Abstract
Study Objectives:
Multiunit electromyogram recordings of genioglossus have demonstrated an abrupt reduction in the muscle's activity at sleep onset. Recent evidence from single motor unit recordings indicates that the human genioglossus muscle consists of motor units with a variety of discharge patterns. The aim of the present study was to determine the effect of sleep onset on the activity of individual motor units as a function of their particular discharge pattern.
Design:
Genioglossus activity was assessed using intramuscular fine-wire electrodes via a percutaneous approach. Sleep onsets (α-to-θ transitions) were identified and the genioglossus electromyogram recordings analyzed for single motor unit activity.
Setting:
Sleep research laboratory.
Participants:
Sleep and respiratory data were collected in 8 healthy subjects (6 men).
Measurements and Results:
One hundred twenty-seven motor units were identified: 23% inspiratory phasic, 45% inspiratory tonic, 4% expiratory phasic, 9% expiratory tonic, 16% tonic, and 3% other. Approximately 50% of inspiratory units (phasic and tonic) ceased activity entirely at sleep onset, whereas those inspiratory units that continued to be active showed a reduction in the proportion of each breath over which they were active. However, the rate of discharge of inspiratory units during the period they did fire was not altered. In contrast, tonic and expiratory units were unaffected by sleep onset, maintaining their discharge pattern over the α-to-θ transition.
Conclusions:
Central control of inspiratory motoneuron output differs from that of tonic and expiratory units during sleep onset, suggesting that the maintenance of airway patency during sleep may become more reliant on the stiffening properties of tonic and expiratory modulated motor units.
Citation:
Wilkinson V; Malhotra A; Nicholas CL; Worsnop C; Jordan AS; Butler JE; Saboisky JP; Gandevia SC; White DP; Trinder J. Discharge patterns of human genioglossus motor units during sleep onset. SLEEP 2008;31(4):525-533.
Keywords: Motor control, upper airway, upper airway muscles, single motor units, obstructive sleep apnea, motoneuron
DISORDERS OF BREATHING DURING SLEEP ARE A MAJOR HEALTH PROBLEM. OF THESE, OBSTRUCTIVE SLEEP APNEA (OSA) IS THE MOST PREVALENT, occurring in at least 4% of men and 2% of women.1 In this disorder, the upper airway collapses during sleep, causing reduction of airflow and oxygen desaturation. The airway occludes because dilator muscles are unable to sustain patency in the face of the negative pressures generated by inspiratory effort. For patency to be reestablished, upper airway muscles must be recruited, a process that is often associated with arousal from sleep. Broadly considered, OSA is most often due to 2 interacting factors: sleep-induced changes in upper airway muscle (UAM) activity and an anatomically narrow upper airway.2 It follows that understanding UAM control during sleep remains critical in understanding the pathophysiology of OSA. A crucial feature of sleep-related changes in UAM activity is that they are initiated abruptly, early in the sleep onset process, at the point when electroencephalographic (EEG) α activity is reduced.3–6
Although it is likely that maintenance of upper airway patency depends on the coordinated activity of all UAMs,7 experimental work has paid particular attention to the major protrudor muscle of the tongue, the genioglossus. This is because the evidence suggests that the genioglossus is particularly important to the maintenance of airway patency during sleep and may be representative of other muscles with respiratory phasic activity.8
Recent studies of genioglossus single motor units in humans have indicated that the components of muscle activity are complex.9,10 Saboisky et al.9 identified 6 different discharge patterns in genioglossus motor units: (1) units that fire throughout the respiratory cycle, but with increased firing frequency during inspiration (these were designated as inspiratory tonic and consisted of 12% of units); (2) units that only fire during inspiration (inspiratory phasic, 39% of units); (3) units that fire throughout the respiratory cycle, but with increased firing frequency during expiration (expiratory tonic, 11%); (4) units that only fire during expiration (expiratory phasic, 5% of units); (5) units that fire with a constant frequency across the respiratory cycle (tonic, 29% of units); and (6) units that modulate their firing rate, but not in phase with the respiratory cycle (tonic other, 4% of units). An earlier study10 also identified 2 types of phasic units in human genioglossus, inspiratory phasic and inspiratory tonic units, whereas a more recent study11 has identified a similar range of discharge patterns to Saboisky et al.9 during both wakefulness and sleep. Finally, another recent study12 has demonstrated that genioglossus motor unit spikes are of longer duration in patients with OSA, suggesting neurogenic changes.
Consistent with these findings, animal studies have shown that the hypoglossal motor nucleus, the motor nerve of which provides innervations to the genioglossus, contains a large pool of inspiratory phasic neurons with variable threshold and precise discharge patterns.13–16 In animals, inspiratory phasic hypoglossal motoneurons13,15 and genioglossus motor units14 appear to respond to increased respiratory drive with both the recruitment of additional units and an increased discharge rate of already active units. There is also evidence of a shift in the phasic component to an earlier, preinspiratory onset time.13–16 Animal studies have also identified neurons that discharge tonically14,15 and that may be less responsive to respiratory stimuli.15
As noted above, multiple unit recordings have indicated that sleep onset, in particular the transition from dominant EEG α to dominant EEG θ, is associated with large and abrupt changes in genioglossus activity. Changes in the motor control of UAMs during sleep onset are likely to be critical in the development of sleep-related airway occlusion. Thus, the identification of single motor unit activity in the genioglossus over the sleep-onset period has the potential to provide unique insight into the effect of sleep on genioglossus motor control. However, no studies, animal or human, have specifically investigated single motor unit activity in genioglossus during sleep onset and only 1 study has previously investigated motor unit activity during sleep.11 In this study, we assessed the effect of the α-to-θ transition on the discharge patterns of individual genioglossus motor units.
METHODS
Subjects
Eight subjects (6 men and 2 women with an average age of 27.6 ± 7.3 years and body mass index of 24.2 ± 1.6 kg/m2) participated in the study. Data were collected on 1 recording night for each subject. The data were collected in the Sleep Disorders Program sleep research laboratory, Division of Sleep Medicine, Brigham and Women's Hospital, Boston (6 subjects), and the sleep laboratory, Department of Psychology, The University of Melbourne (2 subjects), using identical methodology. All subjects were healthy and were without sleep complaints. The protocol conformed to the Declaration of Helsinki and had prior approval of the Human Subjects Committee of the Brigham and Women's Hospital and the University of Melbourne. Informed consent was obtained from each subject.
Equipment and Techniques
Recording techniques and equipment were the same in the 2 laboratories.
Sleep-Wake State
Two channels of EEG (C3-A2 and O1-A2) and electrooculography and a chin electromyogram (EMG) were collected in order to determine sleep-wake state and to identify α-to-θ transitions. Consistent with previous studies,5,6,17,18 transitions were defined as having stable state (α or θ) for at least 3 breaths before and after transition, with up to 4 breaths being analyzed. Further, to remove the confounding effects of arousal and body movement, it was required that the pretransition α breaths be at least 15 seconds after the last arousal and be free of movement and that the posttransition θ breaths be free of movement and microarousals. However, as in previous studies,5,6,17,18 these criteria limited the number and length of data segments available for analyses.
Transitions were identified visually according to previously published criteria17 by an experimenter who was blinded to the results of the EMG recordings. Briefly, α was defined as 8- to 12-Hz activity with a regular sinusoidal pattern, whereas θ was defined as 3- to 8-Hz activity with an irregular, mixed frequency pattern. Visual analysis was chosen, rather than the automated spectral method we have previously developed, because there were insufficient stable data to determine the criterion values required by the automated technique.18 We have used both methods in earlier studies with identical results5,18 and have experimentally shown, using signal detection procedures, that the 2 methods show close agreement, with a d prime value of 2.88. The breath on which the EEG transition occurred was classified as a θ breath if the EEG transition occurred before peak inspiratory flow; otherwise the following breath was classified as the first θ breath.
Ventilation and Airway Mechanics
Subjects wore a nasal mask (Respironics, Inc. Murraysville, PA) connected to a non-rebreathing valve, whereas respiratory airflow was determined by a calibrated pneumotachometer (Fleisch #2) and differential pressure transducer (DP-45, Validyne Corp., Northridge, CA). Subjects breathed through their noses with their mouths taped shut during data collection. In addition, end-tidal co2 was monitored from the mask using an Ametek co2 (CD-3A) analyzer. Pressures were monitored in the mask with an open catheter attached to a pressure transducer (Validyne Corp) and in the airway at the level of the epiglottis using a pressure-tipped catheter (MPC-500, Millar, Houston, TX). One nostril was decongested (oxymetazoline HCl) and anesthetized (lidocaine HCl), and the Millar catheter was inserted through this nostril and located at the epiglottis.
Muscle Activity
The procedures used to record genioglossal EMG activity followed those of Eastwood et al.19 For each subject, the location and depth of the genioglossus muscle was determined using ultrasonography. The genioglossus EMG was recorded using 3 monopolar intramuscular wire electrodes referenced to a common surface electrode positioned over the bony mandible. A large flexible ground strap was placed on the right shoulder. The 3 electrodes were inserted into the muscle using a percutaneous approach,19 guided by the ultrasound measures. Approximately an hour prior to electrode insertions, a local anesthetic cream (Lidocaine-Prilocaine, Fogera, Melville, NY) was applied to the skin. Insertion was via a 25-gauge hypodermic needle, with 2 stainless-steel, Teflon-coated, 0.002-inch diameter, wire electrodes in each needle. Each electrode had 0.5 mm of insulation removed from the recording surface. The insertion sites were distributed over left-right and relatively anterior-posterior positions such that, over the 8 subjects, all 4 sites were similarly sampled. Prior to the start of data collection, the electrode with the best recording from each pair of electrodes was selected for data acquisition and storage.
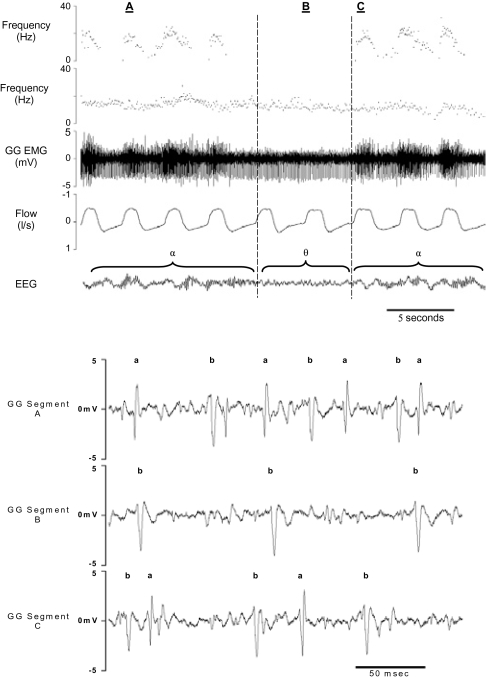
A methodologic consideration with intramuscular recordings is electrode movement. Thus, in the case of single motor unit recordings, it is possible that the onset or cessation of the activity of a unit is an artifact of electrode movement. A number of procedures were adopted to minimize the influence of electrode movement and to determine whether a change in the discharge rate of a unit was likely to be due to movement artifact. Importantly, data were not analyzed over α-to-θ transitions in which there was a body movement, swallow, or similar event. In the absence of such events, the electrodes were highly stable. We reached this conclusion on the basis of the following considerations: (1) there was rarely a change in the morphology of the action potential of a motor unit over a transition; (2) further, in a number of instances in which a unit ceased activity, another unit recorded by the same electrode continued to discharge with constant action potential morphology; and (3) finally, on a number of occasions when units ceased activity at an α-to-θ transition, the unit returned with identical morphology with the return of α activity (these features are illustrated in Figure 1). Thus, we are confident that electrodes rarely moved or changed their recording field as a function of the respiratory cycle or sleep-wake transitions.
Figure 1.
The top panel shows the instantaneous frequency plots for 2 motor units recorded on the same electrode, before and after α-to-θ and θ-to-α transitions. Also shown are the raw electromyogram (EMG), airflow, and electroencephalogram (EEG) recordings. Vertical lines indicate state transitions. The figure illustrates the differential effects of the α-to-θ transition on inspiratory phasic (top tracing) and tonic (second tracing) motor units and shows that the cessation of the inspiratory phasic unit was not a consequence of electrode movement. The bottom panel shows typical individual spikes for the 2 motor units illustrated in the top panel at points A, B, and C (a is the inspiratory phasic unit and b, the tonic unit). The figure illustrates both the spike sorting process and the consistency of spike morphology over transitions. GG refers to genioglossal.
Data Acquisition
Data were stored on computer using Spike 2 acquisition software with a 1401 interface (Cambridge Electronic Design, Cambridge, UK). All signals were amplified and filtered using GRASS amplifiers (Grass Telefactor, West Warwick, RI) prior to analogue-to-digital conversion (ADC) by the 1401 interface using the following values for filtering, amplification and ADC rates, respectively: EEG, 0.3 to 100 Hz, X 10 k, 256 Hz; EOG, 0.3 to 35 Hz, X 10 k, 100 Hz; submental EMG, 10 to 100 Hz, X 10 k, 256Hz; genioglossal EMG, 0.3 to 3 kHz, X 10 k, 10 kHz; airflow, DC-15 Hz, X 20, 100 Hz; end-tidal co2, DC-15 Hz, X 5, 100 Hz; epiglottal pressure, DC-15 Hz, X 15, 100 Hz; mask pressure, DC-15 Hz, X 20, 100 Hz.
General Laboratory Procedures
Subjects arrived at the laboratory 4 hours before their normal bedtime. Upon arriving, they were prepared for bed and the recording equipment attached. Subjects were put to bed approximately 1 hour before their normal bedtime, and the collection of 20 minutes of wakefulness data was completed. The lights were then turned off, and sleep-wake transition data were collected. To maximize the opportunity to collect data over the α-to-θ transitions, subjects were awakened if they remained in Stage 2 sleep or slow-wave sleep for 5 minutes. Subjects were required to maintain a supine position throughout data collection.
Data Reduction
Identification and Quantification of Respiratory Variables
Breath-by-breath values for the pretransition and posttransition breaths were determined for cycle duration (Ttot); tidal volume (Vt); minute ventilation (Vi); epiglottal pressure 200 milliseconds into inspiration (Pepi200); minimum epiglottal pressure during inspiration (Pepimin); upper airway resistance (epiglottis to mask) 200 ms into inspiration (Rau200); and upper airway resistance (epiglottis to mask) at the minimum epiglottal pressure (Raumax) using in-house software.
Identification and Quantification of Genioglossus EMG Activity
The discharge properties of single motor units were determined using Spike 2 analysis software (Cambridge Electronic Design, Cambridge, UK). The steps in the application of the software were as follows.
α-to-θ transitions were identified according to criteria described above.
The EMG of each electrode was inspected for the presence of identifiable motor units within the period of the transition. If it did not contain any motor unit activity, or if it contained too much activity for analysis, it was discarded.
Motor units were extracted from the raw EMG signal based on a spike-triggered threshold voltage and sorted into templates on the bases of their detailed signatures (amplitude and shape).
Subsidiary software was then used to manually inspect and edit the initial software classification, such that action potentials were reclassified to other motor units or removed.
The resulting time series of action potentials were displayed as instantaneous frequency plots, and firing statistics were then generated. The process is illustrated in Figure 1.
In order to identify potential classification errors, we used a procedure analogous to error identification in heart rate data. A change of greater than ±40% from 1 interspike interval to the next identified classification errors. In our experience, such abrupt changes in discharge rate rarely occur in the normal activity of a unit. Such errors persisted after editing because the unit had a poor signal to noise ratio or because it was difficult to distinguish from other units on the same electrode. Thus, we defined a successful identification of the discharge pattern of a genioglossus motor unit as one in which less than 5% of consecutive interspike intervals varied by more than ±40%. Exclusion criteria based on maximum and minimum frequencies were not used because of the wide variability in the discharge frequency of units and because we have insufficient data at this time to choose criteria values.
Classification of Units
The 6 different discharge patterns were identified visually according to procedures reported by Saboisky et al.9
Inspiratory Phasic
These were units that showed peak activity during inspiration and had a minimal firing frequency of less than 2.0 Hz (i.e., were silent for > 500 ms).
Inspiratory Tonic
These were units that showed peak activity during inspiration and maintain a discharge rate of more than 2.0 Hz throughout the respiratory cycle.
Expiratory Phasic
These were units that showed peak activity during expiration and had a minimal firing frequency of less than 2.0 Hz (i.e., were silent for > 500 ms).
Expiratory Tonic
These were units that showed peak activity during expiration and maintained a discharge rate of greater than 2.0 Hz throughout the respiratory cycle.
Tonic
These were units that fired throughout the respiratory cycle but had no obvious respiratory or other modulation.
Tonic Other
These were units that fired throughout the respiratory cycle and showed variation in their discharge rates but not in phase with the respiratory cycle.
We also quantified the degree of respiratory modulation by comparing the within-breath variation in interspike interval to the variation in airflow over the breath using a cross-correlation procedure. Thus, the instantaneous frequency of a unit at each action potential (determined from the interspike interval) was correlated with airflow at the time of the action potential, over all action potentials within a breath (thus the computation would be over 80 pairs of numbers for a 20-Hz firing rate over a 4-second breath). The lag in the cross-correlation was achieved by rotating the airflow values around the breath. The degree of respiratory modulation in the activity of a unit was identified by the maximum positive correlation (the best phase fit of the 2 variables). The computation was determined on a breath-by-breath basis, and values were averaged over α breaths. Orem and Dick20 'η2 statistic could not be used because this statistic requires a relatively large number of consecutive breaths in the relevant state to determine between-breath variance, such data not being available over sleep onset. (A modified version of the Orem and Dick20 technique has been successfully applied to genioglossus motor units during stable wakefulness and sleep,11 where longer data sets were available.)
As has been reported previously,9 our data indicated good agreement between the quantification of the degree of respiratory modulation using the cross-correlation method and blinded visual classification. Respiratory-modulated units had a maximum correlation value of 0.69 (SD = 0.14), and tonic units a value of 0.41 (SD = 0.09). The value used to distinguish between tonic, and inspiratory and expiratory tonic, was 0.49. Units with values below this were classified as tonic. This value was chosen because it minimized the number of discrepancies between the cross-correlation and visual methods.
A number of features of the instantaneous frequency plots were quantified to characterize the discharge pattern of the motor units.
The mean frequency during either inspiration or expiration, depending on the phase of the unit.
The peak frequency during inspiration or expiration, depending on the phase of the unit (200-ms average).
The tonic frequency, which was defined as the last 200 milliseconds before the onset of the inspiratory or expiratory phasic component. This measure did not apply to inspiratory or expiratory phasic units.
The time, with respect to the onset of inspiration, of the first inspiratory phasic action potential. A negative value indicated preactivation of the motor unit. This measure did not apply to expiratory or Tonic motor units.
The proportion of the breath during which the unit was active.
Statistical Analyses
An average transition for each type of motor unit was calculated for each subject, and subjects were used as replications. The analysis compared the average of the 4 pretransition α breaths with the 4 posttransition θ breaths for inspiratory phasic and inspiratory tonic units using 2 X 2 repeated measures analyses of variance. However, for some analyses, insufficient data were available from inspiratory phasic units; in these cases, inspiratory tonic α and θ means were compared with repeated-measures t tests. Expiratory and tonic units were identified in only a small number of subjects, such that group statistics were not meaningful. Where the number of units identified for a subject exceeded 5, within-subject analyses using individual transitions as replications were conducted.
As described in greater detail below, a portion of units ceased activity during posttransition θ. Statistically, such events were dealt with in 2 ways. First, a 0 value was assigned. Using this procedure, averaged data reflected the activity of the muscle, as indicated by single motor unit activity. In the second procedure, motor units that stopped were eliminated from the analysis. Using this procedure, the data reflected the effects of the sleep-wake transition on the discharge characteristics of single motor units that continued to be active.
RESULTS
As indicated in Table 1, respiratory activity over the α-to-θ transition replicated previous studies in normal healthy individuals.5,18 Thus, there was a significant fall in ventilation (Vi and Vt) but no change in respiratory timing (Ttot). Further, there was a significant rise in upper airway resistance at the point of minimum pressure at the epiglottis (Raumax), which, by the fourth posttransition breath, had risen from 5.8 to 10.7 cm h2o·L−1·s−1 (an average of 8.79 cm h2o·L−1·s−1 over the 4 posttransition breaths). However, there was not a change at 200 milliseconds into the inspiratory phase (Rau200), and negative pressure at the epiglottis did not change at either Pepi200 or Pepimin.
Table 1.
Respiratory Activity During the Four Breaths Before and After α-to-θ Transitions for All 8 Subjects
| Variables | α | θ | P |
|---|---|---|---|
| Ttot, ms | 4.09 (0.79) | 4.15 (0.72) | |
| Vt, mL | 498 (119) | 406 (90) | < 0.05 |
| Vi, L/min | 6.82 (2.27) | 5.72 (2.22) | < 0.05 |
| Pepi200, cm h2o | −0.86 (0.42) | −0.82 (0.45) | |
| Pepimin, cm h2o | −2.61 (1.05) | −2.72 (1.12) | |
| Rau200, cm h2o·L−1·s−1 | 2.73 (1.88) | 2.56 (1.61) | |
| Raumax, cm h2o·L−1·s−1 | 5.78 (3.01) | 8.79 (4.58) | < 0.05 |
Data are presented as mean (SD). Ttot refers to respiratory cycle duration; Vt, tidal volume; Vi, minute ventilation; Pepi200, epiglottal pressure 200 ms into inspiration; Pepimin, minimum epiglottal pressure during inspiration; Rau200, upper airway resistance 200 ms into inspiration; Raumax, upper airway resistance at minimum epiglottal pressure.
Table 2 shows the number of α-to-θ transitions and the different types of motor unit activity identified in each subject. Because 3 electrodes were recorded simultaneously, the 61 transitions provided 183 for transition analysis. Motor units were identified and successfully sorted on 76 electrode transitions (38 had 1, 25 had 2, and 13 had 3 units). Of the remaining electrode transitions, a small number were of poor technical quality, whereas others had no detectable units or too many spikes to identify single motor units. The majority of units were classified as inspiratory tonic units followed by inspiratory phasic units, with only 3 subjects having tonic or expiratory units, whereas tonic-other units were rarely observed.
Table 2.
Number of α-to-θ Transitions and the Number of Each Type of Motor Unit Identified in Each of the Subjects
| Subject | Transitions, no. | Motor units, no. |
Total | |||||
|---|---|---|---|---|---|---|---|---|
| IP | IT | EP | ET | TT | TO | |||
| 1 | 5 | 9 | 6 | - | - | - | - | 15 |
| 2 | 7 | 1 | 4 | - | 3 | 6 | - | 14 |
| 3 | 6 | 1 | 7 | - | - | - | - | 8 |
| 4 | 10 | 11 | 9 | - | - | - | - | 20 |
| 5 | 5 | 3 | 6 | - | - | - | - | 9 |
| 6 | 10 | 3 | 7 | - | - | 12 | 1 | 23 |
| 7 | 8 | 1 | 19 | - | - | - | 3 | 23 |
| 8 | 10 | - | - | 5 | 8 | 2 | - | 15 |
| Total | 61 | 29 | 58 | 5 | 11 | 20 | 4 | 127 |
| Distribution, % | 23 | 45 | 3 | 9 | 16 | 3 | 100 | |
IP refers to inspiratory phasic; IT, inspiratory tonic; EP, expiratory phasic; ET, expiratory tonic; TT, tonic; TO, tonic other.
Inspiratory Motor Units
The most dramatic feature of the data was the number of inspiratory phasic and inspiratory tonic motor units that ceased activity entirely at some point during the 4 posttransition θ breaths. Such an event is illustrated in Figure 1. As shown in Table 3, approximately 50% of inspiratory phasic (16 of 29) and inspiratory tonic (29 of 58) units ceased activity. Further, approximately 20% of inspiratory tonic units became inspiratory phasic. Forty-two of the 87 inspiratory modulated units remained active after the EEG transition.
Table 3.
Changes in the Discharge Patterns of Motor Units at the α-to-θ Transition as a Function of Their Pretransition Pattern
| Pretransition state | Units, no. | Posttransition state |
Unit Off | ||||
|---|---|---|---|---|---|---|---|
| IP | IT | EP | ET | TT | |||
| IP | 29 | 13 | - | - | - | - | 16 |
| IT | 58 | 11 | 18 | - | - | - | 29 |
| EP | 5 | - | - | 1 | 3 | - | 1 |
| ET | 11 | - | - | - | 11 | - | - |
| TT | 20 | - | - | - | - | 19 | 1 |
IP refers to inspiratory phasic; IT, inspiratory tonic; EP, expiratory phasic; ET, expiratory tonic; TT, tonic.
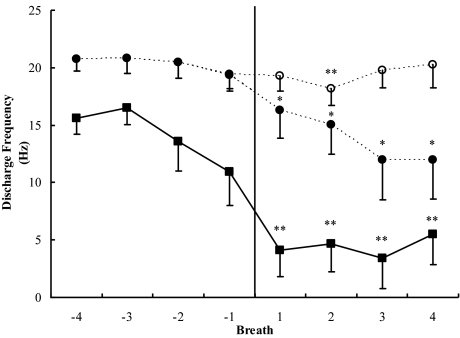
In analyses in which a motor unit that was inactive on a particular breath was assigned a value of 0, inspiratory phasic and inspiratory tonic motor units showed substantial falls in peak and mean motor unit frequency in association with the α-to-θ transition (see Table 4 and Figure 2). The mean discharge rate of inspiratory phasic units began to fall at the second-to-the-last α breath, slightly before the EEG transition. This may mean that respiratory activity is influenced by the state change earlier than the cortex, although it could also reflect variability in the identification of the transition. Both the peak and mean discharge rates were significantly lower during θ breaths, with inspiratory phasic units having significantly lower discharge rates than inspiratory tonic units (see Table 4). In addition, the tonic component of inspiratory tonic units was significantly lower during θ.
Table 4.
Discharge Characteristics of IP and IT Motor Units as a Function of α-to-θ State
| IP (29 units) |
IT (58 units) |
|||
|---|---|---|---|---|
| α | θ | α | θ | |
| Timing of the onset of the phasic component(ms)≈ | 232 (310) | - | −126 (39) | −14 (176)† |
| 0 values included in averages | ||||
| Peak f, Hz * | 14.2 (4.6) | 4.4 (6.1)‡ | 20.3 (3.2) | 13.8 (7.5)‡§ |
| Mean f, Hz * | 11.9 (3.7) | 3.8 (5.2)‡ | 15.6 (2.2) | 10.6 (5.5)‡§ |
| Tonic f, Hz ≈ | - | - | 13.4 (2.5) | 6.3 (4.7)‡ |
| % Duration* | 46.6 (18.5) | 8.6 (10.9)‡ | 89.1 (4.0) | 49.4 (26.4)‡§ |
| IT (29 units) |
||||
|---|---|---|---|---|
| α | θ | |||
| 0 values NOT included in averages | ||||
| Peak f, Hz ≈ | 20.4 (3.1) | 19.4 (3.9) | ||
| Mean f, Hz ≈ | 15.7 (2.2) | 15.0 (2.7) | ||
| Tonic f, Hz ≈ | 13.9 (2.2) | 13.1 (3.7) | ||
| % Duration ≈ | 89.2 (3.9) | 72.4 (12.4)‡ | ||
Data are presented as means over all 7 subjects with inspiratory phasic (IP) and inspiratory tonic (IT) units. Data for IP units where 0 values were not included has not been reported because only 3 subjects had IP units that continued to fire after transition.
1. A dash (-) indicates where data were not available because only 3 subjects had IP units active during θ or because IP units do not have tonic activity.
2. Negative values for the timing of the phasic components indicate preactivation.
3. % Duration − Denotes percentage of breath the unit is active.
4. Asterisk (*) denotes the use of repeated measures analysis of variance. Approximation symbol (≈) denotes the use of repeated measures t-tests over the α / θ transition for IT units.
P < 0.05 over α / θ transition
P < 0.01 over α / θ transition
P < 0.01 for unit type IP - IT
Figure 2.
The functions with solid symbols represent peak discharge frequencies of all inspiratory phasic (solid squares) and inspiratory tonic (solid circles) motor units before and after α-to-θ transitions. A value of 0 was entered when a unit ceased activity, and, thus, the data reflect the contribution of phasic motor units to overall muscle activity. The function with open symbols represents inspiratory tonic (open circles) units that continued to be active following the transition. Asterisks indicate that the posttransition value is significantly different from the mean of the pretransition values (*P < 0.05; **P < 0.01). Error bars indicate SEM.
If only units active throughout the transition were included in subject averages, the pattern of results was somewhat different. Seven subjects had inspiratory tonic units that continued to fire throughout the transition. As indicated in Figure 2 and Table 4, these units maintained approximately the same discharge rate during θ breaths as they had during α. Only 3 subjects had inspiratory phasic units that maintained their activity during θ, and, as a consequence, statistical comparisons were not conducted. In general, the pattern indicated that reductions in genioglossus muscle activity were primarily due to motor units ceasing to fire, rather than to reductions in the frequency of their firing.
There was also a reduction in the proportion of a breath during which a motor unit was active over the α-to-θ transition (see Table 4). The measure reflects both a reduction in the time that inspiratory phasic units fire and the conversion of inspiratory tonic to inspiratory phasic firing. Average values were calculated with and without 0 being entered when a unit ceased firing. When 0 values were included, there was a substantial and significant reduction in the proportion of a breath during which motor units were active. Nevertheless, although the effect was smaller when motor units that became inactive were eliminated from the analysis, it remained statistically significant (statistical analysis being available for inspiratory tonic units only).
Consistent with the reduction in the proportion of the respiratory cycle that inspiratory units were active, there was also a delay in the onset of the phasic component during θ, as compared to α, activity. Inspiratory tonic units had significantly greater preactivation during α, as compared with θ activity (Table 4). Also, the phasic component occurred substantially earlier in inspiratory tonic, compared to inspiratory phasic, units, with 91% of inspiratory tonic and 41% of inspiratory phasic units showing preactivation during α activity.
Expiratory and Tonic Motor Units
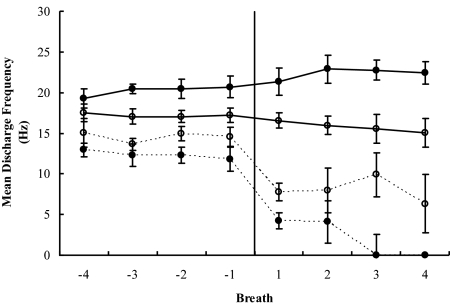
Tonic units were identified in 3 subjects, and, in all 3, the discharge pattern for tonic units was unaffected by the α-to-θ transition. Averaged mean discharge values for α and θ were 20.0 and 22.3 Hz for subject 2, 17.2 and 15.7 Hz for subject 6, and 18.0 and 19.6 Hz for subject 8. An individual tonic unit is illustrated in Figure 1 (second frequency plot); Figure 3 illustrates the absence of change in average tonic unit activity, as compared with the large reduction in average inspiratory unit activity, for the 2 subjects who had both inspiratory and tonic units. The difference between the α and θ mean frequencies for tonic units was not significant for either subject (P > 0.05). Finally, only 1 of the 20 tonic units identified ceased activity during the 4 posttransition θ breaths. Mean discharge frequency values are reported in Table 5.
Figure 3.
Peak discharge frequency of tonic and inspiratory modulated (inspiratory phasic and inspiratory tonic) motor units for the 2 subjects (S2 and S6) in whom both tonic- and inspiratory-modulated units were recorded. Data are presented for breaths before and after α-to-θ transitions. Open circles indicate S2, filled circles S6, whereas solid lines indicate tonic units and dashed lines, inspiratory phasic plus inspiratory tonic. Error bars indicate SEM.
Table 5.
Average Peak, Mean, and Tonic Discharge Rates for Expiratory Phasic, Expiratory Tonic, and Tonic Units During α and θ Activity
| EP |
ET |
TT |
||||
|---|---|---|---|---|---|---|
| α | θ | α | θ | α | θ | |
| Peak f, Hz | 20.7 | 19.9 | 23.0 | 23.5 | - | - |
| Mean f, Hz | 19.1 | 15.7 | 17.9 | 19.1 | 18.4 | 19.2 |
| Tonic f, Hz | - | - | 16.7 | 17.4 | - | - |
The number of subjects contributing to expiratory phasic (EP), expiratory tonic (ET), and tonic (TT) values varies (see text and Table 2).
Expiratory units were identified in 2 subjects (3 in subject 2 and 13 in subject 8). As indicated in Table 3, 15 of the 16 units maintained their activity over the transition, whereas 3 of the 5 expiratory phasic units converted to an expiratory tonic pattern. In general, expiratory units, similar to tonic units, sustained their activity over the sleep-onset transition (see Table 5).
Inspiratory Modulation Correlated with Fall in Discharge Rate
The strength of the inspiratory modulation of the discharge patterns of the units was quantified by the maximum cross-correlation between ventilation and instantaneous frequency plots during α breaths. It was anticipated that the maximum cross-correlation of a unit would be positively correlated with the magnitude of its fall in discharge rate. However, only 2 subjects (S2 and S6, see Table 2) had a sufficient spread of units with different discharge patterns to effectively test the hypothesis. In each of these subjects, as hypothesized, there was a strong positive correlation (S2, df 10, r = 0.75, P < 0.01; S6, df 21, r = 0.77, P < 0.001) between these measures. Five of the remaining subjects had only inspiratory phasic and inspiratory tonic units with a narrow range of maximum cross-correlation values (5 subjects) and, as would be expected, showed low and nonsignificant correlations with the magnitude of the fall in discharge rate (mean r = 0.11, P > 0.05). The final subject had predominantly expiratory units, for which the relationship is not predicted to occur.
DISCUSSION
Our major finding is that the behavior of genioglossus motor units during a transition to sleep depended on the discharge pattern of the unit during wakefulness. Motor units with an inspiratory pattern dramatically reduced their activity in association with the loss of EEG α activity, whereas those units that did not modulate their activity in phase with respiration (tonic units), or showed an expiratory pattern, did not alter their activity at the EEG transition. Consistent with this, within subjects in whom both tonic and inspiratory modulated units were identified, there were strong linear relationships between the degree of inspiratory modulation and the magnitude of the fall in the discharge rate of the unit at sleep onset.
The pattern of change in inspiratory phasic and inspiratory tonic units at the α-to-θ transition had a number of interesting features. Approximately 50% of both forms of inspiratory units completely ceased activity. Further, approximately 20% of the inspiratory tonic units became inspiratory phasic. Furthermore, the proportion of a breath over which units were active fell from 89% to 72%. Similarly, inspiratory phasic units that remained active in θ also reduced the time that they were active from 41% to 32%. Consistent with this, there was a significant fall (126 ms to 14 ms) in the extent to which the onset of the phasic component of inspiratory tonic motor units occurred before the onset of ventilation (preactivation). Put together, inspiratory units either ceased firing or fired for a shorter period of time in each breath after sleep onset. In contrast, there were only small and generally nonsignificant reductions in the rate that units fired when they remained active, an observation that applied to peak, mean, and tonic firing levels. Thus, at sleep onset, the sleep-related reduction in respiratory drive caused a reduction in the duration of discharge of an inspiratory unit, rather than a reduction in its rate of discharge.
Whether this change in firing pattern is characteristic of human genioglossus motor units in other situations in which respiratory drive is altered remains to be determined. Animal studies have indicated that, with increased chemical stimulation, additional hypoglossal motoneurons and genioglossus motor units are recruited, but they also increase their discharge rate and increase the likelihood of preinspiratory firing.13–15 In contrast, in a study in humans, Bailey et al.21 reported that genioglossus motor units activated by voluntary tongue protrusion rapidly saturated and were insensitive to greater protrusion, with increased drive being reflected in the recruitment of additional units. The failure of human inspiratory genioglossus motor units to alter their discharge rate under conditions of altered drive may reflect a species difference. However, it is also possible that, during relaxed wakefulness preceding sleep, active units are only just above their activation threshold, so that, with only slight reductions in drive, they cease firing.
In contrast to inspiratory modulated units, tonic units almost always remained active over sleep onset. It is possible that the activity of such units is unrelated to respiration (e.g., postural). Alternatively, tonic units may act to stiffen the tongue, preventing it from falling into the airway. The presence of respiratory modulation in inspiratory units and its absence in tonic units suggest that different premotoneuronal pathways are involved in their control.
The results of the present study are consistent with the 1 study that measured genioglossus motor unit activity during wakefulness and stable sleep.11 Bailey et al.11 have reported that, although many units that were active during wakefulness were silent during stable non-rapid eye movement sleep, those that remained active had relatively similar discharge patterns in the 2 states. Further, they reported a high proportion of tonic units among those that were active during both wakefulness and stable sleep. This is consistent with our observation that it is inspiratory phasic and inspiratory tonic units that tend to cease activity at sleep onset.
In 1 respect, the results are somewhat unexpected. Orem and colleagues22 have reported that respiratory-related brainstem neurons with weak respiratory-related modulation in their discharge rate are more likely to cease activity during sleep than are neurons with strong inspiratory phasic modulation. On the basis of these data, we would have anticipated that genioglossus motor units with tonic activity patterns would have been more likely to cease activity at sleep onset. The reason for this apparent discrepancy is unknown.
The distribution of different discharge patterns observed in the current study was somewhat different from that observed in a previous study.9 Most notably, in this study, we identified fewer tonic units and more inspiratory-modulated units. Further, the different patterns were less well spread over subjects, with only 3 subjects having tonic units and 1 subject having no inspiratory units. It is likely that this reflects differences in data-collection procedures. Because of the constraints of data collection during sleep, electrodes were inserted and left in the 1 location, whereas, in the earlier study, in which participants were awake, the recording electrode was moved in search of units. The technique used in the current sleep study may restrict the variety of units identified within a subject on a particular recording occasion and, as a consequence, is not an appropriate method to use to identify the distribution of different discharge patterns. On the other hand, the number of units identified was clearly sufficient to determine the effect of sleep onset on genioglossus motor units.
It is also possible that inspiratory-modulated units are elevated in this and in our earlier study by the higher-than-normal airway resistance produced by the recording equipment. We have no data to evaluate this possibility, and, for the moment, any conclusions as to the distribution of units with particular discharge patterns should be regarded as preliminary.
Although it is possible that the absence of change in tonic units reflected idiosyncratic properties of these 3 participants, we consider this unlikely. Inspiratory units were observed in 2 of these participants, and, as with other participants, the inspiratory units reduced their activity at the transition. Further, we have observed tonic units in other data sets, and, as with the current data, their activity has been unchanged at sleep onset.23 Units with expiratory activity patterns behaved in a similar manner at sleep onset to those with tonic patterns, in that they maintained their activity over the transition. Thus, almost 30% of motor units were unaffected by the loss of wakefulness. However, we have insufficient data at this time to determine whether expiratory units and tonic units are influenced by a common drive component.
The subjects were studied in the supine position, and it is possible that, in this position, subjects consciously or unconsciously protrude their tongue in order to maintain airway patency, an act they cannot perform while asleep. Although it is likely that the supine position places enhanced requirements on genioglossus,24 it is unlikely that these drive components are unique to this muscle. Sleep onset is associated with abrupt falls in a range of muscles, including the diaphragm, intercostals, and tensor palatini, indicating a general reduction in respiratory drive, rather than a drive component specific to the supine position.6 Further, voluntary protrusion of the tongue is associated with the activation of tonically behaving genioglossus motor units,21 not the inspiratory-modulated units that reduce their activity at sleep onset.
The genioglossus is considered to be an important muscle for the maintenance of inspiratory airway patency, particularly during sleep. For this reason, a reduction in the activity of genioglossus during sleep, either because of a large reduction in activity at sleep onset or because of poor sensitivity to respiratory stimuli during sleep, is a potential mechanism leading to OSA. The present data suggest that at least 1 of these properties of the muscle, reduction in activity at sleep onset, is a consequence of changes in inspiratory-mediated (inspiratory phasic and inspiratory tonic) motor unit activity. The identification of tonic and expiratory motor units that may influence airway patency by stiffening the airway suggests other mechanisms by which the muscle might fail during sleep. Thus, a high ratio of inspiratory phasic and inspiratory tonic to tonic and expiratory units, or a tendency of tonic units to cease activity during sleep, would potentially compromise the ability of the muscle to maintain airway patency asleep.
In summary, the genioglossus muscle is composed of motor units that have a range of different discharge patterns. Of these, inspiratory phasic and inspiratory tonic units are relatively common and together make up approximately 60% of units in the muscle that are active during quiet breathing. Sleep onset profoundly alters their inspiratory modulation. It reduces their duration of firing to the extent that many units cease activity entirely but does not affect their rate of discharge when active. In contrast, tonic units and units with an expiratory pattern are unaffected by sleep onset. It is likely that motor control of inspiratory phasic and inspiratory tonic units differs from that of tonic and expiratory motor units, although differences in the properties of the motor units themselves cannot be ruled out.
Footnotes
Disclosure Statement
This was not an industry supported study. Dr. Malhotra has received consulting and/or research support from Respironics, Restore Medical, Inspiration Medical, NMT Medical, Pfizer, and Cephalon. Dr. White is Chief Medical Officer of Respironics; has consulted for WideMed, Aspire Medical, PAVAD, and Itamar Medical; and has received research support from WideMed. The other authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.White DP. Pathogenesis of obstructive and central sleep apnea. Am J Respir Crit Care Med. 2005;172:1363–70. doi: 10.1164/rccm.200412-1631SO. [DOI] [PubMed] [Google Scholar]
- 3.Bulow K. Respiration and wakefulness in man. Acta Physiol Scand. 1963;Suppl:209. [PubMed] [Google Scholar]
- 4.Trinder J. Respiratory and cardiac activity during sleep onset. In: Bradley TD, Floras JS, editors. Sleep Apnea: Implications in Cardiovascular and Cerebrovascular Disease. New York: Marcel Dekker; 2000. pp. 337–54. [Google Scholar]
- 5.Trinder J, Whitworth F, Kay A, Wilken P. Respiratory instability during sleep onset. J Appl Physiol. 1992;73:2462–9. doi: 10.1152/jappl.1992.73.6.2462. [DOI] [PubMed] [Google Scholar]
- 6.Worsnop CJ, Kay A, Pierce R, Kim Y, Trinder J. Activity of respiratory pump and upper airway muscles during sleep onset. J Appl Physiol. 1998;85:908–20. doi: 10.1152/jappl.1998.85.3.908. [DOI] [PubMed] [Google Scholar]
- 7.Bailey EF, Fregosi RF. Coordination of intrinsic and extrinsic tongue muscles during spontaneous breathing in the rat. J Appl Physiol. 2004;96:440–9. doi: 10.1152/japplphysiol.00733.2003. [DOI] [PubMed] [Google Scholar]
- 8.Malhotra A, White DP. Obstructive sleep apnea. Lancet. 2002;360:237–45. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 9.Saboisky JP, Butler JE, Fogel RB, Taylor JL, Trinder J, White DP, Gandevia SC. Tonic and phasic respiratory drives to human genioglossus motorneurons during breathing. J Neurophysiol. 2006;95:2213–21. doi: 10.1152/jn.00940.2005. [DOI] [PubMed] [Google Scholar]
- 10.Tsuiki S, Ono T, Ishiwata Y, Kuroda T. Functional divergence of human genioglossus motor units with respiratory-related activity. Eur Respir J. 2000;15:906–10. doi: 10.1034/j.1399-3003.2000.15e16.x. [DOI] [PubMed] [Google Scholar]
- 11.Bailey EF, Fridel KW, Rice AD. Sleep/wake firing patterns of human genioglossus motor units. J Neurophysiol. 2007;98:3284–91. doi: 10.1152/jn.00865.2007. [DOI] [PubMed] [Google Scholar]
- 12.Saboisky JP, Butler JE, Mckenzie DK, Gorman RB, Trinder J, White DP, Gandevia SC. Neural drive to human genioglossus in obstructive sleep apnea. J Physiol. 2007;585:135–46. doi: 10.1113/jphysiol.2007.139584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang JC, Bartlett D, St John WM. Characterisation of respiratory modulated activities of hypoglossal motorneurons. J Appl Physiol. 1983;55:793–8. doi: 10.1152/jappl.1983.55.3.793. [DOI] [PubMed] [Google Scholar]
- 14.John J, Bailey EF, Fregosi RF. Respiratory-related discharge of genioglossus muscle motor units. Am J Respir Crit Care Med. 2005;172:1331–7. doi: 10.1164/rccm.200505-790OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitra J, Cherniack NS. The effects of hypercapnia and hypoxia on single hypoglossal nerve fiber activity. Respir Physiol. 1983;54:55–66. doi: 10.1016/0034-5687(83)90113-5. [DOI] [PubMed] [Google Scholar]
- 16.Withington-Wray D, Mifflin SW, Spyer KM. Intracellular analysis of respiratory modulated hypoglossal motor neurons in the cat. Neuroscience. 1988;25:1041–51. doi: 10.1016/0306-4522(88)90057-7. [DOI] [PubMed] [Google Scholar]
- 17.Colrain IM, Trinder J, Fraser G, Wilson G. Ventilation during sleep onset. J Appl Physiol. 1987;63:2067–74. doi: 10.1152/jappl.1987.63.5.2067. [DOI] [PubMed] [Google Scholar]
- 18.Kay A, Trinder J, Bowes G, Kim Y. Changes in airway resistance during sleep onset. J Appl Physiol. 1994;76:1600–1607. doi: 10.1152/jappl.1994.76.4.1600. [DOI] [PubMed] [Google Scholar]
- 19.Eastwood PR, Allison GT, Shepherd KL, Szollosi I, Hillman DR. Heterogeneous activity of the human genioglossus muscle assessed by multiple bipolar fine-wire electrodes. J Appl Physiol. 2003;94:1849–58. doi: 10.1152/japplphysiol.01017.2002. [DOI] [PubMed] [Google Scholar]
- 20.Orem J, Dick T. Consistency and signal strength of respiratory neuronal activity. J Neurophysiol. 1983;50:1098–107. doi: 10.1152/jn.1983.50.5.1098. [DOI] [PubMed] [Google Scholar]
- 21.Bailey EF, Rice AD, Fuglevand AJ. Firing patterns of human genioglossus motor units during voluntary tongue movement. J Neurophysiol. 2007;97:933–6. doi: 10.1152/jn.00737.2006. [DOI] [PubMed] [Google Scholar]
- 22.Orem J. Respiratory neurons and sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine, 2nd ed. Philadelphia: Saunders; 1994. pp. 177–93. [Google Scholar]
- 23.Jordan AS, Eckert DJ, Steiner MK, Schory K, Dover L, Malhotra D, White DP, Trinder J. Genioglossus motor discharge patterns at sleep onset in patients with obstructive sleep apnea (OSA) Am J Respir Crit Care Med. 2007;175:A754. [Google Scholar]
- 24.Malhotra A, Trinder J, Fogel R, et al. Postural effects on pharyngeal protective reflex mechanisms. Sleep. 2004;27:1105–12. doi: 10.1093/sleep/27.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]