.36 |
| Actigraph | 377 (29) | 371 (49) | 374 (65) | 393 (40) | 0.61 | |
| SE (%) | Diary | 67.1 (15.9) | 84.0 (13.0) | 72.3 (15.0) | 77.6 (15.0) | 0.76 |
| Actigraph | 81.1 (6.1) | 86.4 (5.9) | 82.9 (9.8) | 83.2 (9.1) | 0.48 | |
| QUAL | Diary | 4.9 (1.6) | 6.8 (1.7) | 5.2 (2.1) | 6.1 (2.5) | 0.48 |
ISI = Insomnia Severity Index; TWT= total wake time from lights out to the time out of bed; TST = total sleep time; SE = sleep efficiency; QUAL = Sleep Quality, rated on a 10-point Likert scale; higher number indicates better subjective sleep quality. Cohen's-d effect sizes were computed for the group comparisons of the change from baseline to the last available data.
Relationship between Remission of MDD and Compliance with CBTI
For participants receiving EsCIT + CBTI, the correlation between the therapists' ratings of patients' compliance with CBTI instructions and the change in HRSD17 score from baseline to the last available observation was 0.61 (P = 0.07). Compliance with CBTI was not significantly correlated with changes in sleep (P = 0.13 for the diary rating of sleep quality and P-values ≥ 0.5 for changes in all other sleep diary variables and for the change in ISI).
DISCUSSION
This pilot study provides evidence that the strategy of augmenting an antidepressant medication with a brief, symptom focused cognitive-behavioral therapy for insomnia is promising for individuals with MDD and comorbid insomnia. With respect to the primary aim of improving remission from depression we found that the rate of remission from MDD was markedly higher for those treated with EsCIT + CBTI (62%) than in those treated with EsCIT + CTRL (33%). This is a clinically meaningful finding as remission of depression is the ultimate goal of depression treatment.28 With respect to treating insomnia, we also found a clinically and statistically significant advantage for the augmentation strategy. Treatment with EsCIT + CBTI was associated with a higher rate of remission of insomnia (50%), defined by the ISI, than treatment with EsCIT + CTRL (8%). The substantially higher remission rate of insomnia among those treated with EsCIT + CBTI suggests that CBTI improves sleep above and beyond any improvement associated with the antidepressant medication itself.
The mechanism by which augmentation of the SSRI escitalopram with a brief insomnia therapy enhances antidepressant response cannot be discerned from this pilot study. The small sample size precludes a meaningful test of the possibility that the observed differential remission of MDD is mediated by the differential improvement in sleep. Two findings from this study suggest that the magnitude of the difference in improvement of depression exceeds what would be expected from the differential improvement in sleep alone. The first is the equivalent effect sizes observed for the change in total HRSD scores and for the HRSD scores after removing the three sleep items. The second is related to the magnitude of the difference in depression remission rates between the 2 groups. It suggests a differential impact on symptoms other than sleep. Specifically, the large group difference in the rate of remission from MDD suggests that the 2 therapies differed in their impact on the core symptoms of MDD (sadness and anhedonia), as the absence of both symptoms was required before remission status can be determined. Thus, it appears that the two treatments have differentially affected non-sleep symptoms of MDD. Given that mood, concentration, energy, and coping are often affected by poor sleep, improved sleep could have generalized to improvements in these symptoms. Additional support for the strategy of combining depression treatment with insomnia treatment for patients with MDD and comorbid insomnia comes from a recent study that combined fluoxetine and eszopiclone.44 The primary endpoint of this large study (n = 545) was time awake after sleep onset rather than remission of depression, but the latter was also reported. The study found that relative to fluoxetine and placebo the combination of fluoxetine and eszopiclone produced 9% higher rates of remission of depression (33% versus 42%). Remission of insomnia as measured by the ISI was 17% higher in the group receiving the two active drugs (50% versus 33%).
The possibility that differential expectations of benefit confounded our findings needs to be considered. Our exploratory analysis, using split median, revealed that patients' expectations might have played a greater role in the CTRL treatment than in CBTI. In the CTRL treatment a greater remission rate was observed in those with expectations above the sample median compared to those whose expectations were below the median. In contrast, in the CBTI group a somewhat greater remission rate was observed in those with lower expectations compared to those with higher than median expectations. Together these findings suggest that nonspecific therapeutic factors were more likely to have impacted outcome in the CTRL group than in the CBTI group. Past research that compared CBTI with the same CTRL therapy used in the present study has successfully maintained equivalent credibility for both therapies when delivered by new therapists with no experience in CBTI.45 This was not the case in the present study, possibly because our therapists were more experienced. We selected experienced therapists because we believe that, in the context of depression, it is important that the therapists have sufficient experience in addressing clinical crisis when indicated, yet refrain from providing general psychotherapy. The possibility that differential therapists' expectations could have contributed to differential patient expectations leads us to recommend that future research use different therapists to deliver the CBTI and CTRL therapies, or consider an alternative control therapy.
As is inherent in a pilot study, the results can only be viewed as tentative and will need replication in larger samples before conclusions about the efficacy of this treatment strategy can be generalized. Such studies will need to address several limitations of the current study, including our small sample, the relative homogeneity of the sample with respect to racial distribution, and exclusion of patients with comorbidity. To increase the generalizability of the results, future research will also need to test a range of antidepressant medications.
There are many important unanswered questions related to the treatment of comorbid insomnia and depression that the current design cannot address. Although CBTI seems promising for the treatment of individuals who experience depression and insomnia at baseline, a different study design is needed to determine if CBTI can improve sleep and depression in patients with treatment emergent insomnia or in patients who experience residual symptoms of insomnia at the end of an adequate antidepressant trial.
The experience in the pilot study suggests that CBTI is acceptable to patients, as evidenced by the fact that only one participant did not tolerate treatment. We also found that CBTI is relatively easy to disseminate. In 3 months we successfully trained 2 general psychotherapists naive to sleep medicine and insomnia therapy to deliver both CBTI and CTRL. This indicates that the proposed intervention strategy, should it prove efficacious in larger replication studies, will be relatively simple to implement in the community utilizing licensed mental health providers.
ACKNOWLEDGMENTS
The authors wish to thank the treatment providers, Drs Magdolna Dunai, Brent Solvason, Kimberly Hill, and Kristin Luce, and other research personnel, Chai-Yu Cardell and Viola Arias for their contribution to the research presented in this manuscript. This research was supported by a grant form the National Institute of Metal Health (NIMH), grant number MH066131. Medications for this study were provided by Forest Laboratory.
ABBREVIATIONS
- MDD
major depressive disorder
- CBTI
cognitive behavioral therapy for insomnia
- CTRL
control therapy
- EsCIT
escitalopram
- HRSD17
Hamilton Rating Scale for Depression (17 items)
- DSM-IV-TR
Diagnostic and Statistical Manual of Mental Disorders
- SCID
Structured Clinical Interview for the DSM-IV
- ICSD-2
The International Classification of Sleep Disorders, Second edition
- ISI
Insomnia Severity Index
- TWT
total wake time
- TST
total sleep time
- SE
sleep efficiency
- BMI
Body Mass Index
- RDI
Respiratory Disturbance Index
- PLM
Periodic Limb Movement
- QUAL
Sleep Quality
Footnotes
Disclosure Statement
This was not an industry supported study. Forest Laboratory provided medication used in the study. Dr. Edinger has received research support from Respironics; has consulted for Respironics/MiniMitter Division; has participated in speaking engagements for Sleep Medicine Education Institute; and participated in a advisory panel meeting for Takeda. Dr. Kuo has received research support from Jazz Pharmaceuticals. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Buysse DJ, Reynolds CF, Houck PR, et al. Does lorazepam impair the antidepressant response to nortriptyline and psychotherapy? J Clin Psychiatry. 1997;58:426–32. doi: 10.4088/jcp.v58n1003. [DOI] [PubMed] [Google Scholar]
- 2.Dew MA, Reynolds CF, Houck PR, et al. Temporal profiles of the course of depression during treatment. Predictors of pathways toward recovery in the elderly. Arch Gen Psychiatry. 1997;54:1016–24. doi: 10.1001/archpsyc.1997.01830230050007. [DOI] [PubMed] [Google Scholar]
- 3.Winokur A, Reynolds CF. The effects of antidepressants and anxiolitics on sleep physiology. Prim Psychiatry. 1994;1:22–7. [Google Scholar]
- 4.Thase ME, Buysse DJ, Frank E, et al. Which depressed patients will respond to interpersonal psychotherapy? The role of abnormal EEG sleep profiles. Am J Psychiatry. 1997;154:502–9. doi: 10.1176/ajp.154.4.502. [DOI] [PubMed] [Google Scholar]
- 5.Thase ME. Depression, sleep, and antidepressants. J Clin Psychiatry. 1998;59:55–65. [PubMed] [Google Scholar]
- 6.Nierenberg AA, Keefe BR, Leslie VC, et al. Residual symptoms in depressed patients who respond acutely to fluoxetine. J Clin Psychiatry. 1999;60:221–5. doi: 10.4088/jcp.v60n0403. [DOI] [PubMed] [Google Scholar]
- 7.Carney CE, Segal ZV, Edinger JD, Krystal AD. A comparison of rates of residual insomnia symptoms following pharmacotherapy or cognitive-behavioral therapy for major depressive disorder. J Clin Psychiatry. 2007;68:254–60. doi: 10.4088/jcp.v68n0211. [DOI] [PubMed] [Google Scholar]
- 8.Mouchabac S, Ferreri M, Cabanac F, Bitton M. Residual symptoms after a treated major depressive disorder: in practice ambulatory observatory carried out of city. Encephale. 2003;29:438–44. [PubMed] [Google Scholar]
- 9.Manber R, Rush AJ, Thase ME, et al. The effects of psychotherapy, nefazodone, and their combination on subjective sleep in chronic depression. Sleep. 2003;15:130–6. doi: 10.1093/sleep/26.2.130. [DOI] [PubMed] [Google Scholar]
- 10.Thase ME, Rush AJ, Manber R, et al. Effects of nefazodone and cognitive behavioral analysis system of psychotherapy, singly and in combination, on insomnia associated with chronic depression. J Clin Psychiatry. 2002;63:493–500. doi: 10.4088/jcp.v63n0605. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds CF, Frank E, Houck PR, et al. Which elderly patients with remitted depression remain well with continued interpersonal psychotherapy after discontinuation of antidepressant medication? Am J Psychiatry. 1997;154:958–62. doi: 10.1176/ajp.154.7.958. [DOI] [PubMed] [Google Scholar]
- 12.Perlis ML, Giles DE, Buysee DJ, Tu X, Kupfer DJ. Self-reported sleep disturbance as a prodormal symptom in recurrent depression. J Affect Disord. 1997;42:209–12. doi: 10.1016/s0165-0327(96)01411-5. [DOI] [PubMed] [Google Scholar]
- 13.Benca R. Mood disorders. In: Kryger MH, Roth T, Dement WC, editors. Principles and practices of sleep medicine. 3rd ed. Philadelphia: W.B. Saunders; 2000. pp. 1140–57. [Google Scholar]
- 14.Morin C, Culbert J, Schwartz S. Nonpharmacological interventions for insomnia: A meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151:1172–80. doi: 10.1176/ajp.151.8.1172. [DOI] [PubMed] [Google Scholar]
- 15.Murtagh D, Greenwood K. Identifying effective psychological treatments for insomnia: A meta-analysis. J Consult Clin Psychol. 1995;63:79–89. doi: 10.1037//0022-006x.63.1.79. [DOI] [PubMed] [Google Scholar]
- 16.Nowell P, Mazumdar S, Buysse D, Dew M, Reynolds CF, Kupfer D. Benzodiazepines and zolpidem for chronic insomnia: a meta-analysis of treatment efficacy. JAMA. 1997;278:2170–7. [PubMed] [Google Scholar]
- 17.Smith MT, Perlis ML, Park A, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159:5–11. doi: 10.1176/appi.ajp.159.1.5. [DOI] [PubMed] [Google Scholar]
- 18.Morin CM, Colecchi C, Stone J, Sood RM. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA. 1999;281:991–9. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 19.Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive behavioral therapy for treatment of chronic primary insomnia: a randomized controlled trial. JAMA. 2001;285:1856–1864. doi: 10.1001/jama.285.14.1856. [DOI] [PubMed] [Google Scholar]
- 20.Murtagh DR, Greenwood KM. Identifying effective psychological treatments for insomnia: A meta-analysis. J Consult Clin Psychol. 1995;63:79–89. doi: 10.1037//0022-006x.63.1.79. [DOI] [PubMed] [Google Scholar]
- 21.Lichstein KL, Wilson NM, Johnson CT. Psychological treatment of secondary insomnia. Psychol Aging. 2000;15:232–240. doi: 10.1037//0882-7974.15.2.232. [DOI] [PubMed] [Google Scholar]
- 22.Perlis MM, Aloia M, Millikan, A, et al. Behavioral treatment of insomnia: a clinical case series study. J Behav Med. 1999;23:149–161. doi: 10.1023/a:1005413117932. [DOI] [PubMed] [Google Scholar]
- 23.Kuo T, Manber R, Loewy D. Insomniacs with comorbid conditions achieved comparable improvement in a cognitive behavioral group treatment program as insomniacs without comorbid depression. Sleep. 2001;14:A62. [Google Scholar]
- 24.Buysse DJ, Reynolds CF, 3rd, Houck PR, et al. Does lorazepam impair the antidepressant response to nortriptyline and psychotherapy? J Clin Psychiatry. 1997;58:426–32. doi: 10.4088/jcp.v58n1003. [DOI] [PubMed] [Google Scholar]
- 25.Taylor DJ, Lichstein KL, Weinstock J, Sanford S, Temple JR. A pilot study of cognitive-behavioral therapy of insomnia in people with mild depression. Behav Ther. 2007;38:49–57. doi: 10.1016/j.beth.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Morin C, Colecchi C, Stone J, Sood R. Behavioral and Pharmacological Therapies for Late-Life Insomnia: A Randomized Controlled Trial. JAMA. 1999;281:991–9. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 27.Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4:104–13. doi: 10.1207/s15402010bsm0402_3. [DOI] [PubMed] [Google Scholar]
- 28.Rush AJ, Kraemer HC, Sackeim HA, et al. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology. 2006;31:1841–53. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- 29.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th, Text Revision ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 30.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders - Patient edition (SCID-I/P, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 31.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Psychol. 1967;6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 32.AASM. diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. ICSD - 2 The International classification of sleep disorders. [Google Scholar]
- 33.Fawcett J, Epstein P, Fiester SJ, Elkin I, Autry JH. Clinical management - imipramine/placebo administration manual: NIMH Treatment of Depression Collaborative Research Program. Psychopharmacol Bull. 1987;23:309–24. [PubMed] [Google Scholar]
- 34.Spielman A, Saskin P, Thorpy M. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10:45–56. [PubMed] [Google Scholar]
- 35.Bootzin R, Epstein D, Wood J. Stimulus control instructions. In: Hauri P, editor. Case studies in insomnia. New York: Plenum Press; 1991. pp. 19–28. [Google Scholar]
- 36.Morin C. Insomnia. New York: Guilford Press; 1993. [Google Scholar]
- 37.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 38.Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. An American Academy of Sleep Medicine report. Sleep. 2006;29:1415–9. [PubMed] [Google Scholar]
- 39.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 40.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 41.Gaston L, Marmar C. Manual of California Psychotherapy Alliance Scales: Unpublished manuscript. 1991.
- 42.Marmar CR, Gaston L, Gallagher D, Thompson LW. Alliance and outcome in late-life depression. J Nerv Ment Dis. 1989;177:464–72. doi: 10.1097/00005053-198908000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Shoham-Salomon V, Avner R, Neeman R. You are changed if you do and changed if you don't: Mechanisms underlying paradoxical interventions. J Consult Clin Psychol. 1989;57:590–8. [Google Scholar]
- 44.Fava M, McCall WV, Krystal A, et al. Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol Psychiatry. 2006;59:1052–60. doi: 10.1016/j.biopsych.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 45.Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive behavioral therapy for treatment of chronic primary insomnia: a randomized controlled trial. JAMA. 2001;285:1856–64. doi: 10.1001/jama.285.14.1856. [DOI] [PubMed] [Google Scholar]