Abstract
Aim:
To analyze the heart rate (HR) response to traffic noise during sleep and the influence of acoustic parameters, time of night, and momentary sleep stage on these responses.
Participants:
Twelve women and 12 men (19–28 years).
Measurements and Results:
The participants slept in the laboratory for 4 consecutive nights in each of 3 consecutive weeks and were exposed to aircraft, road, or rail traffic noise with weekly permutations. The 4 nights of each week consisted of a random sequence of a quiet night (32 dBA) and 3 nights during which aircraft, rail traffic, or road traffic noises occurred with maximum levels of 45–77 dBA. The polysomnogram and the electrocardiogram were recorded during all nights.
In case of awakenings, the HR alterations consisted of monophasic elevations for >1 min, with mean maximum HR elevations of 30 bpm. Though obviously triggered by the noise events, the awakenings per se rather than the acoustical parameters determined the extent and pattern of the response. Without awakenings, HR responses were biphasic and consisted of initial accelerations with maximum HR elevations of about 9 bpm followed by decelerations below the baseline. These alterations were clearly influenced by the acoustic parameters (traffic mode, maximum level, rate of rise) as well as by the momentary sleep stage.
Conclusions:
Cardiac responses did not habituate to traffic noise within the night and may therefore play a key role in promoting traffic noise induced cardiovascular disease. If so, these consequences are more likely for responses accompanied by awakenings than for situations without awakenings.
Citation:
Griefahn B; Bröde P; Marks A; Basner M. Autonomic arousals related to traffic noise during sleep. SLEEP 2008;31(4):569-577.
Keywords: Traffic noise, sleep, polysomnogram, event-related autonomic arousals, acoustic and situational influences
TRANSIENT EXCITATIONS OF THE CENTRAL AND OF THE AUTONOMIC NERVOUS SYSTEM WITH A COMMON ORIGIN IN THE BRAINSTEM OCCUR FREQUENTLY and spontaneously (with no obvious reason) during normal sleep. Cortical arousals, which might lead to sleep stage changes or awakenings, are usually accompanied by autonomic arousals. As the reverse is not true, the latter may occur alone.1,2 Autonomic arousals are transient elevations of the sympathetic tone. They encompass increases of ventilation, of systolic and diastolic blood pressure, and of peripheral resistance; but they but are most often indicated by alterations of heart rate (HR). These cardiac arousals start to increase well before the visually detectable onset of cortical arousals.1–3 Their extent and patterns vary with the duration of the cortical arousals. With cortical arousals lasting up to 10 s, cardiac arousals are typically biphasic: an initial acceleration is followed by a deceleration below the baseline. The baseline is then regained after a gradual increase 15–30 s after stimulus onset.1,3–5 With longer lasting arousals the deceleration becomes gradually flatter, thus leading to a monophasic elevation of heart rate.1,4–5
Similar alterations are evoked by various external stimuli, in particular by acoustic stimuli.6–8 Research in this area was usually performed with artificial sounds, mostly with tones of up to 4 kHz and durations up to 5 s.6–12 The extents and the patterns of these responses were analyzed in detail and were shown to depend at least on stimulus intensity and on the sleep stage at the time of stimulation.
Traffic noises are a major cause of extrinsic sleep disturbances with after effects on mood, performance, and health.13 Despite this, cardiac responses to traffic noise have only occasionally been studied.14–17 A detailed analysis of these responses was performed only for sonic booms, which evoked the typical biphasic cardiac arousals described above.18
This paper deals with the cardiac responses of 24 persons to noises from aircraft, rail, and road vehicles during sleep in the laboratory. It investigates possible influences of acoustical parameters, time of night and momentary sleep stage. Such an analysis is highly relevant as numerous residents living in the vicinity of airports, along busy streets, and along railway tracks are permanently exposed to these noises while sleeping. Long-term exposure to these noises is assumed to contribute to the genesis of cardiovascular diseases.19
MATERIALS AND METHODS
Participants
Twenty-four healthy subjects with normal hearing thresholds (12 women, 12 men, 19–28 years) participated and gave their written informed consent to the study that was approved by the local Ethics Committee. None of the participants stated a more than usual exposure to any of the traffic noises applied in the study.
Design
After a habituation night from Sunday to Monday the participants slept in 3 consecutive weeks, 4 consecutive nights each week in the laboratory (Monday evening to Friday morning). They were exposed to aircraft, road, and rail traffic noise with weekly permutations. The 4 nights of each week consisted of a random sequence of a quiet and 3 noisy nights.
Technical Equipment
The participants slept in separate sound shielded rooms that are located in the basement of the Institute. The temperature was adjusted to 20 °C. All rooms were equipped with 2 bi-amplified active monitoring speakers (Genelec 1031A 48 Hz to 22 kHz, free field frequency response ± 2 dB). Noise presentations were controlled by NUENDO-software (Steinberg Media Technologies) using a Soundcard DMX 6.fire Wave. The noise levels reported here refer to the levels measured at the ear of the participants.
Acoustic Stimuli
Recordings of real traffic sounds were presented via loud speakers. These were, 24 pass-bys of road vehicles (private cars and trucks), 18 pass-bys of trains (passenger and freight trains), and 18 flyovers.
These out-of-door recordings were then filtered to simulate a tilted window. Thus the indoor levels at the ear of the sleeper to which this paper refers were 15 dBA lower. These noises were arranged to 8-h scenarios with 195 aircraft, 162 rail-, or 262 road traffic noises, respectively. The maximum noise levels varied in each scenario within a range of approximately 12 dBA. The rate of applied stimuli decreased from 23:00 to 01:00 and increased again from 04:00 to 07:00. Table 1 shows the distribution of noise events per hour. Each of these scenarios was presented with equivalent sound levels of 39, 44, and 50 dBA, where maximum sound levels of the single noises were accordingly amplified or attenuated. The rates of rise, the durations of the noises and of the noise-free intervals are presented in Table 2.
Table 1.
Hourly Number of Vehicle Pass-Bys of Rail and Road Vehicles and of Flyovers
| Time (h) | 23–24 | 0–1 | 1–2 | 2–3 | 3–4 | 4–5 | 5–6 | 6–7 | 23–7 |
|---|---|---|---|---|---|---|---|---|---|
| Road traffic | 38 | 32 | 16 | 16 | 16 | 35 | 54 | 54 | 261 |
| Rail traffic | 26 | 22 | 10 | 10 | 10 | 24 | 30 | 30 | 162 |
| Air traffic | 32 | 18 | 16 | 16 | 16 | 25 | 36 | 36 | 195 |
Table 2.
Means, Standard Deviations, Minima and Maxima of Physical Parameters of the Noise Scenarios
| Road |
Rail |
Aircraft |
||||
|---|---|---|---|---|---|---|
| Max. level [LAF max] | 55.8 ± 8.0 | 43.8–74.1 | 53.8 ± 7.9 | 39.5–71.9 | 54.7 ± 7.8 | 43.2–75.7 |
| Rate of rise [dBA/s] | 4.8 ± 1.9 | 1.5–8.4 | 3.3 ± 1.9 | 0.8–8.6 | 1.6 ± 0.5 | 0.8–4.2 |
| Duration [s] | 13.9 ± 9.8 | 3.4–51.4 | 22.3 ± 19.0 | 6.0–92.2 | 23.5 ± 9.8 | 7.6–59.0 |
| Noise-free interval [min] | 1.7 ± 1.6 | 0.4–7.0 | 2.7 ± 2.0 | 0.5–8.0 | 2.2 ± 1.2 | 0.5–5.4 |
A 32 dBA broad band noise with amplitudes decreasing by 6 dB per octave (red noise) was continuously applied throughout all nights. This background noise masked the sounds from the air conditioning and the very beginning (fading in) of the various traffic sounds during the noisy nights. Thus the latter were definitely inaudible until emerging from the background noise level.
Experimental Procedure
Two hours prior to bedtime, electrodes were fixed for the recording of the polysomnogram (PSG: 2 EEG, 2 EOG, 1 EMG) and the electrocardiogram. At 23:00, light was extinguished and noise exposure and the recording of the physiological data began. The participants were woken up at 07:00 the next morning.
Data Analysis
The PSGs were evaluated adhering to the criteria of Rechtschaffen and Kales.20 Movement time was regarded as wake, and sleep stages S3 and S4 were combined to slow wave sleep (SWS). A total of 45,026 acoustic stimuli were applied. As the stimuli occurred in random intervals, i.e., not synchronized to the beginning of a PSG epoch, and as the evaluators were blinded concerning the occurrence of the stimuli, event-related awakenings were defined as follows: If a noise event started within ±15 s relative to the start of a 30-s sleep epoch, this epoch was defined as the first epoch under the influence of noise. This and the following epoch were defined as the noise window and scanned for sleep stage changes. Awakenings were defined as changes from stages SWS, S2, or REM to wake, MT, or S1; the trials where no awakening occurred were determined by stage S2, SWS, or REM sleep. “Abrupt” awakenings in the first epoch under the influence of noise may therefore include a certain number of (spontaneous) arousals starting before stimulus onset. “Delayed” awakenings in the second epoch can be considered as true noise effects. Both cases were analyzed separately.
After the electrocardiograms were digitized at a sampling rate of 256 s−1, the HR and the time of each consecutive heart beat were determined from R-R intervals. Misclassifications due to movement artifacts were corrected by visual inspection. The HR time series were then linked to the polysomnographic recordings and synchronized to the respective noise onsets. Noise onset was defined as the time when a noise event emerged from the background level of 32 dBA. After interpolation using cubic splines and equidistant resampling with a rate of 2 s−1 for the period from 20 s before to 60 s after noise onset, the average over the period from 20 s to 10 s prior to each noise onset3 was subtracted from the data 10 s before to 60 s after each noise onset. HRs above 130 min−1 and below 35 min−1 were excluded, as well as a few noise events with a noise-free interval less than 90 s relative to the end of the previous event.
Two parameters of the transient HR elevations, the maximum elevation (amplitude) and the corresponding latency, were then determined from the raw, i.e., non-interpolated RR-interval data for each noise event. The window within which the maximum was searched was 60 s in the presence and 30 s in the absence of an awakening, respectively. The same analyses were performed for “virtual noises,” i.e., the HR courses that occurred during noise-free control nights at the same time as the noise stimuli in the noisy nights.
Concerning awakenings, a random subject effect logistic regression was calculated to reveal the probability of awakenings over the time of night.
Repeated measurement ANOVAs applying linear mixed models with an unstructured covariance structure21 were calculated for the maximum HR elevation and the corresponding latency, where the following possible categorized influences were regarded.
awakening (yes/no)
abruptness of awakening (first, second epoch)
duration of awakening (1, 2, >2 epochs)
traffic mode (aircraft-, rail-, road traffic noise)
maximum noise level (LAmax <50, 50–60, >60 dBA)
dynamics (rate of rise, dBA/s). As the increase of noise levels in dBA/s differed considerably between traffic modes, the cut points for flatter and steeper rates of rise were set individually (1.5 dBA/s for aircraft, 2.7 dBA/s for rail noise, and 4.8 dBA/s for road vehicles)
sleep stage prior to stimulation (S2, SWS, and REM)
time of night—because almost the same number of vehicles occurred between 23:00 and 04:00 and between 04:00 and 07:00, the cut point was set to 04:00.
gender.
RESULTS
A first analysis showed that the response pattern was mainly determined by the absence or presence of awakenings. Thus the analyses were performed separately for both cases.
Cardiac Arousals in Case of Awakenings
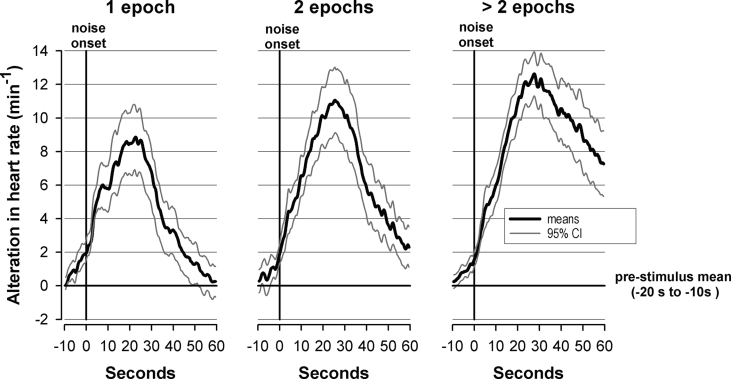
Table 3 shows the proportion of noise windows with awakenings, depending on traffic mode and the abruptness of the awakening. Two-thirds of awakenings occurred rather abruptly in the same epoch in which the noise was presented (first epoch), one-third in the following epoch (delayed awakenings). The arousal patterns in Figure 1 reveal monophasic HR elevations that varied according to abruptness. With abrupt awakenings, HR elevations seem to start before stimulus onset; the accelerations and decelerations were steeper, the maxima occurred earlier, and the baseline was regained earlier than with delayed awakenings. The response is considerably higher than in case of the “virtual noises” (Table 4b).
Table 3.
Mean Percentage and Standard Error (SE) of Noise Windows with Awakenings Depending on Traffic Mode and Abruptness of Awakening
| Noises emitted by | Awakenings (%), total mean ± SE | Abrupt awakenings in the first epoch (%) mean ± SE | Delayed awakenings in the second epoch (%) mean ± SE |
|---|---|---|---|
| Aircraft | 9.0 ± 0.5 | 5.5 ± 0.4 | 3.3 ± 0.2 |
| Road vehicles | 8.4 ± 0.5 | 5.2 ± 0.3 | 3.2 ± 0.2 |
| Rail vehicles | 10.8 ± 0.6 | 7.5 ± 0.4 | 3.2 ± 0.3 |
| Total | 9.4 ± 0.3 | 6.1 ± 0.2 | 3.2 ± 0.1 |
Figure 1.
Mean increases and 95% confidence intervals (CI) of heart rates after transportation noises (aircraft, rail, and road traffic noise) that were associated with awakenings. Black line: abrupt awakenings that occurred in the same epoch as the stimulus. Grey line: delayed awakenings that occurred during the following epoch.
Table 4b.
Means and Standard Errors (SE) for the Various Categories of Influences on the Maximum HR Elevation and the Corresponding Latency, Separately for Abrupt and Delayed Awakenings
| category | Abrupt awakenings |
Delayed awakenings |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Maximum HR-elevation (bpm) |
Latency (s) |
Maximum HR-elevation (bpm) |
Latency (s) |
||||||
| Mean | SE | mean | SE | mean | SE | mean | SE | ||
| Real noises | 29.3 | 1.07 | 24.7 | 0.49 | 29.4 | 1.13 | 41.6 | 0.84 | |
| Virtual noises (control) | - | 14.8 | 0.99 | 31.3 | 0.85 | 15.0 | 0.88 | 34.3 | 0.77 |
| Duration of awakening | 1 epoch | 26.8 | 1.31 | 19.8 | 0.86 | 28.9 | 1.31 | 39.6 | 1.29 |
| 2 epochs | 29.8 | 1.18 | 24.1 | 0.78 | 29.1 | 1.18 | 42.3 | 1.00 | |
| >2 epochs | 32.6 | 1.12 | 29.8 | 0.74 | 31.3 | 1.18 | 44.3 | 1.17 | |
| Maximum level | <50 dBA | 30.5 | 1.37 | 23.8 | 1.10 | 29.4 | 1.22 | 45.2 | 1.00 |
| 50–60 dBA | 30.3 | 1.13 | 24.9 | 0.68 | 29.0 | 1.13 | 41.6 | 1.08 | |
| >60 dBA | 28.4 | 1.09 | 25.0 | 0.78 | 30.9 | 1.08 | 39.5 | 1.25 | |
| Rate of rise | flatter | 29.5 | 1.02 | 25.3 | 0.71 | 30.5 | 1.21 | 41.3 | 0.97 |
| steeper | 30.0 | 1.17 | 23.9 | 0.74 | 29.1 | 0.99 | 42.8 | 0.76 | |
| Sleep stage prior | S2 | 29.7 | 1.23 | 24.1 | 0.74 | 28.6 | 1.22 | 40.7 | 0.88 |
| Stimulation | SWS | 34.4 | 1.24 | 26.4 | 1.26 | 35.4 | 1.33 | 43.3 | 1.28 |
| REM | 25.1 | 1.11 | 23.3 | 0.59 | 25.3 | 1.18 | 42.2 | 1.21 | |
| Time of night | <04:00 | 27.8 | 1.10 | 24.9 | 0.64 | 27.7 | 1.00 | 42.5 | 1.16 |
| ≥04:00 | 31.7 | 1.13 | 24.3 | 0.88 | 31.9 | 1.19 | 41.6 | 0.75 | |
Due to the limited number of awakenings, the inclusion of all possible influences into one ANOVA was not possible. Thus ANOVAs were calculated for the effect variables “maximum HR elevation” and the corresponding “latency,” with “gender,” “abruptness,” “duration of awakening,” and `“traffic mode” as possible influences. Because neither gender nor traffic mode revealed a significant effect, further ANOVAs were calculated separately for abrupt and for delayed awakenings and with “duration of awakening,” “maximum level,” “rate of rise,” “sleep stage prior awakening,” and “time of night” as possible influences.
As shown in Table 4a, the duration of the awakening, the sleep stage prior to awakening, and the time of night had a highly significant influence on the maximum HR elevation of abrupt and of delayed awakenings. Table 4b presents means and standard errors for the categories of the various possible influences. In both abrupt and delayed awakenings the amplitude of HR elevations and the latency to maximum HR increased significantly with awakening duration (Fig. 2). Concerning situational influences, the maximum HR elevation was largest after awakening from SWS and lowest after awakening from REM sleep. The extent of the response was about 4 bpm greater during the last 3 h than in the first 5 h of sleep time. In addition, a random subject effect logistic regression revealed a significant increase of awakening probability with the time of night (P < 0.001). Among the acoustical parameters only the maximum noise level had an influence on the response latency in case of delayed awakenings: the greater the maximum noise level the earlier occurred the maximum HR increase.
Table 4a.
Results of a Repeated Measures ANOVA (df: Degree of Freedom, F- and P-Value) Concerning the Maximum HR Elevation and the Corresponding Latency (Time in Seconds After Which the Maximum HR Elevation Occurred), Separately for Abrupt and Delayed Awakenings
| Possible influences | Abrupt awakenings |
Delayed awakenings |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maximum HR-elevation (bpm) |
Latency (s) |
Maximum HR-elevation (bpm) |
Latency (s) |
|||||||||
| df | F | P | df | F | P | df | F | P | df | F | P | |
| Duration of awakening | 46 | 14.35 | <0.001 | 46 | 49.24 | <0.001 | 46 | 3.92 | 0.027 | 46 | 3.50 | <0.039 |
| Maximum level | 46 | 2.18 | 0.125 | 46 | 0.56 | 0.572 | 46 | 2.54 | 0.090 | 46 | 5.48 | 0.007 |
| Rate of rise | 23 | 0.42 | 0.522 | 23 | 2.96 | 0.099 | 23 | 2.45 | 0.132 | 23 | 2.57 | 0.122 |
| Sleep stage prior awakening | 45 | 60.11 | <0.001 | 45 | 2.71 | 0.077 | 45 | 26.32 | <0.001 | 45 | 2.15 | 0.128 |
| Time of night | 23 | 23.74 | <0.001 | 23 | 0.46 | 0.505 | 23 | 24.81 | <0.001 | 23 | 0.45 | 0.507 |
Figure 2.
Mean increases and 95% confidence intervals of heart rates after transportation noises (aircraft, rail and road traffic noise) that were associated with awakenings that lasted 1 epoch (left panel), 2 epochs (middle panel), or more than 2 epochs (right panel).
Cardiac Arousals without Awakenings
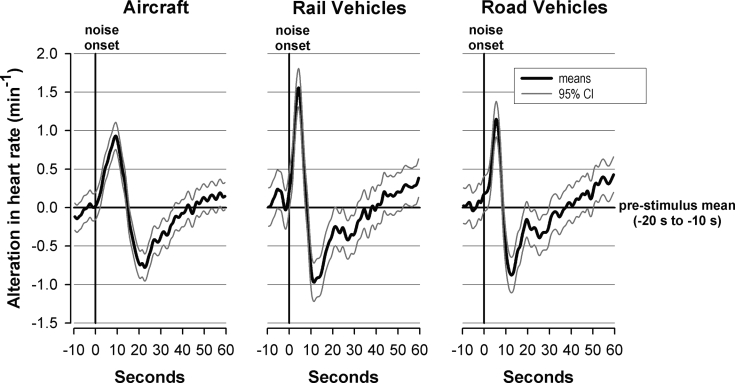
In the absence of awakening responses noise caused, as shown in Figure 3, biphasic alterations. HR increased immediately after stimulus onset, reached a maximum after 4–11 s, followed by a decrease to a minimum below baseline. After a slow increase the baseline was regained after 30–40 s. Whereas the maximum HR increase after real and virtual noises were the same, the maxima after real noises occurred significantly earlier. For virtual noises, the maxima lay in the middle of the 30-s window within which the maximum heart rate was searched (Table 5b).
Figure 3.
Mean alterations and 95% confidence intervals of heart rates after transportation noises without awakenings. Aircraft noise (left panel), railway noise (middle panel), or road traffic noise (right panel).
Table 5b.
Means and Standard Errors (SE) for the Various Categories of Influences on the Maximum HR Elevation and the Corresponding Latency on Responses Without Awakenings
| category | Maximum HR elevation (bpm) |
Latency (s) |
|||
|---|---|---|---|---|---|
| mean | SE | mean | SE | ||
| Real noises | 9.4 | 0.53 | 13.2 | 0.17 | |
| Virtual noises (control) | 9.2 | 0.67 | 15.4 | 0.08 | |
| Gender | men | 8.5 | 0.73 | 13.5 | 0.26 |
| women | 9.7 | 0.66 | 12.7 | 0.29 | |
| Traffic mode | aircraft | 8.9 | 0.52 | 13.6 | 0.24 |
| road | 9.0 | 0.59 | 13.2 | 0.27 | |
| rail | 9.4 | 0.49 | 12.5 | 0.39 | |
| Maximum level | <50 dBA | 8.4 | 0.45 | 13.2 | 0.27 |
| 50–60 dBA | 9.2 | 0.51 | 13.1 | 0.24 | |
| >60 dBA | 9.7 | 0.58 | 13.0 | 0.29 | |
| Rate of rise | flatter | 8.8 | 0.45 | 13.8 | 0.21 |
| steeper | 9.3 | 0.54 | 12.4 | 0.27 | |
| Sleep stage prior | S2 | 9.5 | 0.54 | 13.1 | 0.21 |
| Stimulation | SWS | 8.0 | 0.49 | 14.3 | 0.33 |
| REM | 9.8 | 0.56 | 11.9 | 0.29 | |
| Time of night | <4 h | 8.7 | 0.55 | 12.7 | 0.24 |
| ≥4 h | 9.4 | 0.47 | 13.5 | 0.22 | |
The maximum HR elevations and the corresponding latencies were submitted to an ANOVA where the following possible influences were included: “gender,” “traffic mode,” “maximum noise level,” “dynamics (flatter and steeper rates of rise),” “sleep stage prior stimulation,” and “time of night.” According to the data presented in Tables 5a and 5b, gender had no significant influence on the response. Concerning the acoustical parameters the traffic mode per se had a significant influence only on the latency of the maximum HR elevation. This elevation was longest for aircraft and shortest for railway noise (see also Fig. 3). The maximum noise level revealed a significant influence only on the maximum HR elevation increasing with the maximum levels. The dynamics, i.e., the rate of rise was decisive for the maximum HR elevation as well as for the corresponding latency. Steeper rates of rise caused, irrespective of traffic mode, greater maximum HR elevations that occurred later in case of aircraft and earlier in case of railway noises (see also Fig. 4). Concerning situational parameters, the sleep stage prior stimulation as well as the time of night influenced significantly the maximum HR elevation as well as the corresponding latency. The maximum HR elevation was largest in case of REM sleep and lowest in SWS. The extents of these responses were not related to baseline HR, which was (mean ± standard error) 58.8 ± 1.4 bpm, 60.9 ± 1.4 bpm, and 62.0 ± 1.4 bpm for S2, SWS, and REM sleep, respectively. The latency increased inversely to the extent of the response. Concerning the time of night, maximum HR elevations were by 0.7 bpm larger and the latency by 0.8 s longer in the last 3 h than the first 5 h of the night.
Table 5a.
Results of a Repeated Measures ANOVA Concerning the Maximum HR Elevation and the Corresponding Latency on Responses Without Awakenings
| Possible influences | Maximum HR elevation (bpm) |
Latency (s) |
||||
|---|---|---|---|---|---|---|
| Df | F | P | df | F | P | |
| Gender | 22 | 1.45 | 0.242 | 22 | 3.93 | 0.060 |
| Traffic mode | 44 | 1.54 | 0.225 | 44 | 4.93 | 0.012 |
| Maximum level | 44 | 10.98 | <0.001 | 44 | 0.21 | 0.814 |
| Rate of rise | 22 | 6.90 | 0.015 | 22 | 23.39 | <0.001 |
| Sleep stage prior stimulation | 44 | 21.14 | <0.001 | 44 | 20.56 | <0.001 |
| Time of night | 22 | 5.58 | 0.027 | 22 | 13.08 | 0.002 |
Figure 4.
Mean alterations of heart rates after transportation noises without awakenings. Black line flatter, grey line steeper rate of rise. Aircraft noise (left panel), railway noise (middle panel), or road traffic noise (right panel).
DISCUSSION
Acoustically induced autonomic arousals which indicate transient elevations of the sympathetic tone encompass decreases of peripheral blood flow, elevations of heart rate, of ventilation and of blood pressure.6–10,12,22 These responses were described in detail for artificial sounds, e.g., for short tones. Our analyses focused on transportation noises to which numerous persons living in the vicinity of airports, busy streets, or along railway tracks, are exposed to.
A first analysis showed that awakenings had a major influence on the extent and the pattern of the cardiac arousals. In case of awakenings the noises evoked monophasic HR elevations for more than a minute. Without awakenings, HR showed biphasic responses with considerably lower HR elevations than with awakenings. Thus the analyses were performed separately for conditions with and without simultaneous awakenings and the influences of traffic mode, maximum level, rise time, time of night, and momentary sleep depth were investigated. As the HR elevation in both these situations differed considerably from the response to virtual noises, the alterations ascertained here were most likely caused by noise.
Cardiac Arousals in Case of Awakenings
The HR elevations observed here, yielded an average maximum increase of about 30 bpm (Table 4b). This strong response is supported by several studies on the relation between the extent and the pattern of autonomic arousals on one hand and the duration of cortical arousals on the other hand.1,4–6 The response is most likely caused by an increase of the sympathetic tone and an elevated release of cortisol that usually accompanies awakenings.23–25
The awakening responses were subdivided into those that occurred abruptly in the first epoch of the screening window or delayed in the following epoch. The maximum HR elevation was comparable between both conditions, but occurred 18 s later with delayed as compared to abrupt awakenings. In case of abrupt awakenings there was a gradual HR elevation even before stimulus onset. This was probably due to the fact that the HR courses were synchronized to the onsets of the randomly occurring stimuli. Classifications of awakenings were, due to the rules of Rechtschaffen and Kales,20 however, bound to the 30-s epoch scheme. Thus, some spontaneous awakenings may have occurred prior to stimulus onset and unrelated to noise events. This assumption is supported by the significantly higher heart rate prior to stimulus onset in case of virtual noises with awakenings compared to those without awakenings. This particular finding leads, on the other hand, to the hypothesis that awakenings may preferably be evoked if a stimulus coincides with a spontaneous autonomic arousal, i.e., a sympathetic excitation that occurs repeatedly during the night.26–29 The assumption that the stimuli might have been heard before emerging from the background noise of 32 dBA is not likely. The red noise was particularly chosen and applied because it reliably masks the traffic noise. Further Basner et al.30 have shown in a field study that awakenings occurred only if maximum levels reach at least 33 dBA and exceed the background level by at least 6 dBA. However, it is certainly advisable for the design of future studies to synchronise the noise onsets with the start of a 30-s epoch used in sleep stage classification.20
Gender did not influence the HR response. Whether this is true in general needs to be studied systematically in a larger sample. However, this finding seems to be plausible as the probability of noise-induced awakenings was found to be essentially the same in men and in women.30,31
The sleep stage prior to awakenings clearly influenced the extent of the response. The greatest and the smallest HR elevation occurred with awakenings from SWS and from REM sleep, respectively. This was expected and corresponds to the extent of excitation that is largest and lowest for awakenings from SWS and REM-sleep, respectively.
The extent of the HR response increased with the time of night, although only marginally (4 bpm). This seems to indicate a sensitization as also the probability of awakenings increased slowly during the night.
The physical parameters of the noise, i.e., the rate of rise and the maximum level had no effect on the maximum HR elevation. This leads to the conclusion that, while the noise triggers the awakening response, only the latter determines the extent and the pattern of the HR response via strong sympathetic excitations.
Cardiac Arousals without Awakenings
Noises not accompanied by an awakening caused biphasic HR-alterations, where the initial acceleration was followed by a deceleration below the baseline and then by a slow increase back to prestimulus levels. The initial acceleration is certainly caused by a vagal inhibition, the consecutive deceleration corresponds to a counter-regulation and is caused by an increase of the vagal tone and a simultaneous inhibition of the sympathetic excitation.18,32,33 This pattern is characteristic for autonomic excitations that are accompanied by cortical arousals with a duration of 3–10 s.1,4–7
Mean maxima and minima of the heart rate response varied by less than +2 bpm and −1 bpm, respectively. The separate consideration of the maximum HR elevations revealed, however, elevations of about 9 bpm, corroborated by reports of other researchers.16,17,34,35
The significance of this reaction is still unknown. Muzet36 argued that “cardiovascular responses are particularly dangerous as they do not habituate, they are pure reflexes and they do not correspond to any energetic need of the sleeping body.” The vegetative reactions are therefore assumed to bear a pathogenic potential for the genesis of cardiovascular diseases. Whether this conclusion is valid remains open as similar alterations occur spontaneously, i.e., without external stimulation frequently during each night.28 Acute consequences for the consecutive period of wakefulness are questionable as well. Guilleminault et al.11 on one hand found increased tiredness only if the autonomic arousals were accompanied by cortical arousals. On the other hand, noise-related maximum HR elevations scarcely exceeded those of the virtual noises. The temporal mean of its occurrence, however, was identical with the center of the window within which the maximum heart rate was searched. The noise-related HR elevation occurred significantly earlier. This observation is strongly supported by a study, in which spontaneous arousals (phases d'activations transitoires) decrease in favor of evoked arousals, meaning that noises cause a redistribution of cardiac arousal.37
The Influence of Situational Conditions on the HR Response
Concerning situational influences the lowest maximum HR elevations together with the longest latencies were found for stimuli that occurred during SWS. The highest maxima along with the shortest latencies were observed during REM sleep. The least alterations during SWS are supported by all studies that concerned acoustically induced autonomic reactions. The largest response was almost equal during stage S2 and during REM sleep.7,15–18 A previous study on cardiac effects of sonic booms has shown a significant inverse relation between the prestimulatory heart rate and the extent of the HR elevation.22 Here baseline HR which was lowest in S2 and highest in REM could not explain the different responses in the different stages.
Time of Night
In agreement with other reports, this study did not show any decrease of the HR response during the course of the night, thus indicating a lack of habituation.12,15,16,34 Instead, there was a minimal increase, which may also be related to the increasing percentage of stage S2 and REM sleep, where autonomic responses are significantly stronger than during SWS, which occurs primarily during the first part of the night.
The Influence of Noise Parameters on the HR Response
Traffic Mode
The influence of the traffic mode on the HR response concerned the latency rather than the maximum of the HR elevation that were earliest reached after railway and latest after aircraft noise. The few studies applying noises of different traffic modes as well, support this.16,34 The most likely cause for this is probably the rate of rise (see below). The novelty of stimuli which were reported to influence the response to noise38 even during sleep cannot explain these results. The participants are in their daily life with decreasing frequency exposed to road, rail and aircraft noise. Thus, the strongest response would have been expected for aircraft noise.
Maximum Levels
Increasing maximum noise levels (that were almost the same for the 3 traffic modes) were associated with increasing HR maxima, whereas the latencies remained unaffected. This is supported by Muzet et al.,39 whereas most other authors denied such a relation.15,18 But as the HR alterations caused by largely varying maximum noise levels (45–77 dBA) varied by not more than 1.3 bpm (Table 5b), the present results do not really contradict previous studies. Significance here was probably achieved due to the much higher number of noises applied and analyzed compared to other studies.
Dynamics
The increase of noise levels per second (rate of rise) clearly determined the HR response. This concerns, as shown in Figure 4, particularly the noises from pass-bys of rail and road vehicles. Faster increases in noise levels caused steeper HR accelerations with earlier and higher maxima and with steeper decelerations. This is supported by Carter et al.,14 who reported greater increases of the systolic and the diastolic blood pressure due to noises with rapid onsets (military aircraft and tones) as compared to noises with slowly increasing levels (trucks, civil aircraft). This is most likely related to adaptation processes. The latter start immediately after stimulus onset and thus are less effective for noises with short rise times (steep rates of rise) but prevent abrupt and excessive HR accelerations when noise levels increase slowly.22 This applies particularly to aircraft noises, where the maximum levels were reached after longer rise times, thus explaining the lower HR maxima as compared to the cardiac responses to rail and road traffic noises. The undoubtedly decisive influence of the dynamics was, however, not true across the 3 traffic modes. Otherwise road traffic noise with the steepest increase would have caused stronger effects than railway noise.
CONCLUSIONS
This report concerned HR alterations during sleep evoked by noises emitted from aircraft, rail-, and road vehicles with maximum levels of 45–77 dBA. The response patterns were mainly determined by the occurrence or absence of awakenings.
In case of awakenings the cardiac responses consisted of monophasic HR elevations of more than one minute. This response was scarcely influenced by the acoustic parameters. The most significant influence arose from the momentary sleep stage at the time of stimulation. The HR elevation corresponded to the extent of the increase of the sympathetic tone that is largest and lowest when waking up from SWS and REM, respectively.
Without awakenings the HR response showed a biphasic pattern where an initial acceleration with a maximum after 4 to 11 s was followed by a deceleration to a minimum below the baseline after 12 to 23 s, followed by a consecutive increase towards baseline values. The extent and the pattern of this response were significantly determined by the traffic mode where railway noise caused the earliest and aircraft noise the latest increase. The strongest influence arose again from the sleep stage at the time of stimulation with largest alterations in REM sleep and lowest in SWS.
Neither of these responses decreased with the time of night indicating that habituation is not likely. A lack of habituation is the essential precondition for the interpretation of these responses as potentially pathogenetic. Whether there is a pathogenetic effect has to be proven in future studies. If so this is more likely for the strong heart rate response in case of simultaneous awakenings. This supports the view of those authors who regard awakenings as the strongest reaction to nocturnal noises and base their concepts for the protection of residents against the impact of nocturnal traffic noise on this criterion.13,30,40
ACKNOWLEDGMENTS
This study was supported by the Virtual Institute “Transportation Noise – Effects on Sleep and Performance” of the Helmholtz Gemeinschaft (HGF) under the grant-n° VH-VI-111.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no conflicts of interest.
REFERENCES
- 1.Sforza E, Jouny C, Ibanez V. Cardiac activation during arousal in humans: further evidence for hierarchy in arousal response. Clin Neurophysiol. 2000;111:1611–19. doi: 10.1016/s1388-2457(00)00363-1. [DOI] [PubMed] [Google Scholar]
- 2.Togo F, Cherniack NS, Natelson BH. Electroencephalogram characteristics of autonomic arousals during sleep in healthy men. Clin Neurophysiol. 2006;117:2597–603. doi: 10.1016/j.clinph.2006.07.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muzet A, Michel C. Heart rate preceding short activation phases in sleep. Waking Sleeping. 1977;1:175–9. [Google Scholar]
- 4.Sforza E, Chapotot F, Lavoie S, et al. Heart rate during spontaneous arousal from sleep: effect of sleep deprivation. Clin Neurophysiol. 2004;115:2442–51. doi: 10.1016/j.clinph.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Trinder J, Allen N, Kleiman J, et al. On the nature of cardiovascular activation at an arousal from sleep. Sleep. 2003;26:543–51. [PubMed] [Google Scholar]
- 6.Catcheside PG, Orr RS, Chiong SC, et al. Noninvasive cardiovascular markers of acoustically induced arousal from non-rapid-eye-movement sleep. Sleep. 2002;25:67–74. doi: 10.1093/sleep/25.7.797. [DOI] [PubMed] [Google Scholar]
- 7.Kato T, Montplaisie JY, Lavigne GJ. Experimentally induced arousals during sleep: a cross-modality matching paradigm. J Sleep Res. 2004;13:229–38. doi: 10.1111/j.1365-2869.2004.00409.x. [DOI] [PubMed] [Google Scholar]
- 8.Nalivaiko E, Catcheside PG, Adams A, et al. Cardiac changes during arousals from non-REM sleep in healthy volunteers. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1320–7. doi: 10.1152/ajpregu.00642.2006. [DOI] [PubMed] [Google Scholar]
- 9.Ackner B, Pampiglione G. Some relationships between peripheral vasomotor and E.E.G-changes. J Neurol Neurosurg Psychiatry. 1955;20:58–64. doi: 10.1136/jnnp.20.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carley DW, Applebaum R, Basner RC, et al. Respiratory and arousal responses to acoustic stimulation. Chest. 1997;112:1567–71. doi: 10.1378/chest.112.6.1567. [DOI] [PubMed] [Google Scholar]
- 11.Guilleminault C, Abad VC, Philip P, et al. The effect of CNS activation versus EEG arousal during sleep on heart rate response and daytime tests. Clin Neurophysiol. 2006;117:731–9. doi: 10.1016/j.clinph.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 12.Williams HL, Hammack JT, Daly RL, et al. Responses to auditory stimulation, sleep loss and the EEG states of sleep. EEG Clin Neurophysiol. 1964;16:269–79. doi: 10.1016/0013-4694(64)90109-9. [DOI] [PubMed] [Google Scholar]
- 13.Griefahn B. Noise control during the night. Acoust Aust. 1992;20:43–7. [Google Scholar]
- 14.Carter NL, Henderson R, Lal S, et al. Cardiovascular and autonomic response to environmental noise during sleep in night shift workers. Sleep. 2002;25:457–64. [PubMed] [Google Scholar]
- 15.Hume KI, Whitehead C. Aircraft noise and measures of sleep arousals; Internoise 2004; Prague. 2004. [Google Scholar]
- 16.Muzet A, Weber LD, Di Nisi J, et al. Comparaison de la reactivite cardiovasculaire au bruit au cours de la veille et du sommeil. CNRS Strasbourg: Centre d'etude bioclimatique; 1985. Conv. No 82243. [Google Scholar]
- 17.Whitehead C, Hume K, Muzet A. In: Cardiovascular responses to aircraft noise in sleeping subjects. Carter N, Job S, editors. Sydney: Noise effects 1998; 1998. pp. 471–4. [Google Scholar]
- 18.Griefahn B. Effects of sonic booms on fingerpulse amplitudes during sleep. Int Arch Occup Environ Health. 1975;36:57–66. doi: 10.1007/BF01267852. [DOI] [PubMed] [Google Scholar]
- 19.Babisch W. Transportation noise and cardiovascular risk. Review and synthesis of epidemiological studies. Dose-effect curve and risk estimation. 2006. WaBoLu-Hefte 01/06. 2006. [DOI] [PubMed]
- 20.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 21.Littell RC, Milliken GA, Stroup WW, et al. SAS System for Mixed Models. Cary, NC: SAS Institute Inc; 1996. [Google Scholar]
- 22.Griefahn B. Zur Ermittlung reizbedingter Pulsfrequenzänderungen. Eur J Appl Physiol. 1977;37:13–16. doi: 10.1007/BF00421594. [DOI] [PubMed] [Google Scholar]
- 23.Ekstedt M, Åkerstedt T, Söderström M. Microarousals during sleep are associated with increased levels of lipids, cortisol, and blood pressure. Psychosom Med. 2004;66:925–31. doi: 10.1097/01.psy.0000145821.25453.f7. [DOI] [PubMed] [Google Scholar]
- 24.Hucklebridge F, Clow A, Rahman H, et al. The cortisol response to normal and nocturnal awakening. J Psychophysiol. 2000;14:24–8. [Google Scholar]
- 25.Born J, Kern W, Bieber K, Fehm-Wolfsdorf G, Schiebe M, Fehm HL. Night time plasma cortisol secretion is associated with specific sleep stages. Biol Psychiatry. 1986;21:1415–24. doi: 10.1016/0006-3223(86)90333-1. [DOI] [PubMed] [Google Scholar]
- 26.Bonnet MH, Arand DL. Heart rate variability: sleep stage, time of night, and arousal influences. EEG Clin Neurophysiol. 1997;102:390–6. doi: 10.1016/s0921-884x(96)96070-1. [DOI] [PubMed] [Google Scholar]
- 27.Halász P, Terzano M, Parrino L, et al. The nature of arousal in sleep. J Sleep Res. 2004;13:1–23. doi: 10.1111/j.1365-2869.2004.00388.x. [DOI] [PubMed] [Google Scholar]
- 28.Terzano MG, Parrino L. Clinical applications of cyclic alternating pattern. Physiol Behav. 1993;54:807–13. doi: 10.1016/0031-9384(93)90096-x. [DOI] [PubMed] [Google Scholar]
- 29.Terzano MG, Parrino L, Smerieri A, et al. CAP and arousals are involved in the homeostatic and ultradian sleep processes. J Sleep Res. 2005;14:359–68. doi: 10.1111/j.1365-2869.2005.00479.x. [DOI] [PubMed] [Google Scholar]
- 30.Basner M, Samel A, Isermann U. Aircraft noise effects on sleep: Application of the results of a large polysomnographic field study. J Acoust Soc Am. 2006;119:2772–84. doi: 10.1121/1.2184247. [DOI] [PubMed] [Google Scholar]
- 31.Griefahn B, Marks A, Robens S. Noise emitted from road, rail and air traffic and their effects on sleep. J Sound Vib. 2006;295:129–40. [Google Scholar]
- 32.Baust W, Marbaise J. Phasische Herzfrequenzänderungen nach Schallreizen im natürlichen Schlaf des Menschen. Pflügers Arch. 1971;324:165–75. doi: 10.1007/BF00592661. [DOI] [PubMed] [Google Scholar]
- 33.Griefahn B. Cardiac responses caused by shots of tanks during sleep. J Sound Vib. 1989;128:109–19. [Google Scholar]
- 34.Di Nisi J. Différences interindividuelles au cours de la veille et du sommeil. Strasbourg: Thèse, Université Louis Pasteur; 1987. Modifications de la fréquence cardiaque et de la vasomotricité digitale provoquées par le bruit. [Google Scholar]
- 35.Whitehead C, Hume K. A field experiment on the effect of aircraft noise on heart rate during sleep. Programme – Abstract Book; Int Symp on Noise Pollution & Health; April 6–8 2001; Cambridge, UK. 2001. p. 53. [Google Scholar]
- 36.Muzet A. Research on noise-disturbed sleep since 1978. In: Rossi G, editor. Noise as a Public Health Problem. Milano: Edizioni Tecniche a cura del Centro Ricerche e Studi Amplifon; 1983. pp. 883–93. [Google Scholar]
- 37.Muzet A, Schieber JP, Olivier-Martin N, Ehrhart J, Metz B. Relationship between subjective and physiological assessments of noise-disturbed sleep. In: Ward WD, editor. 1st Int Congr on Noise as a Public Health Problem. Washington DC: 1973. pp. 575–86. 20460: EPA 550/9-73-008. [Google Scholar]
- 38.Strauch I, Schneider-Düker M, Zayer H, et al. Der Einfluss sinnvoller akustischer Signale auf das Schlafverhalten. Archiv für Psychologie. 1976;128:75–95. [PubMed] [Google Scholar]
- 39.Muzet A, Ehrhart J, Eschenlauer R, et al. Modifications vegetatives entrainees par le bruit au cours du sommeil. Ministère de l'Environnement et du Cadre de Vie, Comité Bruit et Vibration; 1980. Convention no 76.22. [Google Scholar]
- 40.Basner M, Buess H, Elmenhorst D, et al. Nachtflugwirkungen. www.dlr.de/me/institut/abteilungen/flugphysiologie/fluglärm/Lit_laermw_reports/BerichtBas6FB2004-07-e.pdf.