Abstract
Study Objectives:
Insomnia and depressive disorders are significant health problems in the elderly. Persistent insomnia is a risk factor for the development of new-onset and recurrent major depressive disorder (MDD). Less clear is whether persistent insomnia may perpetuate MDD and/or dysthymia. The present longitudinal study examines the relationship of insomnia to the continuation of depression in the context of an intervention study in elderly subjects.
Design:
Data were drawn from Project IMPACT, a multisite intervention study, which enrolled 1801 elderly patients with MDD and/or dysthymia. In the current study, subjects were assigned to an insomnia-status group (Persistent, Intermediate, and No Insomnia) based on insomnia scores at both baseline and 3-month time points. Logistic regressions were conducted to determine whether Persistent Insomnia was prospectively associated with increased risk of remaining depressed and/or achieving a less than 50% clinical improvement at 6 and at 12 months compared with the No Insomnia reference group. The Intermediate Insomnia group was compared with the other 2 groups to determine whether a dose-response relationship existed between insomnia type and subsequent depression.
Setting:
Eighteen primary clinics in 5 states.
Participants:
Older adults (60+) with depression.
Measurements and Results:
Overall, patients with persistent insomnia were 1.8 to 3.5 times more likely to remain depressed, compared with patients with no insomnia. The findings were more robust in patients receiving usual care for depression than in patients receiving enhanced care. Findings were also more robust in subjects who had MDD as opposed to those with dysthymia alone.
Conclusions:
These findings suggest that, in addition to being a risk factor for a depressive episode, persistent insomnia may serve to perpetuate the illness in some elderly patients and especially in those receiving standard care for depression in primary care settings. Enhanced depression care may partially mitigate the perpetuating effects of insomnia on depression.
Citation:
Pigeon WR; Hegel M; Unützer J; Fan MY; Sateia MJ; Lyness JM; Phillips C; Perlis ML. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort?. SLEEP 2008;31(4):481-488.
Keywords: Insomnia, depression, elderly, treatment response, risk factor, primary care
LATE-LIFE DEPRESSION AND INSOMNIA ARE SIGNIFICANT PUBLIC HEALTH ISSUES,1 WITH AS MANY AS 42% OF OLDER ADULTS REPORTING TROUBLES associated with sleep.2 A recent review of community and epidemiologic studies conducted exclusively in or including older-age cohorts (total n = 43,070) reported the prevalence of depression to be approximately 9% and that of insomnia to be approximately 17%.3 The estimates were lower in studies with more stringent criteria, approximately 5% and 10% for depression and insomnia, respectively. Longitudinal studies that have evaluated depression and sleep in the elderly have found that insomnia confers an increased risk for depression.4–7 As might be expected, it is not the only significant risk factor. A meta-analysis of studies in older adults found that recent bereavement, with an odds ratio (OR) of 3.3, was the largest risk factor for late-life depression and that sleep disturbance was second (OR 2.6).8
Nonetheless, insomnia has historically been considered a symptom, as opposed to a disorder. When it occurred with psychiatric illness, insomnia was viewed as a natural consequence of mood dysregulation in which sleep-onset insomnia and early-morning insomnia were considered the cardinal symptoms of anxiety and depression. As a symptom, insomnia was often viewed as a secondary phenomenon that would resolve with remission of, or recovery from, the parent disorder.
In recent years, this point of view has partially given way to the perspective that insomnia may exist as a primary disorder9,10 and, when it occurs with psychiatric illnesses, it may be viewed as a comorbid condition.9,11 This change in perspective has occurred owing to several considerations. First, to date, one longitudinal study has shown that insomnia symptoms worsen as patients with recurrent major depressive disorder (MDD) approach new-onset episodes of depression,12 suggesting that insomnia may be a prodromal symptom of depression and may trigger or precipitate new episodes.
Second, antidepressants can exert their clinical effects without ameliorating the patients' insomnia complaints. e.g., 13–16 For instance, in a fluoxetine trial, disturbed sleep and fatigue were the most common residual symptoms among depression remitters, (present in 44% and 38% of remitters, respectively).15 In a trial of nortriptyline, although depression remitters had significant decreases in mean sleep disturbance scores on the Pittsburgh Sleep Quality Index,17 their mean score remained above the clinical cutoff and higher than that of healthy controls.18 Third, similar findings have been observed in the cognitive behavioral treatment of depression.19,20 For example, in two separate randomized trials comparing cognitive behavioral treatment for depression to antidepressant medication, approximately 50% of those with remitted depression had residual insomnia, and this was evenly distributed between intervention groups.21,22 Fourth, in significant subsets of patients, insomnia becomes chronic, despite successful resolution of the psychiatric illness.23–27 Fifth, Fava et al. recently reported that coadministration of eszopiclone with fluoxetine resulted in greater sleep improvements and antidepressant effects than fluoxetine alone.28 Finally, there are a number of longitudinal studies showing that insomnia confers an increased risk for depression over time frames of between 6 months and 3 years.4–6,12,29–36 There are also studies that show that insomnia can confer risk over periods that extend over decades.7,37,38 In general, patients with persistent insomnia are approximately 3.5 times more likely to develop depression, as compared with subjects without insomnia complaints.
Despite a set of findings suggesting that insomnia is more than a symptom of depression, it does not completely rule out the possibility. Another interpretation of the above findings is that cognitive behavioral treatment for depression and a variety of antidepressant medication therapies improve sleep in a large number of patients.
It may be impossible to determine whether insomnia is purely a symptom or a marker of depression severity or if it is purely a separate disorder. More realistically, not all depressed individuals have an insomnia complaint, and, for a large percentage of depressed patients with an insomnia complaint, the insomnia does, in fact, resolve. It may be that, for some individuals, insomnia is simply a symptom that does not transition to a persistent comorbid insomnia. For others, insomnia does represent a comorbid condition that may or may not resolve without targeted intervention. One open question, therefore, is whether acutely depressed patients who already demonstrate persistent insomnia are more likely to remain depressed than patients with no insomnia complaints or than patients with only acute or mild insomnia. In the present study, we specifically evaluate the proposition that persistent insomnia may be a perpetuating factor for depression.
METHODS
Data were drawn from project IMPACT (Improving Mood-Promoting Access to Collaborative Treatment), a multisite randomized controlled trial of an enhanced care program for late-life depression in primary care that was found to significantly improve depression outcomes when compared with usual care.39 Study protocols were approved by the institutional review boards of all 7 participating sites and the study coordinating center. For the current analysis, we used the prospective data collected at the study baseline, and at 3-, 6-, and 12-month time points.
Parent Sample
A total of 1801 elderly patients with depression from 18 primary care clinics were enrolled in the study. All participants completed a written informed consent form and a structured baseline interview conducted by trained lay interviewers according to structured format. Initial diagnoses of MDD were made using the Structured Clinical Interview for DSM-III-R (SCID),7,40 which was also administered at the 6-month assessment. At each assessment (0, 3, 6, and 12 months), subjects were administered a battery of self-report questionnaires, including the 20 depression items from the Hopkins Symptom Checklist (HSCL-20).41 Additional details regarding recruitment and sample aggregation may be found in Unützer et al.42
Participants were randomly assigned to a collaborative care management intervention program or to usual care at their regular primary care clinic. All patients were identified as having MDD and/or dysthymia, and all patients were made aware of the diagnosis. Patients in the intervention arm were assigned to a depression clinical specialist (typically a nurse trained in the intervention). In their initial visit with the specialist, a brief video and brochure about depression were viewed and discussed, and the patient engaged in a discussion of treatment options, including brief problem-solving therapy and/or antidepressant therapy. The specialist met regularly with treating physicians to discuss cases. Finally, if depression remission was not achieved, the patient was considered for a change in treatment approach and/or dosage and/or a psychiatric consultation. Patients assigned to the usual-care arm were informed of their diagnosis and encouraged to follow-up with their primary care providers. Patients in this arm (and their physicians) were allowed to use all primary care or specialty mental health services available to them apart from the depression-care specialist.
Study Sample
Subjects
The original sample (n = 1801) of subjects meeting SCID criteria for MDD and/or dysthymia had a mean age of 71.2 (± 7.5) years; 77% were white/non-Hispanic, and 65% were women. Of these, 17 were excluded for completely missing data at 6 months, and a total of 27 were excluded at 12 months. Additional analyses were conducted excluding a subset of 544 subjects with dysthymia only (i.e., no MDD) at baseline.
Sample Categorization for Insomnia Status
Following the methodologic precedent set by Ford and Kamerow29 and used by other investigators,e.g., 30 insomnia status was categorized on the basis of item responses pertaining to insomnia on the HSCL. Recent 3 empiric support exists for the validity of single-item sleep measures derived from depression scales.43
In specific, the 3 HSCL questions are related to the degree of complaint associated with “trouble falling asleep,” (Item #7) “early morning awakening,” (Item #8), and “restless or disturbed sleep” (Item #9) for the month prior to the interview. Scores from these items (which ranged from 0–4) were summed (possible range 0–12) and then averaged to arrive at an insomnia score with a possible range of 0 to 4.
Subjects with a mean insomnia score of 1.0 or less at both baseline and 3 months were defined as having “No Insomnia” (n = 293); subjects scoring 2.5 or higher at both time points were defined as having “Persistent Insomnia” (n = 207). These cutoffs were chosen in order to strike a balance between each group's sample size and sample purity. More stringent cutoffs led to increasingly small group sizes, whereas less stringent cutoffs were felt to dilute both groups with subjects who may have had transient or modest insomnia. One alternative would be to choose a reasonable clinical cutoff and dichotomize the entire sample. Instead, a No Insomnia and a Persistent Insomnia group were aggregated per the above criteria, whereas all other subjects were defined as “Intermediate Insomnia” (n = 1301); these were excluded from the primary analyses but entered into secondary analyses, which allowed a dose-response relationship to be assessed.
In addition, further analyses were undertaken in a more restricted sample with insomnia status determined on the basis of having insomnia (n = 107) or no insomnia (n = 282) across 3 consecutive time points (0,3, and 6 months), with 1412 subjects falling into the intermediate category.
Sample Categorization for Depression Status
As was undertaken in the original IMPACT study,39 3 measures of depression were employed. First, the 6-month SCID assessment was used to categorize subjects as Remitted or Un-Remitted at 6 months (note that the SCID was not administered at 12 months). Second, the 20-item HSCL depression score was recalculated absent the 3 sleep items and then divided by 17 to arrive at a revised mean HSCL score with a possible range of 0 to 4. As was done in the parent study, subjects scoring a mean of less than 0.5 on the resulting HSCL score at 6 months were defined as “Remitted” and those scoring 0.5 or greater were defined as “Un-Remitted.” The same procedure was used to identify remission status at 12 months. Third, clinical improvement was defined as a 50% or greater reduction in the HSCL score (absent sleep items) from baseline to the 6-month assessment, with the groups classified as “Improved” or “Not Improved.” Again, the same procedure was used to identify improvement status at 12 months (percentage of HSCL reduction from baseline to 12-month assessment).
Statistical Considerations
Data Management
The parent IMPACT study used a multiple imputation technique for missing data. Rates of missing data ranged from 0% to 2% at the item level. The results across 5 imputed data sets were combined by averaging, and standard errors were adjusted to reflect both within-imputation variability and between-imputation variability. Additional details of the imputation techniques are available elsewhere.44 The resulting dataset was used as a starting point for the current study in which preliminary analyses were conducted using SPSS 15.0 (SPSS, Inc., Chicago, IL), whereas all outcome analyses were performed using SAS 9.1 (SAS Institute Inc., Cary, NC).
Insomnia Group Characteristics
Demographic characteristics of the Persistent Insomnia and No Insomnia groups were compared using t-tests for continuous variables (e.g., age) and were assessed using contingency analyses for categorical variables (e.g., sex). As displayed in Table 1, the groups did not differ with respect to race, sex, education, or marital status. They also did not differ with respect to clinical characteristics, including alcohol use, history of posttraumatic stress disorder, and antidepressant use. The groups did differ with respect to baseline severity of depression, the number of chronic illnesses at baseline, and the proportions of each group randomized to the IMPACT intervention versus care as usual. These 3 baseline differences are controlled for in all analyses. Depression severity (0–4.0) was stratified into 3 equal-sized strata, and chronic illness (0–11) into strata of 0 to 2, 3 to 4, and 5+ illnesses. In addition, age was significantly lower in the Persistent Insomnia group (70.0 versus 72.1 years), but age was not correlated with mean sleep scores, individual sleep item scores, change in sleep scores from baseline to 3 months, or total HSCL scores (minus sleep items) at 6 or 12 months and was therefore not included as a control variable. Instead, a propensity score was calculated and added as a control variable to adjust for a number of baseline variables (described in more detail below).
Table 1.
Demographic and Clinical Characteristics at Study Intake Comparing the Parent Sample to the Primary Study Sample and Comparing Persistent Insomnia to no Insomnia Status
| Variable | Parent sample (n = 1801) | Study sample (n = 500) | Persistent insomnia (n = 207) | No insomnia (n = 293) | P value |
|---|---|---|---|---|---|
| Age, y, mean (SD) | 71.2 (7.5) | 71.1 (7.4) | 70.0 (7.0) | 72.1 (7.6) | 0.001 |
| Female sex, no. (%) | 65% | 326 (65) | 134 (65) | 192 (66) | 0.86 |
| Ethnic minority, no. (%) | 23% | 112 (22) | 56 (27) | 56 (19) | 0.05 |
| Married, no. (%) | 46% | 229 (46) | 94 (45) | 135 (46) | 0.78 |
| High-school graduate, no. (%) | 79% | 412 (81) | 168 (81) | 244 (83) | 0.32 |
| Enhanced-care arm, no. (%) | 50% | 252 (50) | 92 (44) | 160 (54) | 0.02 |
| Depression score, mean (SD) | 1.7 (0.6) | 1.6 (0.6) | 1.9 (0.6) | 1.4 (0.6) | < 0.001 |
| Comorbid medical illnesses, no. (mean) | 3.6 (1.9) | 3.7 (2.0) | 4.1 (2.1) | 3.4 (1.8) | 0.001 |
| Alcohol problem, no. (%) | 65 (3) | 25 (5) | 12 (6) | 15 (4) | 0.72 |
| PTSD history, no. (%) | 193 (11) | 42 (8) | 22 (11) | 20 (7) | 0.13 |
| Using antidepressant, no. (%) | 771 (43) | 228 (46) | 91 (46) | 148 (45) | 0.91 |
Test statistics were t-tests for continuous data and χ2 for categorical data. PTSD refers to posttraumatic stress disorder.
*There were no group difference between the parent sample and the study sample. P values represent values for group differences between the persistent insomnia group and the no insomnia group.
Depression Outcomes
Common OR estimates with 95% confidence intervals (CI) for both Un-Remitted depression and for achieving less than a 50% improvement in depression severity were calculated at both 6 months and 12 months for the Persistent Insomnia group, with No Insomnia as the reference group in a series of logistic regressions. This was done when insomnia status was defined by 2 time points (baseline and 3 months) and by 3 time points (baseline, 3 months, and 6 months). As indicated above, adjusted ORs controlled for baseline differences on depression severity, medical burden, and intervention arm.
In addition, because subjects were not randomly assigned to Insomnia Status groups, the above covariates might not adequately adjust for potential differences between these 2 insomnia-status groups. In order to adjust for the effects of those variables, we used propensity scores to address the issue of potential selection bias.45,46 A logistic regression model was run with Persistent Insomnia as the dependent variable (yes/no) and all baseline characteristics that might be associated with the Persistent Insomnia as the independent variables. The independent variables included age, sex, ethnicity, education, marital status, alcohol screening score (CAGE), use of antidepressants, NEO score, number of chronic conditions, posttraumatic stress disorder, quality of life, 17-item HSCL score, and the 3 items of the Sheehan Disability Scale. The predicted probabilities derived from the logistic-regression model were the propensity scores. In the multiple logistic regression models in which we evaluated the adjusted effect of persistent insomnia on the 6-and 12-month depression outcomes, we included the propensity scores as a covariate in addition to the categorized depression severity (by strata), the number of chronic conditions at baseline (by strata), and assigned intervention arm (enhanced care vs usual care) to further adjust for the likelihood of a patient having persistent insomnia.
In order to assess the effect of intervention arm on outcomes, the enhanced-care arm and the usual-care arm were also analyzed separately. In order to assess whether subjects with baseline MDD differed from those with dysthymia-only at baseline, the entire set of analyses was repeated in this smaller sample. Finally, in order to assess whether there was any dose-response relationship with respect to insomnia severity, we report a simple χ2 analysis of Insomnia status (Persistent, Intermediate, and No Insomnia) and the depression outcomes at 6 and 12 months.
RESULTS
Primary Outcomes
As assessed by the SCID at 6 months and the HSCL at both 6 and 12 months, subjects with Persistent Insomnia were more likely to exhibit Un-Remitted depression and less than 50% improvement on the HSCL, in contrast to the No Insomnia reference group on 5 of the 7 measures of depression. As presented in Table 2, adjusted ORs ranged from 1.8 to 3.5. These outcomes were more robust when the sample was restricted by excluding subjects who were dysthymic only (i.e., did not have MDD). In this MDD sample, Persistent Insomnia was a significant predictor in 6 of 7 the models, with adjusted ORs ranging from 2.5 to 5.9.
Table 2.
Adjusted Odds Ratio Estimates of Variables Predicting Depression Status at 6 and 12 Months
| Model Measure of DV IVs in Model | Entire Sample |
Enhanced Care Arm |
Usual Care Arm |
|||||
|---|---|---|---|---|---|---|---|---|
| β | SE | P | Adjusted OR (95% CI) | P | Adjusted OR (95% CI) | P | Adjusted OR (95% CI) | |
| 1 SCID MDD at 6 Mo | ||||||||
| Persistent Insomnia (2x) | 1.02 | 0.25 | < 0.0001 | 2.77 (1.7–4.5) | 0.055 | 2.13 (.98–4.6) | < 0.0001 | 3.61 (1.8–7.2) |
| Intervention arm | −0.58 | 0.23 | 0.010 | 0.56 (0.4–0.9) | - | - | - | - |
| SCL17 (strata 2 vs 1) | 0.32 | 0.32 | 0.322 | 1.37 (0.7–2.6) | 0.515 | 1.38 (0.5–3.6) | 0.189 | 1.72 (0.8–3.8) |
| SCL17 (strata 3 vs 1) | 0.63 | 0.41 | 0.124 | 1.88 (0.8–4.2) | 0.023 | 4.39 (1.2–15.0) | 0.176 | 1.89 (0.8–4.7) |
| No. diseases (2 vs 1) | 0.10 | 0.30 | 0.729 | 1.11 (0.6–2.0) | 0.429 | 1.45 (0.6–3.7) | 0.950 | 1.03 (0.5–2.3) |
| No. diseases (3 vs 1) | 0.53 | 0.31 | 0.092 | 1.69 (0.9–3.1) | 0.003 | 4.43 (1.7–11.7) | 0.975 | 0.99 (0.4–2.2) |
| Propensity score | 0.88 | 0.76 | 0.244 | 2.40 (0.5–10.6) | 0.478 | 0.45 (0.1–4.1) | 0.139 | 3.30 (0.7–16.0) |
| 2 SCL Un-Remitted at 6 Mo | # | # | ||||||
| Persistent Insomnia (2x) | 1.04 | 0.27 | < 0.0001 | 2.83 (1.7–4.8) | 0.127 | 1.74 (0.9–3.6) | < 0.0001 | 4.65 (2.0–10.7) |
| 3 < 50% improved at 6 Mo | ||||||||
| Persistent Insomnia (2x) | 1.13 | 0.23 | < 0.0001 | 3.09 (2.0–4.9) | 0.054 | 1.87 (0.99–3.5) | < 0.0001 | 5.26 (2.5–10.8) |
| 4 SCL Un-Remitted at 12 Mo | # | # | ||||||
| Persistent Insomnia (2x) | 0.12 | 0.28 | 0.670 | 1.13 (0.6–2.0) | 0.258 | 0.65 (0.3–1.4) | 0.113 | 2.19 (0.8–5.8) |
| 5 < 50% improved at 12 Mo | ||||||||
| Persistent Insomnia (2x) | 0.57 | 0.24 | 0.018 | 1.78 (1.1–2.9) | 0.574 | 1.20 (0.6–2.3) | 0.008 | 2.89 (1.3–6.3) |
| 6 SCL Un-Remitted at 12 Mo | # | # | # | |||||
| Persistent Insomnia (3x) | 0.59 | 0.42 | 0.166 | 1.81 (0.8–4.2) | 0.893 | 0.92 (0.3–2.8) | 0.042 | 5.56 (1.1–29.1) |
| 7 < 50% improved at 12 Mo | ||||||||
| Persistent Insomnia (3x) | 1.24 | 0.35 | < 0.0001 | 3.47 (1.7–6.9) | 0.028 | 2.91 (1.1–7.5) | 0.009 | 4.55 (1.5–14.1) |
In models 1–5, insomnia status is consistent across 2 time points (2x): baseline and 3 months (No Insomnia is the reference group); whereas, in models 6–7, insomnia status is consistent across 3 time points (3x): baseline, 3 months, and 6 months. In models 2–7, the same variables as in Model 1 were entered, but only the values for Persistent Insomnia are displayed. Besides intervention arm, no other variables significantly predicted depression status except for Symptom Checklist (SCL) strata 2 vs. SCL strata 1 (denoted by #). DV refers to Dependent Variable; IV, Independent Variables; SCID, Structured Clinical Interview for DSM-III-R; OR, odds ratio; CI, confidence interval.
Effect of Intervention Arm
When the entire sample was analyzed, intervention arm was a significant predictor in all models when entered as an independent variable. We then ran all models with an interaction variable (between Persistent Insomnia and Intervention), but the interaction variable was not a significant predictor in any of the models. We then removed both the interaction term and the intervention variable from each model in order to reanalyze data by intervention arm. As presented in Table 2, the findings with respect to persistent insomnia were more robust in the usual-care group (and less robust in the enhanced-care group).
Dose-Response Relationship
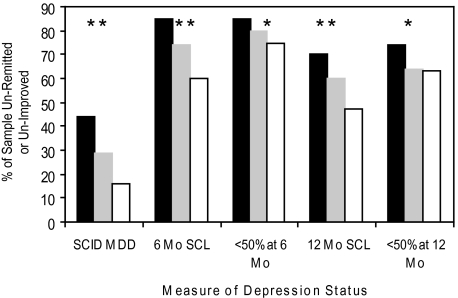
χ2 analyses revealed significant associations between “insomnia dose” (Persistent, Intermediate, or No Insomnia) and depression status. At the 6-month time point, SCID-assessed MDD remained in 44% of subjects with Persistent Insomnia, 29% of those with Intermediate Insomnia, and 16% of those with No Insomnia (P < 0.001). Figure 1 graphically depicts these findings and those from the other measures of depression status; all analyses were significant at P < 0.05.
Figure 1.
Dose-Response Relationship of Insomnia Status Across Depression Outcomes. The Persistent Insomnia, Intermediate Insomnia and No Insomnia groups are represented by the black, grey, and white bars, respectively. The measures of depression are defined as: SCID MDD (Structured Clinical Interview for DSM-III-R diagnosis of major depressive disorder at 6 months); 6 Mo SCL (scoring above the depression cutoff on the 17-item version of the Hopkins Symptom Checklist [HSCL] at 6 months); < 50% at 6 Mo (a less than 50% improvement on the 17-item version of the HSCL from baseline to 6 months); 12 Mo SCL (scoring above the depression cutoff on the 17-item version of the HSCL at 12 months); and < 50% at 12 Mo (a less than 50% improvement on the 17-item version of the HSCL from baseline to 6 months). For each measure of depression, χ2 analyses conducted where * denotes P < 0.05 and ** denotes P < 0.001.
Posthoc Descriptive Results
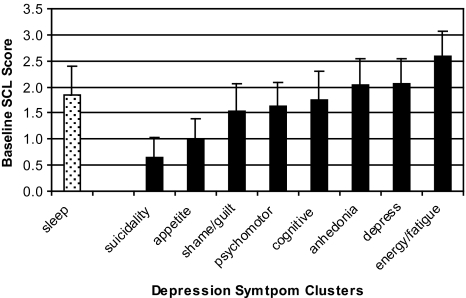
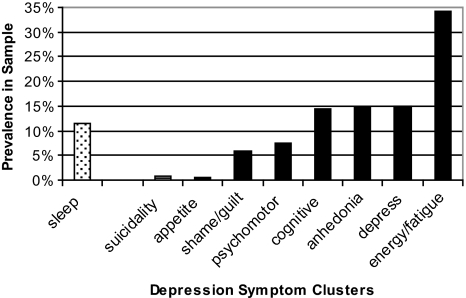
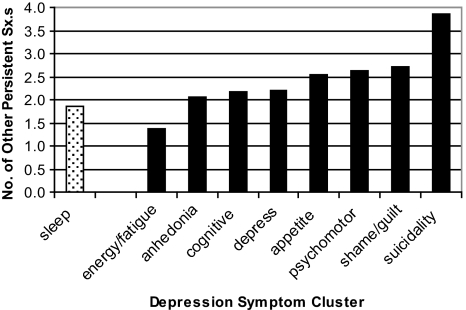
In order to place insomnia within a context of other symptom clusters of depression, we provide some simple descriptive statistics. For the entire parent sample (n = 1801), mean HSCL scores for each of the 9 depression-symptom clusters at baseline are displayed in Figure 2a (0–4.0 range possible). As can be seen, insomnia had the fourth-highest mean baseline value and the largest standard deviation. In terms of persistent symptom clusters (with persistence defined as a mean symptom score of 2.5 or greater at both baseline and 3 months), Persistent Insomnia was observed in 11.6% of the parent sample, which is fifth of the 9 symptom clusters (see Figure 2b). Finally, we wondered whether any particular persistent symptom would occur in the absence of other persistent symptoms (or in the presence of multiple other persistent symptoms). Figure 2c shows, for each persistent symptom cluster, how many other persistent symptoms co-occur. The number of other persistent symptoms ranged from 1.4 to 3.9 additional persistent symptoms. Persistent Insomnia is associated with the second-smallest number of persistent symptoms (1.9) and, when present, Persistent Insomnia is most often associated with persistent fatigue (57% of the time).
Figure 2a.
Mean Symptom Score at Baseline. Mean symptom cluster scores of the parent sample (n = 1801) at baseline with a possible range of 0 to 4.0.
Figure 2b.
Prevalence of Persistent Symptom. Percentage of the parent sample that exhibited an elevated symptom score at both baseline and the 3-month time point.
Figure 2c.
Co-occurring Persistent Symptoms. For each individual symptom, the bar represents the mean number of other persistent symptoms that are co-occurring. For example, subjects with persistent insomnia had a mean of 1.9 co-occurring persistent depressive symptoms.
DISCUSSION
In the present study, after adjusting for baseline differences, it was found that depressed older primary care patients with persistent insomnia were more likely to remain depressed or fail to achieve a benchmark of clinically significant improvement, as compared with subjects with no insomnia. These results are in keeping with prior longitudinal studies that indicated that insomnia was a risk factor for both first and recurrent episodes of major depression. The present study adds to this body of literature by providing evidence that insomnia may also serve to perpetuate depression.
The finding that this risk was higher in the usual-care group, suggests that enhanced depression may partially mitigate the perpetuating effects of insomnia on depression. In addition, the finding that, as insomnia moves from not present to acutely or moderately present to persistently present, the prevalence of ongoing depression increases in a dose-response fashion is an expected outcome especially if persistent insomnia is different than insomnia as a symptom. The fact that the primary findings were more robust in the MDD population, than in those with dysthymia alone, is less straightforward. This is perhaps owing to underlying differences in these clinical entities. Finally, comparing insomnia to other depressive symptoms provides some interesting, albeit inconclusive, data. Insomnia is no more or less prevalent than other symptoms either at baseline or when the symptoms are classified as persistent by meeting the same criteria across 2 consecutive time points, yet, when it does present as a persistent symptom, it is less often part of a larger set of persistent symptoms than are other symptom clusters. Certainly this may be because depression presents across time with a changing array of persistent symptoms. Nonetheless, if persistent insomnia were merely a marker for more entrenched or more severe depression, one would expect it to occur more regularly with other persistent depressive symptoms. Overall, the set of findings from this study support the hypothesis that insomnia may serve as a perpetuating factor in depression.
What these data do not unequivocally tell us is whether insomnia that presents with depression is a symptom or a comorbid disorder. Although we have primarily argued for the latter perspective, it is more likely, as noted in the introduction, that insomnia is simply a symptom in some cases and clearly a disorder requiring its own treatment focus in other cases. In the absence of a definitive causal study, it will take a continued amalgamation of varying kinds of evidence to make a causal claim with respect to insomnia perpetuating the course of depression. The findings from the current study do begin to build the case that persistent insomnia (as defined in this study) may blunt treatment response and serves as a barrier to remission from depression in a particular population and that this is especially true in older patients receiving standard primary care-based treatment of depression.
This study has some of the same limitations that are true of the epidemiologic studies that preceded it. First, the measure used consisted of 3 sleep-related items as opposed to a validated insomnia instrument, though the use of item measures has recently been validated as an index of insomnia.43 Second, removing the 3 sleep items from the HSCL to assess clinical status and depression severity created an unvalidated index of depression. Third, the time between measurements (3–6 months) only allows for an approximation of acute versus persistent insomnia, as the HSCL asks for answers to be tied to the prior month; by default this does not allow us to adequately characterize the difference among what may be transient, recurrent, or persistent insomnia. Similarly, the instruments used are retrospective and subjective for the prior month (as opposed to a daily sleep diary that provides such data much more proximally). Fourth, we were unable to control for type and number of medications used, including antidepressant type. Finally, our data do not speak to the issue of whether the effects observed in this study generalize to other age cohorts.
In order to further evaluate the proposition that insomnia serves to perpetuate depression, several lines of research are possible. First, a large-scale longitudinal study using full, validated insomnia instruments with monthly time points could be deployed to replicate and extend the present findings. Second, intervention trials (both pharmacologic and behavioral) may be conducted that treat persistent insomnia in either current or remitted depression to assess whether this improves depression or delays or aborts recurrent episodes of depression. There are 2 uncontrolled studies that have shown that patients presenting with insomnia and depression who completed a course of cognitive behavioral therapy for insomnia had improvements in both sleep and depression.47,48 Controlled trials are needed to replicate these preliminary findings. Third, an intervention model could be used to determine whether adjunctive treatment for insomnia (in addition to standard treatment for depression) affects the clinical course of MDD. To date, 1 such study has shown that patients treated concomitantly with fluoxetine and eszopiclone exhibited less illness severity over the course of an 8-week intervention and a faster time to recovery than subjects treated with fluoxetine only.21 If replicated and extended to include other adjuvant interventions (e.g., cognitive behavioral therapy) such data would test the perspective that persistent insomnia is a comorbid condition that, when untreated, prolongs illness and, when treated, hastens recovery. If possible, it would also be helpful to determine if there are levels of insomnia severity and/or duration that are empiric thresholds for chronicity that seems to be a risk for ongoing depression. Similarly, data that could establish the patient characteristics for insomnia that does versus does not resolve when depression ameliorates could help guide clinical decision-making during the course of depression.
ACKNOWLEDGMENTS
We would like to acknowledge the contributions and support of patients, primary care providers, investigators, and staff at the study coordinating center and at all participating study sites, which include Duke University, Durham, NC; The South Texas Veterans Health Care System, The Central Texas Veterans Health Care System, and The San Antonio Preventive and Diagnostic Medicine Clinic; Indiana University School of Medicine, Indianapolis, IN; Health and Hospital Corporation of Marion County; Group Health Cooperative of Puget Sound in cooperation with the University of Washington, Seattle, WA; Kaiser Permanente of Northern California, Oakland and Hayward, CA; Kaiser Permanente of Southern California, San Diego, CA; Desert Medical Group, Palm Springs, CA. This study is the result of work supported in part with patients, resources, and the use of facilities at the South Texas Veterans Health Care System and the Central Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Support: The IMPACT study, JU, and MH are supported by grants from the John A. Hartford Foundation, the California Healthcare Foundation, the Hogg Foundation, and the Robert Wood Johnson Foundation. WRP is supported by National Institutes of Health grant NS049789 and the American Sleep Medicine Foundation. JML is supported by National Institutes of Health grant MH71509.
A preliminary version of this paper was presented at the Associated Professional Sleep Societies annual meeting in Denver, Colorado, on June 21, 2005.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Perlis has received research support from Cephalon, and Sanofi-Aventis; has consulted for MiniMitter-Respironics, Gerson Lehrman Group, Elan-King Pharmaceuticals; has participated in speaking engagements for Sanofi-Aventis; is on the advisory board of Elan-King Pharmaceuticals; and is the Managing Member of Internet Didactics Services. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Lyness JM, Caine ED, King DA, Conwell Y, Duberstein PR, Cox C. Depressive disorders and symptoms in older primary care patients: one-year outcomes. Am J Geriatr Psychiatry. 2002;10:275–82. [PubMed] [Google Scholar]
- 2.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–42. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 3.Pigeon WR, Perlis ML. Sleep & psychiatric illness. In: Avidon AY, Alessi C, editors. Geriatric Sleep Medicine. New York: Informa Health Care; 2008. [Google Scholar]
- 4.Brabbins CJ, Dewey ME, Copeland JR, Davidson IA. Insomnia in the elderly: Prevalence, gender differences and relationships with morbidity and mortality. Int J Geriatr Psychiatry. 1993;8:473–80. [Google Scholar]
- 5.Roberts RE, Shema SJ, Kaplan GA, Strawbridge WJ. Sleep complaints and depression in an aging cohort: A prospective perspective. Am J Psychiatry. 2000;157:81–8. doi: 10.1176/ajp.157.1.81. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy GJ, Kelman HR, Thomas C. Persistence and remission of depressive symptoms in late life. Am J Psychiatry. 1991;148:174–8. doi: 10.1176/ajp.148.2.174. [DOI] [PubMed] [Google Scholar]
- 7.Mallon L, Broman JE, Hetta J. Relationship between insomnia, depression, and mortality: a 12-year follow-up of older adults in the community. Int Psychogeriatr. 2000;12:295–306. doi: 10.1017/s1041610200006414. [DOI] [PubMed] [Google Scholar]
- 8.Cole MG, Dendukuri N. Risk factors for depression among elderly community subjects: A systematic review and meta-analysis. Am J Psychiatry. 2003;160:1147–56. doi: 10.1176/appi.ajp.160.6.1147. [DOI] [PubMed] [Google Scholar]
- 9.Rochester, MN: American Sleep Disorders Association; 1997. The International Classification of Sleep Disorders: Diagnostic and Coding Manual - Revised. [Google Scholar]
- 10.4th ed. Washington: American Psychiatric Association; 1994. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 11.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 12.Perlis M, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4:104–13. doi: 10.1207/s15402010bsm0402_3. [DOI] [PubMed] [Google Scholar]
- 13.Karp JF, Buysse DJ, Houck PR, Cherry C, Kupfer DJ, Frank E. Relationship of variability in residual symptoms with recurrence of major depressive disorder during maintenance treatment. Am J Psychiatry. 2004;161:1877–84. doi: 10.1176/ajp.161.10.1877. [DOI] [PubMed] [Google Scholar]
- 14.Opdyke KS, Reynolds CF, III, Frank E, et al. Effect of continuation treatment on residual symptoms in late-life depression: how well is “well”? Depress Anxiety. 1996;4:312–9. doi: 10.1002/(SICI)1520-6394(1996)4:6<312::AID-DA7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 15.Nierenberg AA, Keefe BR, Leslie VC, et al. Residual symptoms in depressed patients who respond acutely to fluoxetine. J Clin Psychiatry. 1999;60:221–5. doi: 10.4088/jcp.v60n0403. [DOI] [PubMed] [Google Scholar]
- 16.Paykel ES, Ramana R, Cooper Z, Hayhurst H, Kerr J, Barocka A. Psychol Med. 1995. Residual Symptoms After Partial Remission: An Important Outcome In Depression; pp. 1171–80. [DOI] [PubMed] [Google Scholar]
- 17.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds CF, Hoch CC, Buysse DJ, et al. Sleep in late-life recurrent depression. Changes during early continuation therapy with nortriptyline. Neuropsychopharmacology. 1991;5:85–96. [PubMed] [Google Scholar]
- 19.Simons AD, Murphy GE, Levine JL, Wetzel RD. Cognitive therapy and pharmacotherapy for depression: Sustained improvement over on year. Arch Gen Psychiatry. 1986;43:43–8. doi: 10.1001/archpsyc.1986.01800010045006. [DOI] [PubMed] [Google Scholar]
- 20.Thase ME, Simons AD, Cahalane JF, McGeary J. Cognitive behavior therapy of endogenous depression: I. An outpatient clinical replication series. Behavior Therapy. 1991;22:457–467. [Google Scholar]
- 21.Carney CE, Segal ZV, Edinger JD, Krystal AD. A comparison of rates of residual insomnia symptoms following pharmacotherapy or cognitive-behavioral therapy for major depressive disorder. J Clin Psychiatry. 2007;68:254–60. doi: 10.4088/jcp.v68n0211. [DOI] [PubMed] [Google Scholar]
- 22.Manber R, Rush J, Thase ME, et al. The effects of psychotherapy, nefazodone, and their combination on subjective assessment of disturbed sleep in chronic depression. Sleep. 2003;26:130–6. doi: 10.1093/sleep/26.2.130. [DOI] [PubMed] [Google Scholar]
- 23.Lichstein K, Wilson NM, Johnson CT. Psychological treatment of secondary insomnia. Psychol Aging. 2000;15:232–40. doi: 10.1037//0882-7974.15.2.232. [DOI] [PubMed] [Google Scholar]
- 24.Mendelson WB. Long-term follow-up of chronic insomnia. Sleep. 1995;18:698–701. doi: 10.1093/sleep/18.8.698. [DOI] [PubMed] [Google Scholar]
- 25.Ohayon M. Epidemiological study on insomnia in the general population. Sleep. 1996;19:S7–15. doi: 10.1093/sleep/19.suppl_3.s7. [DOI] [PubMed] [Google Scholar]
- 26.Harvey AG. Insomnia: Symptom or diagnosis? Clinical Psychology Review. 2001;21:1–22. doi: 10.1016/s0272-7358(00)00083-0. [DOI] [PubMed] [Google Scholar]
- 27.Hauri P, Chernik D, Hawkins D, Mendels J. Sleep of depressed patients in remission. Arch Gen Psychiatry. 1974;31:386–91. doi: 10.1001/archpsyc.1974.01760150090013. [DOI] [PubMed] [Google Scholar]
- 28.Fava M, McCall WV, Krystal A, et al. Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol Psychiatry. 2006;59:1052–60. doi: 10.1016/j.biopsych.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 30.Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411–8. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 31.Dryman A, Eaton WW. Affective symptoms associated with the onset of major depression in the community: findings from the US National Institute of Mental Health Epidemiologic Catchment Area Program. Acta Psychiatr Scand. 1991;84:1–5. doi: 10.1111/j.1600-0447.1991.tb01410.x. [DOI] [PubMed] [Google Scholar]
- 32.Livingston G, Blizard B, Mann A. Does sleep disturbance predict depression in elderly people? A study in inner London [see comments] Br J Gen Pract. 1993;43:445–8. [PMC free article] [PubMed] [Google Scholar]
- 33.Vollrath M, Wicki W, Angst J. The Zurich study. VIII. Insomnia: association with depression, anxiety, somatic syndromes, and course of insomnia. Eur Arch Psychiatry Neurol Sci. 1989;239:113–24. doi: 10.1007/BF01759584. [DOI] [PubMed] [Google Scholar]
- 34.Weissman MM, Greenwald S, Nino-Murcia G, Dement WC. The morbidity of insomnia uncomplicated by psychiatric disorders. Gen Hosp Psychiatry. 1997;19:245–50. doi: 10.1016/s0163-8343(97)00056-x. [DOI] [PubMed] [Google Scholar]
- 35.Livingston G, Watkin V, Milne B, Manela MV, Katona C. Who becomes depressed? The Islington community study of older people. J Affect Disord. 2000;58:125–33. doi: 10.1016/s0165-0327(99)00103-2. [DOI] [PubMed] [Google Scholar]
- 36.Hohagen F, Rink K, Kappler C, Schramm E. Prevalence and treatment of insomnia in general practice: A longitudinal study. Eur Arch Psychiatry Clin Neurosci. 1993;242:329–36. doi: 10.1007/BF02190245. [DOI] [PubMed] [Google Scholar]
- 37.Paffenbarger RS, Lee IM, Leung R. Physical-activity and personal characteristics associated with depression and suicide in American-college men. Acta Psychiatr Scand. 1994;89:16–22. doi: 10.1111/j.1600-0447.1994.tb05796.x. [DOI] [PubMed] [Google Scholar]
- 38.Chang PP, Ford DE, Mead LA, Cooper-Patrick L, Klag MJ. Insomnia in young men and subsequent depression. The Johns Hopkins Precursors Study. Am J Epidemiol. 1997;146:105–14. doi: 10.1093/oxfordjournals.aje.a009241. [DOI] [PubMed] [Google Scholar]
- 39.Unutzer J, Katon W, Callahan CM, et al. Collaborative care management of late-life depression in the primary care setting - A randomized controlled trial. JAMA. 2002;288:2836–45. doi: 10.1001/jama.288.22.2836. [DOI] [PubMed] [Google Scholar]
- 40.Spitzer R, Williams J, Gibbon R, First M. The structured clinical interview for DSM-III-R (SCID): I. History, rationale and description. Arch Gen Psychiatry. 1992;49:624–9. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 41.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HCSL): A self-report symptom inventory. Behav Sci. 1974;19:1–15. doi: 10.1002/bs.3830190102. [DOI] [PubMed] [Google Scholar]
- 42.Unutzer J, Katon W, Williams JW, et al. Improving primary care for depression in late life - The design of a multicenter randomized trial. Med Care. 2001;39(8):785–99. doi: 10.1097/00005650-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Manber R, Blasey C, Arnow B, et al. Assessing insomnia severity in depression: comparison of depression rating scales and sleep diaries. J Psychiatr Res. 2005;39:481–8. doi: 10.1016/j.jpsychires.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Tang L, Song J, Belin TR, Unutzer J. A comparison of imputation methods in a longitudinal randomized clinical trial. Stat Med. 2005;24:2111–28. doi: 10.1002/sim.2099. [DOI] [PubMed] [Google Scholar]
- 45.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for casual effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 46.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516–24. [Google Scholar]
- 47.Morawetz D. Behavioral self-help treatment for insomnia: a controlled evaluation. Behav Therapy. 1989;20:365–79. [Google Scholar]
- 48.Taylor DJ, Lichstein K, Weinstock J, Temple J, Sanford S. A pilot study of cognitive-behavioral therapy of insomnia in people with mild depression. Behav Therapy. 2007;38:49–57. doi: 10.1016/j.beth.2006.04.002. [DOI] [PubMed] [Google Scholar]