Abstract
Study Objectives:
GABAergic transmission in the oral part of the pontine reticular formation (PnO) increases wakefulness. The hypothalamic peptide hypocretin-1 (orexin A) promotes wakefulness, and the PnO receives hypocretinergic input. The present study tested the hypothesis that PnO administration of hypocretin-1 increases PnO GABA levels and increases wakefulness. This study also tested the hypothesis that wakefulness is either increased or decreased, respectively, by PnO administration of drugs known to selectively increase or decrease GABA levels.
Design:
A within-subjects design was used for microdialysis and microinjection experiments.
Setting:
University of Michigan.
Patients or Participants:
Experiments were performed using adult male Crl:CD® (SD)IGS BR (Sprague-Dawley) rats (n = 46).
Interventions:
PnO administration of hypocretin-1, nipecotic acid (a GABA uptake inhibitor that increases extracellular GABA levels), 3-mercaptopropionic acid (a GABA synthesis inhibitor that decreases extracellular GABA levels; 3-MPA), and Ringer solution (vehicle control).
Measurements and Results:
Dialysis administration of hypocretin-1 to the PnO caused a statistically significant, concentration-dependent increase in PnO GABA levels. PnO microinjection of hypocretin-1 or nipecotic acid caused a significant increase in wakefulness and a significant decrease in non-rapid eye movement (NREM) sleep and REM sleep. Microinjecting 3-MPA into the PnO caused a significant increase in NREM sleep and REM sleep and a significant decrease in wakefulness.
Conclusions:
An increase or a decrease in PnO GABA levels causes an increase or decrease, respectively, in wakefulness. Hypocretin-1 may promote wakefulness, at least in part, by increasing GABAergic transmission in the PnO.
Citation:
Watson CJ; Soto-Calderon H; Lydic R; Baghdoyan HA. Pontine reticular formation (PnO) administration of hypocretin-1 increases PnO GABA levels and wakefulness. SLEEP 2008;31(4):453-464.
Keywords: Nipecotic acid, 3-mercaptopropionic acid, in vivo microdialysis, orexin, non-rapid eye movement sleep, rapid eye movement sleep, microinjection
Γ-AMINOBUTYRIC ACID (GABA) IS THE MAJOR INHIBITORY NEUROTRANSMITTER IN BRAIN, AND MOST CLINICALLY USED DRUGS THAT POTENTIATE GABAergic transmission produce a loss of normal wakefulness.1–4 Chronic insomnia, which occurs in about 10% of the US population,5 is frequently treated by drugs that act as agonists at the benzodiazepine binding site on the GABAA receptor complex.1 However, microinjection of the GABAA receptor agonist muscimol into the oral part of the pontine reticular formation (PnO) increases wakefulness and decreases sleep.6–9 Consistent with these findings are data showing that PnO microinjection of the GABAA receptor antagonist bicuculline decreases wakefulness and increases rapid eye movement (REM) sleep.8–10 Taken together, these data support the interpretation that within the PnO, GABAergic transmission promotes wakefulness and inhibits sleep.
Hypocretin-1 (orexin A) is an endogenous hypothalamic peptide that contributes to the regulation of behavioral and electroencephalographic (EEG) arousal.11 Defects in hypocretinergic signaling underlie the human sleep disorder narcolepsy12 and cause a narcoleptic-like phenotype in dog13 and mouse.14 Hypocretinergic neurons project to every major arousal-promoting nucleus in the brain, including the PnO.15–17 Hypocretin receptors are present18 and functionally active19 in rat PnO. Hypocretin-1 directly excites PnO neurons in cat, and microinjection of hypocretin-1 into cat PnO during non-REM (NREM) sleep increases REM sleep.20 No previous studies have investigated the effects on sleep and wakefulness of microinjecting hypocretin-1 into rat PnO.
The present study tested the hypothesis that PnO administration of hypocretin-1 increases PnO GABA levels and increases wakefulness. This hypothesis was examined with 2 approaches. First, in vivo microdialysis in anesthetized rat and high performance liquid chromatography (HPLC) were used to quantify the effects of hypocretin-1 on PnO GABA levels. Second, PnO microinjection in unanesthetized rat was used to assess the effects of hypocretin-1 on sleep and wakefulness. This study also tested the hypothesis that PnO administration of drugs known to selectively increase or decrease GABA levels causes an increase or decrease, respectively, in wakefulness. PnO microinjection of nipecotic acid, a GABA uptake inhibitor known to increase extracellular GABA levels in vivo,21,22 was predicted to increase wakefulness. PnO microinjection of 3-mercaptopropionic acid (3-MPA), a glutamic acid decarboxylase inhibitor that decreases extracellular GABA levels in vivo,22 was predicted to decrease wakefulness. Preliminary portions of this report have been presented as abstracts.23–25
EXPERIMENTAL PROCEDURES
Animals and Chemicals
Experiments conformed to the US Public Health Service Policy on Humane Care and Use of Laboratory Animals (National Institutes of Health Publication 80-23, National Academy of Sciences Press, Washington DC, 1996) and were approved by the University of Michigan Committee on Use and Care of Animals. Adult (235 to 250 g) male Crl:CD® (SD) IGS BR (Sprague-Dawley) rats (n = 46) purchased from Charles River Laboratories (Wilmington, MA) were housed in the Unit for Laboratory Animal Medicine facilities that provided a temperature- and humidity-controlled environment with ad libitum access to food and water and a 12-hour light/dark cycle (lights on at 0600). Rats were given at least 1 week to adapt to this environment before being used for either in vivo microdialysis studies or PnO microinjections followed by electrophysiologic recordings to quantify sleep and wakefulness.
Salts for Ringer solution (147.0 mM NaCl, 2.4 mM CaCl2, 4.0 mM KCl, 1.0 mM MgSO4, pH 6.0) and 85% o-phosphoric acid came from Fisher Scientific (Pittsburgh, PA). Sodium phosphate dibasic was obtained from Mallinckrodt (St. Louis, MO). Human hypocretin-1 was purchased from California Peptide Research, Inc. (Napa, CA), and o-phthaldialdehyde (Fluoraldehyde) from Pierce Biotechnology, Inc. (Rockford, IL). All other chemicals were purchased from Sigma Aldrich (St. Louis, MO).
In Vivo Microdialysis
Published and preliminary data show that endogenous GABA levels vary significantly as a function of arousal state in numerous brain regions, including the posterior hypothalamus,26 dorsal raphe nucleus,27 locus coeruleus,28 and pontine reticular formation.29–31 Hypocretin levels also vary as a function of arousal state,32 and hypocretin levels in rat lateral hypothalamus increase with increased wakefulness.33 Therefore, in vivo microdialysis experiments (n = 18 rats) were conducted using general anesthesia as a method for holding behavioral state constant. This approach has been used successfully for quantifying drug effects on acetylcholine release.34–36 This method helped ensure that measured changes in GABA levels did not result from spontaneous alterations between sleep and wakefulness. Extracellular GABA collected using microdialysis may originate from neurons and glia.37, 38 Therefore, this report refers to changes in GABA levels, rather than changes in GABA release.
Microdialysis experiments followed methods described previously.19, 21 Briefly, anesthesia was induced with 3.5% halothane (Halocarbon Laboratories, River Edge, NJ) in 100% O2 delivered at a flow rate of 1 L/minute. Delivered halothane concentration was measured continuously by spectrometry (Cardiocap™/5, Datex-Ohmeda, Louisville, CO). Once fully anesthetized, a rat was placed in a small-animal stereotaxic instrument fitted with a rat anesthesia mask (David Kopf Instruments, Tujunga, CA), and delivered halothane concentration was reduced to 2%. Core body temperature was monitored continuously using a rectal thermometer, and body temperature was maintained at 37°C throughout the experiment. Using aseptic surgical techniques, 1 CMA/11 microdialysis probe (cuprophane membrane, 1 mm in length, 0.24 mm in diameter, with a 6 kDa cutoff; CMA, North Chelmsford, MA) was aimed for the PnO using stereotaxic coordinates 8.4 mm posterior to bregma, 1.0 mm lateral to bregma, and 9.2 mm ventral to skull surface.39 Delivered halothane concentration was reduced to 1.5% at a flow rate of 0.6 L/minute and held constant during dialysis sample collection. Dialysis probes were perfused continuously at a flow rate of 2.0 μL/minute. Dialysis samples were collected on ice every 7 minutes (14 μL/sample) for subsequent quantification of GABA. Rats were kept for a minimum of 2 days before their brains were examined for histologic confirmation of dialysis probe placement. Each rat was used for only 1 microdialysis experiment.
The first series of in vivo microdialysis experiments aimed to determine the amount of time required to achieve stable levels of GABA following PnO insertion of the microdialysis probe. For these experiments, the dialysis probe was perfused with Ringer solution (control), and collection of dialysis samples began immediately after the dialysis probe was placed in the PnO. The second series of in vivo microdialysis experiments administered hypocretin-1 to the PnO while quantifying the effects on PnO GABA levels. Each of these experiments consisted of collecting a total of 18 sequential dialysis samples during 3 sequential treatment conditions (6 samples per condition). During condition 1, the dialysis probe was perfused with Ringer solution (control). For condition 2, the probe was perfused with Ringer solution containing a known concentration of hypocretin-1 (0, 1, 3, 10, 30, or 100 μM). Condition 3 was a return to perfusing the probe with Ringer solution. The change between dialysis treatment conditions was made via a CMA/110 liquid switch. Studies using neuropeptides of similar molecular mass to hypocretin-1 have shown that less than 0.1% of the peptide crosses the dialysis membrane.40 Therefore, approximate concentrations of hypocretin-1 delivered to the PnO in the present study ranged from 1 to 100 nM. Successful microdialysis delivery of hypocretin-1 to the brain has been demonstrated.19, 34
To ensure that intraexperimental changes in microdialysis probe performance did not confound experimental results, probe recovery of GABA was tested in vitro before and after each experiment as previously described.21, 34 The mean ± standard error of the mean (SEM) percentage of probe recovery of GABA for all microdialysis probes used in this study was 5.3% ± 0.4% before insertion into the brain and 5.7% ± 0.4% after removal from the brain. These recoveries were not significantly different by t-test.
Quantification of GABA Using HPLC and Electrochemical Detection
Dialysis samples were analyzed using an HPLC system described previously.21 Briefly, a Shiseido CAPCELL PAK C-18 separation column (3 μm particle diameter, 3 mm inner diameter, 5 cm length; JM Science Inc., Grand Island, NY) was used for analyte separation. Dialysate was derivatized with a solution consisting of 5.0 mM o-phthaldialdehyde, 1.8 mM β-mercaptoethanol, 97.1 mM borate buffer, 2.5% (v/v) methanol at pH 9.3, and then injected onto the separation column using an autosampler. GABA was isolated using a 100 mM sodium phosphate, 25% (v/v) methanol, and 3% (v/v) acetonitrile (pH 6.75) mobile phase at a flow rate of 0.6 mL/minute.
GABA peak determination in brain dialysis samples was based on the retention time of GABA standards used during standard curve construction. Seven concentrations (ranging from 0.011 pmol/10 μL to 0.913 pmol/10 μL injected volume) of GABA were used for the standard curve and bracketed all dialysate GABA concentrations. Standard curves were generated before and after dialysis sample analysis to confirm that instrument sensitivity did not decrease during sample analysis.
In vivo Microinjection and Arousal State Quantification
Three series of microinjection studies were performed to determine whether PnO administration of hypocretin-1, nipecotic acid, or 3-MPA alters sleep and wakefulness. Surgical and experimental methods have been reported previously.21 Briefly, rats used for the microinjection studies (n = 28) were anesthetized with isoflurane and implanted with a guide cannula (Plastics One, Roanoke, VA) aimed 1 mm above the PnO.39 Three screw electrodes (Plastics One) for recording the cortical EEG were implanted on the surface of the cortex, and 2 additional electrodes were implanted bilaterally in the dorsal neck muscles for monitoring the electromyogram (EMG). The electrode leads were inserted into a 6-pin multichannel electrode pedestal (Plastics One). The guide cannula, electrodes, and pedestal were fixed to the skull by dental acrylic (Lang Dental Manufacturing Company, Inc., Wheeling, IL) and 3 anchor screws (Small Parts, Inc., Miami Lakes, FL). At the end of each surgery, the guide tube was capped with a Plastics One obturator.
Each rat was conditioned to a recording chamber (Raturn®; Bioanalytical Systems (BAS), West Lafayette, IN) and a white-noise generator for a minimum of 7 days after surgery. For conditioning and for experiments, rats were tethered in the chamber. All microinjections were performed a minimum of 1 week apart, and each rat was tethered in the Raturn for a minimum of 18 hours before any microinjection or recording. Microinjections were always performed between 0930 and 1030. All but 3 rats received 1 microinjection (60 second duration) of Ringer solution (0.1 μL; vehicle control) and 1 microinjection of either hypocretin-1 (35.6 ng/0.1 μL; 10 pmol; 0.1 mM), nipecotic acid (1.29 μg/0.1 μL; 10 nmol; 100 mM), or 3-MPA (1.06 μg/0.1 μL; 10 nmol; 100 mM). Three rats received both hypocretin-1 and nipecotic acid. The order of the microinjections was randomized. A 2 hour baseline recording was performed 3 days prior to the first microinjection to ensure signal integrity. Polygraphic signals were scored in 10 second epochs as wakefulness, NREM sleep, or REM sleep using Icelus Analysis software.41
Histologic Localization of Microdialysis and Microinjection Sites
Animals were deeply anesthetized and decapitated, and brains were rapidly removed, frozen, and serially cut in 40-μm thick sections with a cryostat (Leica Microsystems, Nussloch, Germany). Coronal sections spanning the pontine brainstem were stained with cresyl violet. All sections containing microdialysis or microinjection sites were compared to a rat brain atlas39 to determine the 3-dimensional coordinates (mm) of the site relative to bregma. Only experiments in which the microdialysis membrane or the tip of the microinjection cannula was located in the PnO were included in the group data.
Statistical analyses of microdialysis and microinjection data
GABA levels are expressed as percentage of control (dialysis with Ringer solution), calculated for each experiment. Data were analyzed using descriptive and inferential statistics (GBStat™ v.6.5.6, Dynamic Microsystems, Inc., Silver Spring, MD; and SPSS v.11.0.3, SPSS Inc., Chicago, IL). The time required to achieve stable GABA levels following dialysis probe insertion was assessed by linear regression analysis. Time-course dialysis data were analyzed by nonparametric Kruskal-Wallis 1-way analysis of variance (ANOVA) and posthoc t-tests with a Bonferroni correction. Dialysis concentration response data were analyzed using a completely randomized 1-way ANOVA with Dunnett's posthoc multiple comparisons test. Significant effects of drug microinjections on sleep and wakefulness were determined by paired t-test. A probability (P) value of < 0.05 was considered statistically significant. All data are reported as mean ± SEM.
RESULTS
GABA Levels Were Stable Within 42 Minutes After Dialysis Probe Insertion into the PnO
Figure 1 summarizes the results of experiments designed to determine the amount of time needed for GABA levels to stabilize following placement of the dialysis probe in the PnO. Figure 1A demonstrates that GABA is detectable in brain dialysis samples with the use of in vivo microdialysis and HPLC. Figure 1B plots GABA levels during the first 84 minutes after dialysis probe insertion into the PnO. Linear regression analysis of GABA levels in the first 6 dialysis samples (minutes 7-42) resulted in a line equation of y = −0.0451x + 0.2271 and a slope that was significantly different than 0 (t = −4.8; P = 0.0089), indicating that GABA levels were decreasing significantly during this time. The regression equation for the last 6 dialysis samples (minutes 49-84) was y = −0.0021x + 0.0613. The slope was not significantly different from 0, demonstrating that GABA levels had stabilized. Figure 1C shows that during anesthesia, PnO GABA levels remained stable for up to 210 minutes of dialysis with Ringer solution (control). Average GABA levels during minutes 43 to 210 were 0.029 ± 0.001 pmol/10 μL.
Figure 1.
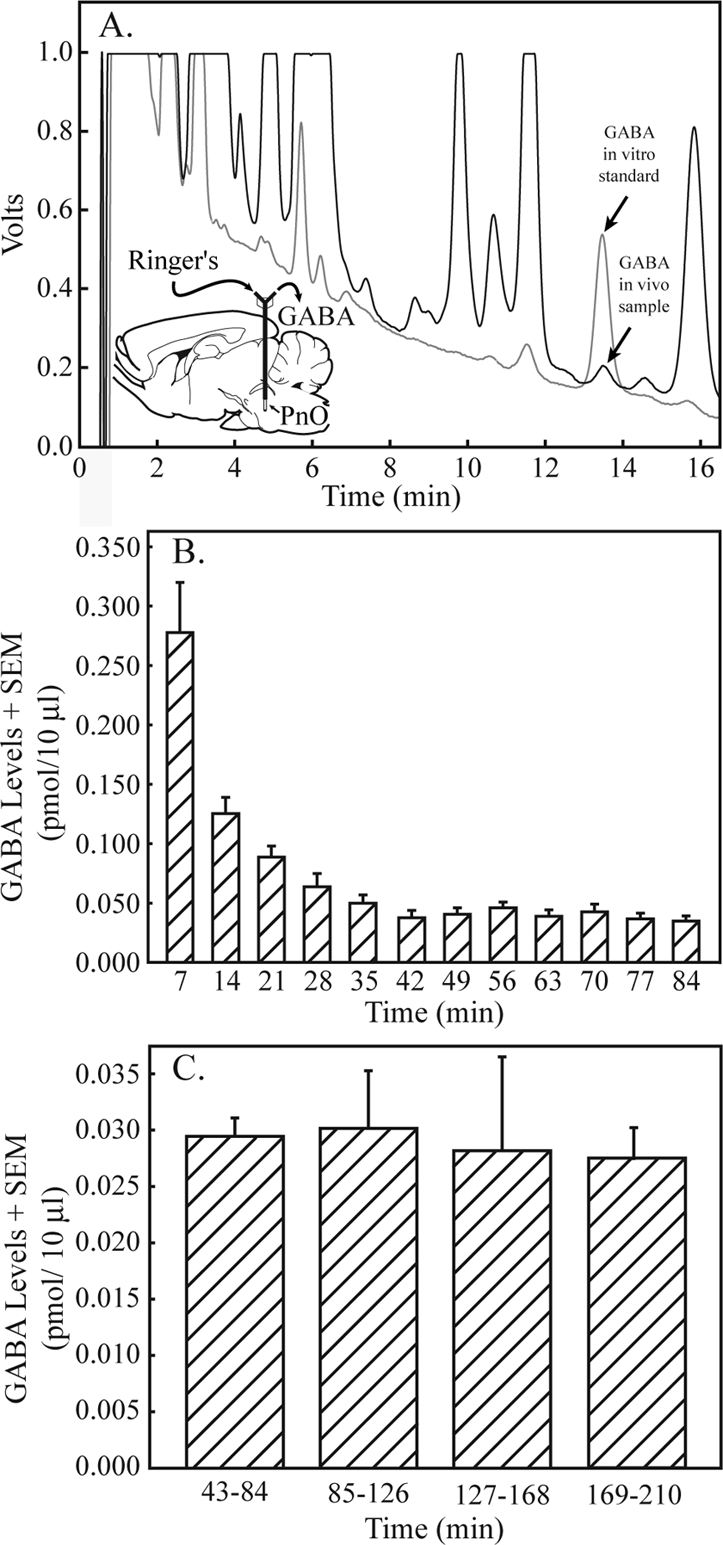
GABA levels in the pontine reticular nucleus, oral part (PnO) became stable by 42 minutes after dialysis probe insertion and remained stable for 2.8 hours. A. A representative chromatogram generated from a known quantity of GABA (in vitro standard, gray line, 0.913 pmol/10 μL) is shown superimposed on a typical chromatogram obtained by dialysis of the PnO (in vivo sample, black line; 0.107 pmol/10 μL), indicating the ability to identify GABA in brain samples. A sagittal diagram of the rat brain39 has been modified by adding a schematized microdialysis probe inserted in the PnO. The dialysis membrane at the tip of the probe is drawn to scale. Curved arrows indicate that Ringer solution was delivered to the probe through the inlet port and GABA from the PnO was collected at the outlet port. B. GABA levels in sequentially collected dialysis samples are plotted for the first 84 minutes after dialysis probe insertion into the PnO. Each bar indicates average GABA levels from 18 rats, plotted in 7 minute intervals. Thus, time 7 indicates minutes 1–7, and time 84 indicates minutes 78–84. C. GABA levels in the PnO remained stable for 2.8 hours of dialysis with Ringer solution. Each bar represents average PnO GABA levels in 18 dialysis samples (6 samples per rat x 3 rats).
Hypocretin-1 Caused a Concentration-Dependent Increase in PnO GABA Levels
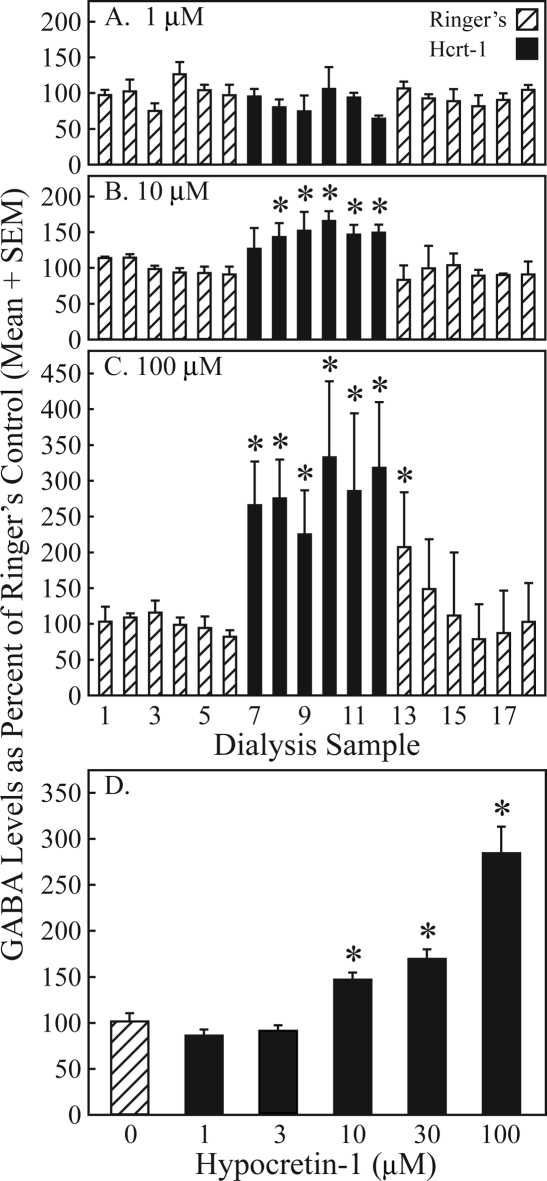
The effects of 3 hypocretin-1 concentrations on GABA levels across time are summarized by Figure 2A–C. Dialysis with 1 μM of hypocretin-1 (Figure 2A, solid bars, samples 7–12) did not significantly change GABA levels compared to dialysis with Ringer solution (Figure 2A, hatched bars, samples 1-6). Dialysis with 10 μM of hypocretin-1 (Figure 2B, solid bars, samples 7-12) significantly (*) increased GABA levels after 7 minutes of drug administration (Figure 2B; χ2 = 24.2; df = 12; P = 0.019). GABA returned to predrug control levels within the first 7 minutes after dialysis administration of hypocretin-1 was discontinued (Figure 2B, hatched bars, samples 13-18). Dialysis with 100 μM of hypocretin-1 (Figure 2C, solid bars, samples 7-12) significantly (*) increased PnO GABA levels within the first 7 minutes of drug administration (Figure 2C; χ2 = 25.5; df = 12; P = 0.013). When the dialysis solution was switched back to Ringer solution without hypocretin-1 (Figure 2C, hatched bars, samples 13-18), GABA levels remained elevated (*) during the first 7-minute sampling period (Figure 2C, sample 13). Figure 2D depicts average GABA levels during dialysis with Ringer solution (0 μM hypocretin-1) and 5 concentrations of hypocretin-1. ANOVA revealed a significant effect of hypocretin-1 concentration on PnO GABA levels (F = 29.12; df = 5, 97; P < 0.0001). Dunnett's posthoc multiple comparisons test showed that GABA levels were significantly (*) increased over control (0 μM hypocretin-1) by 46.5% ± 7.5%, 69.0% ± 10.4%, and 183.8% ± 28.9% during perfusion of the dialysis probe with 10, 30, and 100 μM hypocretin-1, respectively.
Figure 2.
Hypocretin-1 caused a concentration-dependent increase in pontine reticular nucleus, oral part (PnO) GABA levels. A-C. Each bar represents average GABA levels from 3 rats plotted in sequential 7 minute intervals during dialysis with Ringer solution (hatched bars), hypocretin-1 (solid bars), and Ringer solution (hatched bars). Dialysis samples 1–6 correspond in time to minutes 49–84 in Figure 1B. Asterisks indicate a significant increase compared to the average GABA level measured during dialysis with Ringer solution before delivery of hypocretin-1 (samples 1–6). D. Each concentration of hypocretin-1 was tested in 3 rats. The number of dialysis samples used to calculate mean ± SEM GABA levels was 16, 17, 17, 18, 18, and 18 for hypocretin-1 concentrations of 0, 1, 3, 10, 30, and 100 μM, respectively. Average GABA levels significantly (*) increased during dialysis with 10, 30, and 100 μM hypocretin-1 as compared to control dialysis with Ringer solution (0 μM hypocretin-1).
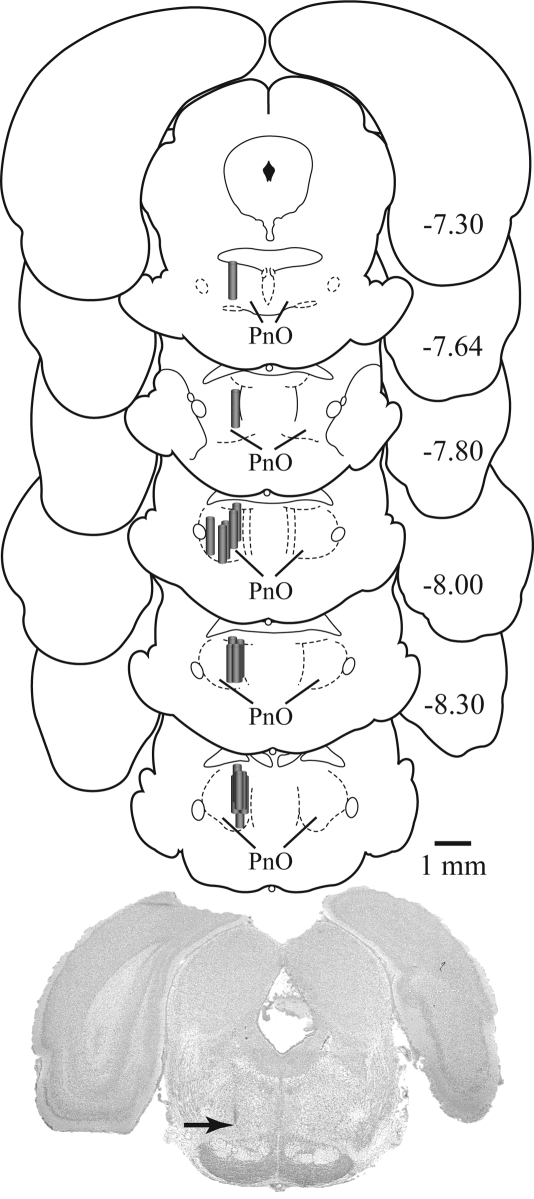
Histologic analyses confirmed that all GABA measures reported in Figures 1 and 2 were obtained from the PnO. Figure 3 schematizes the location of each microdialysis membrane on a series of coronal drawings of the rat brainstem.39 The average stereotaxic coordinates of the dialysis membranes were 7.96 ± 0.06 mm posterior to bregma, 1.07 ± 0.05 mm lateral to the midline, and 8.66 ± 0.06 mm ventral to the skull surface.39
Figure 3.
Microdialysis sites were localized to the pontine reticular formation, oral part (PnO). The vertical cascade of coronal sections shows the location of each microdialysis probe. Probe membranes are denoted by shaded cylinders and are drawn to scale. The digitized image of a cresyl-violet-stained section shows a typical microdialysis probe tract. The arrow points to the most ventral portion of the dialysis site. The coronal diagrams were modified from a rat brain atlas,39 and numbers at the right of each diagram indicate mm from bregma.
Hypocretin-1 and Endogenous GABA in the PnO Increased Wakefulness and Decreased Sleep
Having demonstrated that hypocretin-1 increases GABA levels in the PnO, this study then quantified the effects on sleep and wakefulness of microinjecting hypocretin-1 into the PnO. In addition, the effects on sleep and wakefulness of microinjecting nipecotic acid and 3-MPA into the PnO were examined. Nipecotic acid increases GABA levels in rat PnO,21 and 3-MPA decreases GABA levels in rat striatum.22 Figure 4 shows the temporal distribution of sleep and wakefulness from representative experiments following PnO microinjection of Ringer solution (vehicle control, Figure 4A), hypocretin-1 (Figure 4B), nipecotic acid (Figure 4C), and 3-MPA (Figure 4D). Hypocretin-1 and nipecotic acid increased wakefulness, decreased sleep, and increased REM latency, whereas 3-MPA decreased wakefulness and increased NREM sleep and REM sleep. The effects on sleep and wakefulness shown in these single case examples are representative of the group data, summarized by Figures 5 to 7.
Figure 4.
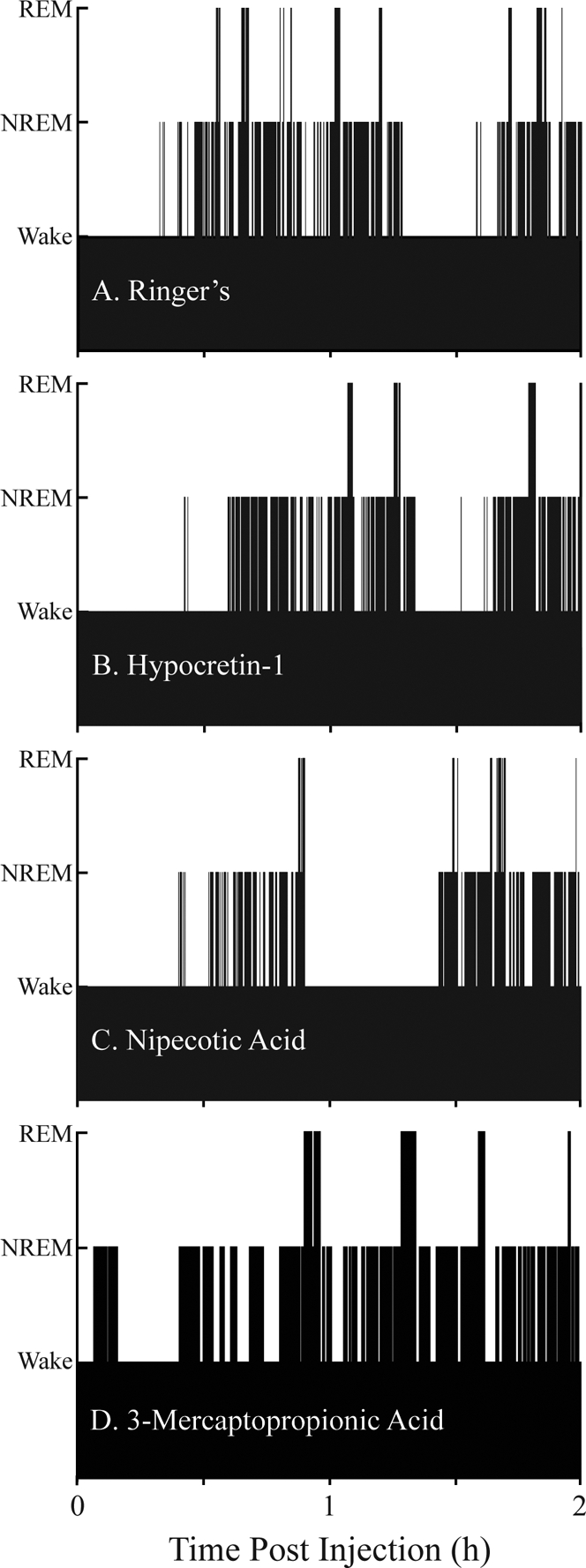
The temporal organization of sleep and wakefulness was normal following pontine reticular nucleus (PnO) microinjection of Ringer solution (A), hypocretin-1 (B), nipecotic acid (C), and 3-mercaptopropionic acid (D). Each graph plots the time course of sleep and wakefulness for 2 hours following a single microinjection, which was made during wakefulness. Bar height indicates states of wakefulness (lowest bars), non-rapid eye movement (NREM) sleep (intermediate height bars) and rapid eye movement (REM) sleep (highest bars). Sleep architecture was normal following drug administration, in that REM sleep was always preceded by NREM sleep and REM sleep was most frequently followed by an episode of wakefulness.
Figure 5.
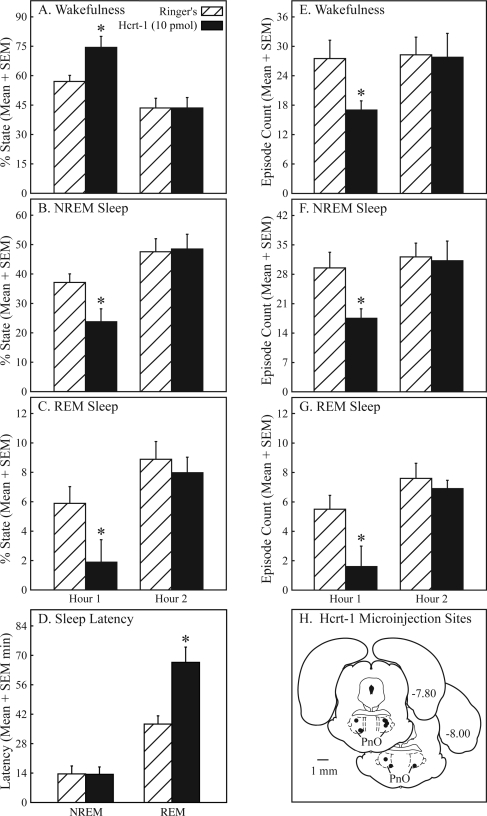
Pontine reticular nucleus, oral part (PnO) microinjection of hypocretin-1 caused a reversible increase in wakefulness and decrease in sleep. In the first hour after microinjection, the percentage of wakefulness (A) increased by 30%, and the percentage of NREM sleep (B) and REM sleep (C) decreased by 36% and 68%, respectively. Hypocretin-1 increased REM latency (D) by 79% but did not affect NREM latency (D). Microinjection of hypocretin-1 decreased the number of episodes of wakefulness (E, 38%), NREM sleep (F, 41%), and REM sleep (G, 71%). All dependent measures of sleep and wakefulness returned to control (Ringer solution) levels during the second hour of recording. Hypocretin-1 microinjection sites (H) were localized to the PnO and are depicted by filled circles on 2 coronal diagrams modified from a rat brain atlas.39 Numbers at the right of each diagram indicate mm posterior to bregma. Hypocretin-1 microinjection sites (n = 10) spanned from −7.80 to −8.26 mm from bregma.
Figure 6.
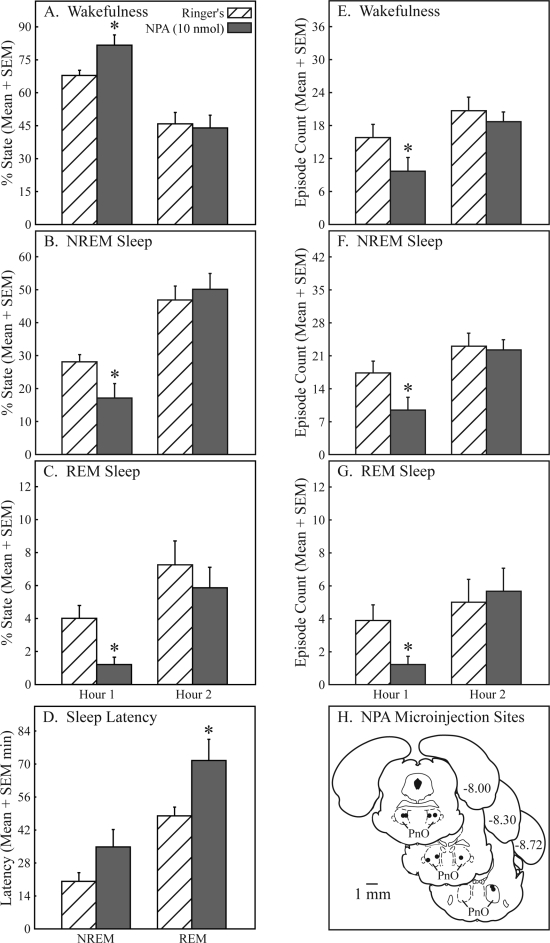
Nipecotic acid significantly (*) increased wakefulness and decreased sleep in the first hour after microinjection into the pontine reticular nucleus, oral part (PnO). Nipecotic acid caused a 20% increase in wakefulness (A), a 39% decrease in non-rapid eye movement (NREM) sleep (B), and a 70% decrease in rapid eye movement (REM) sleep (C). Nipecotic acid also increased latency to onset of REM sleep by 49% (D). Nipecotic acid decreased the number of episodes of wakefulness (E, 39%), NREM sleep (F, 46%), and REM sleep (G, 68%). Nipecotic acid microinjection sites (H) were localized to the PnO and are represented by filled circles on 3 coronal diagrams modified from a rat brain atlas.39 Numbers at the right of each diagram indicate mm posterior to bregma. Sites where nipecotic acid was microinjected ranged from −7.82 to −8.73 mm from bregma.
Figure 7.
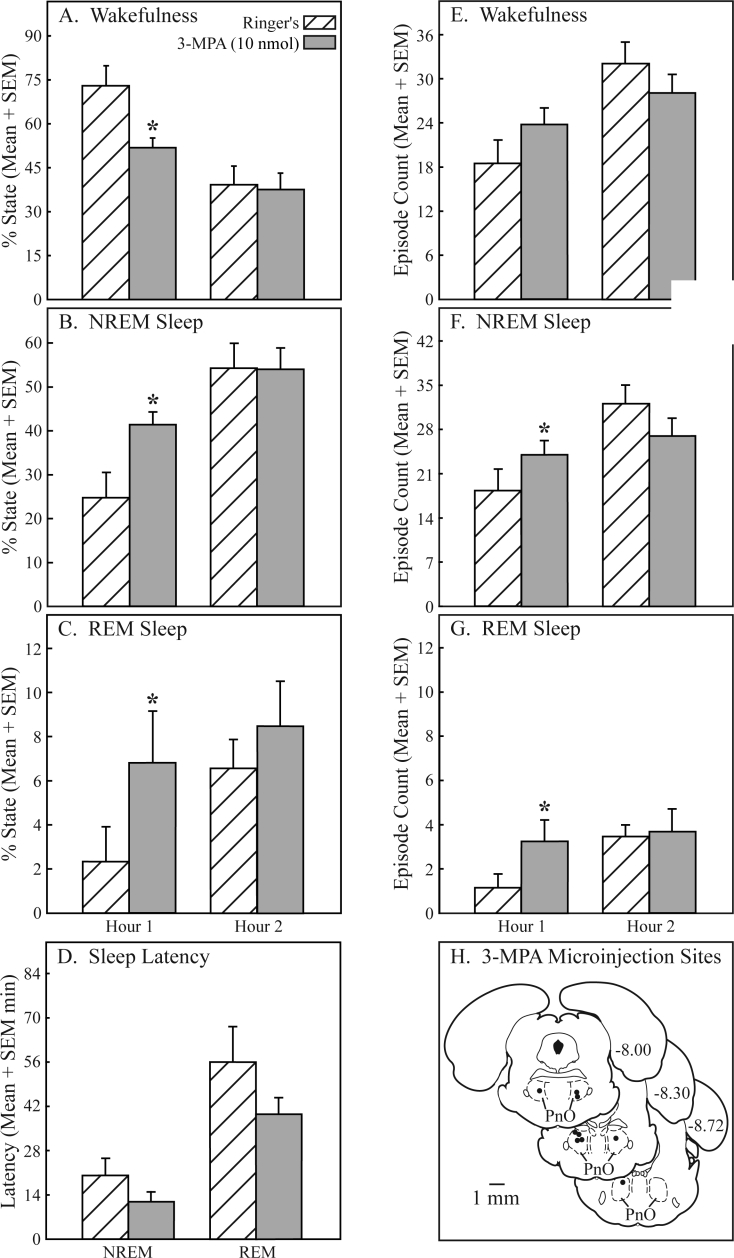
Pontine reticular nucleus, oral part (PnO) microinjection of 3-mercaptopropionic acid (3-MPA) significantly (*) and reversibly increased sleep and decreased wakefulness. Microinjection of 3-MPA decreased wakefulness by 29% (A), increased non-rapid eye movement (NREM) sleep by 68% (B), and increased rapid eye movement (REM) sleep by 193% (C) during the first hour after PnO microinjection. Although the effects did not reach statistical significance, 3-MPA showed a trend toward decreasing the latency to onset of NREM sleep and REM sleep (D) by 42% and 29%, respectively. During the first hour, an increase in the number of episodes of wakefulness (E, 28%), NREM sleep (F, 31%), and REM sleep (G, 190%) was also observed after 3-MPA microinjection, but only the increase in the number of NREM sleep and REM sleep episodes achieved statistical significance. 3-MPA microinjection sites (H) were localized to the PnO and are indicated by filled circles on 3 coronal diagrams modified from a rat brain atlas.39 Numbers at the right of each diagram indicate mm posterior to bregma. The PnO sites where 3-MPA was microinjected extended from −7.97 to −8.51 mm from bregma.
Figure 5 reports the increase in wakefulness and decrease in sleep caused by microinjecting hypocretin-1 into the PnO of awake rat (n = 10). During the first hour after injection, hypocretin-1 significantly (*) increased the percentage of time spent in wakefulness (Figure 5A; t = 4.1; P = 0.0014) and significantly decreased the percentage of time spent in NREM sleep (Figure 5B; t = 3.8; P = 0.0022) and REM sleep (Figure 5C; t = 2.7; P = 0.0116). The amount of wakefulness, NREM sleep, and REM sleep returned to control levels during the second hour after injection (Figures 5A–C). Hypocretin-1 also caused an increase in REM latency (Figure 5D; t = 3.5; P = 0.0033). During the first hour after microinjection, hypocretin-1 decreased the number of wake episodes (Figure 5E, t = 3.3; P = 0.0046) and increased the average duration of wakefulness from 1.53 ± 0.27 to 3.31± 0.77 minutes (data not graphed; t = 2.9; P = 0.0093). Hypocretin-1 also decreased the number of NREM sleep episodes (Figure 5F; t = 3.6; P = 0.0029), with no change in NREM sleep episode duration. A significant decrease in the number of REM episodes (Figure 5G; t = 3.0; P = 0.007) and average REM episode duration from 0.71 ± 0.20 to 0.17 ± 0.12 minutes (data not graphed; t = 1.97; P = 0.04) was also caused by PnO microinjection of hypocretin-1. The number of state transitions decreased by 42.9% during the first hour after microinjection of hypocretin-1 into the PnO (data not graphed; t = 3.7; P = 0.0022). All values returned to control levels (Ringer solution) during the second hour after injection. Figure 5H summarizes microinjection sites for hypocretin-1, indicated by filled circles on schematic coronal drawings from a rat brain atlas.39 Mean ± SEM stereotaxic coordinates39 for hypocretin-1 microinjection sites were 7.95 ± 0.05 mm posterior to bregma, 1.18 ± 0.07 mm lateral to the midline, and 8.36 ± 0.12 mm ventral to the skull surface.
Figure 6 shows that, similar to hypocretin-1, microinjection of the GABA uptake inhibitor nipecotic acid into the PnO of awake rat (n = 9) increased wakefulness and decreased both NREM sleep and REM sleep. Nipecotic acid significantly (*) increased the percentage of wakefulness (Figure 6A; t = 2.2; P = 0.03) and the REM latency (Figure 6D; t = 2.1; P = 0.0359). Nipecotic acid significantly decreased the percentage of time spent in NREM sleep (Figure 6B; t = 1.9; P = 0.0445) and REM sleep (Figure 6C; t = 2.9; P = 0.0100). Also decreased by nipecotic acid were the number of episodes of wakefulness (Figure 6E; t = 2.8; P = 0.0121), NREM sleep (Figure 6F; t = 2.9; P = 0.0086), and REM sleep (Figure 6G; t = 2.7; P = 0.0143). Nipecotic acid showed a trend for increasing the average duration of wakefulness episodes from 2.99 ± 0.379 to 17.43 ± 8.14 minutes (data not graphed; t = 1.8; P = 0.0570). PnO microinjection of nipecotic acid resulted in a 46.3% decrease in the number of state transitions during hour 1 (data not graphed; t = 3.02; P = 0.0083). Similar to hypocretin-1, the effects of nipecotic acid on sleep and wakefulness were observed only during the first hour after injection. All dependent measures of sleep and wakefulness returned to control (Ringer solution) levels during the second hour of recording. The filled circles in Figure 6H localize the nipecotic acid microinjection sites. Mean ± SEM stereotaxic coordinates39 for these sites were 8.19 ± 0.09 mm posterior to bregma, 1.30 ± 0.08 mm lateral to the midline, and 8.10 ± 0.07 mm ventral to the skull surface.
Figure 7 reveals that microinjection of 3-MPA, a GABA synthesis inhibitor, into the PnO of awake rat (n = 9) decreased wakefulness and increased NREM sleep and REM sleep. During the first hour after injection, 3-MPA significantly (*) decreased the percentage of wakefulness (Figure 7A; t = 4.5; P = 0.00095) and the average duration of wakefulness from 3.22 ± 0.86 to 1.42 ± 0.23 minutes (data not graphed; t = 2.4; P = 0.02). Microinjection of 3-MPA also caused a significant increase in the percentage of NREM sleep (Figure 7B; t = 3.2; P = 0.0059) and percentage of REM sleep (Figure 7C; t = 5.0; P = 0.0005). The number of episodes of NREM sleep (Figure 7F; t = 1.9; P = 0.0491) and REM sleep (Figure 7G; t = 4.1; P = 0.00165) significantly increased after administration of 3-MPA. Microinjection of 3-MPA also significantly increased the average duration of NREM sleep from 0.81 ± 0.14 to 1.08 ± 0.09 minutes (data not graphed; t = 2.2; P = 0.0312) and REM sleep from 0.55 ± 0.26 to 1.38 ± 0.23 minutes (data not graphed; t = 3.0; P = 0.0080) in the first hour after microinjection. The decreases in NREM latency and REM latency (Figure 7D) and the increase in the number of wake episodes (Figure 7E) were not statistically significant. PnO microinjection of 3-MPA had no effect on the number of state transitions. All sleep and wake measures monitored returned to Ringer solution (control) values during the second hour after injection. Microinjection sites for 3-MPA are plotted in Figure 7H and were localized to the following mean ± SEM stereotaxic coordinates:39 8.27 ± 0.06 mm posterior to bregma, 1.22 ± 0.04 lateral to the midline, and 8.08 ± 0.09 below the skull surface.
DISCUSSION
This study aimed to further explore the finding that, despite the widespread clinical use of GABAmimetic drugs to produce sleep, sedation, and general anesthesia,1,3–5 GABAergic neurotransmission within the pontine reticular formation promotes wakefulness and inhibits sleep.6–10 Specifically, this study tested the hypothesis that PnO administration of the arousal-promoting peptide hypocretin-1 increases PnO GABA levels and increases wakefulness. This study also tested the hypothesis that PnO administration of drugs that selectively increase or decrease extracellular GABA levels causes an increase or decrease, respectively, in wakefulness. The hypotheses were supported by the data. Following an overview of methodologic issues and study limitations, the results are discussed below with respect to the regulation of sleep and wakefulness by hypocretinergic and GABAergic transmission in the PnO.
Methodologic Considerations and Limitations
Several methodologic issues must be considered when measuring brain GABA levels using in vivo microdialysis and HPLC. First, a variety of GABAergic neurons may contribute to extracellular changes in PnO GABA levels, including afferent neurons,42–45 efferent neurons,46,47 and interneurons.42,48 Identifying the sources of GABA measured in the present study was not feasible. Second, it is unlikely that GABA released into the synapse diffuses out to the extrasynaptic space.49 Third, the spatial resolution of microdialysis probes does not allow for differentiating sources of neurotransmitter changes at the level of the synapse. Instead, microdialysis sampling monitors changes occurring in the extracellular space.37 Increased extracellular levels of neurotransmitters such as GABA may indirectly reflect neuronal release because at least some GABA is released from glia that envelop neuronal terminals and form a tripartite synapse (reviewed in49–52). GABA from nonvesicular sources plays an important role in inhibition via volume transmission, which occurs in the extracellular space (reviewed in37, 38, 49, 53). Volume transmission targets extrasynaptic GABAA receptors, which are activated via changes in GABAergic tone (reviewed in54–56). Similar to microdialysis probes, these extrasynaptic GABAA receptors cannot distinguish the source of GABA but can determine whether GABA levels increase or decrease. Because the exact origins of GABA in the extracellular space remain unclear, the present report takes the conservative approach of referring only to GABA levels and not GABA release.
This is the first study to determine the amount of time needed for GABA levels to stabilize following placement of a microdialysis probe into rat PnO. In a prior study measuring GABA levels in PnO of awake rat,21 the dialysis probe was inserted 17 to 18 hours prior to collection of dialysis samples in an attempt to minimize the effects of tissue trauma on GABA levels. Figure 1 shows that GABA levels in the PnO of halothane-anesthetized rat stabilized within 42 minutes of microdialysis probe insertion. This finding suggests that, for future in vivo microdialysis experiments using unanesthetized rat, it will be possible to decrease the time between probe insertion and measurement of GABA levels in dialysis samples. Figure 1 also clearly demonstrates that GABA levels do not change significantly as a function of time for up to 210 minutes of dialysis during general anesthesia.
Nipecotic acid is a selective GABA uptake inhibitor, and PnO administration of nipecotic acid had the same effects on sleep and wakefulness (Figure 6) as did hypocretin-1 (Figure 5). 3-MPA is a selective inhibitor of GABA synthesis, and 3-MPA caused effects on sleep and wakefulness (Figure 7) that were opposite to those caused by hypocretin-1 (Figure 5). Therefore, the present data suggest that hypocretin-1 increased wakefulness by increasing GABA levels in the PnO. However, a causal relationship has not been established. Demonstrating that the hypocretin-1-induced increase in wakefulness is blocked by a GABAA receptor antagonist would provide direct support for this interpretation.
Hypocretin-1 Delivered to the PnO Increases GABA Levels and Increases Wakefulness
Hypocretinergic neurons originate in the lateral hypothalamus57,58 and project to all major arousal-promoting nuclei in the brain, including the PnO.16 Keen interest in the physiologic roles of hypocretin was sparked by the discovery that defects in hypocretinergic signaling contribute to the human sleep disorder narcolepsy (reviewed in11). A large body of evidence now supports a role for hypocretin in activating motor systems and promoting wakefulness.59–66
Hypocretin may modulate behavioral arousal, in part, by increasing GABAergic transmission. Hypocretin increases GABA-mediated currents in hypothalamic slices67 and activates GABAergic terminals in the tuberomammillary nucleus.68 The effect of hypocretin on GABAergic neurons in the PnO is not known, but hypocretin-1 has been shown to inhibit the spontaneous firing of PnO neurons in urethane-anesthetized rat.15 This inhibition was reversed by pretreatment with the GABAA receptor antagonist bicuculline, suggesting that the hypocretin-induced neuronal inhibition was secondary to a hypocretin-induced increase in GABA release.15 The present data showing that hypocretin-1 caused a concentration-dependent increase in PnO GABA levels (Figure 2) are consistent with this interpretation. Hypocretin has also been shown to depolarize and increase the spontaneous firing rate of PnO neurons.20 Activation of GABAergic neurons in PnO would increase wakefulness, and GABAergic neurons48 and terminals17, 47 are present within the PnO. Although a direct causal effect has not been shown, the localization of hypocretin-1 and hypocretin-2 receptors on PnO GABA neurons69 coupled with the findings that PnO administration of hypocretin-1 increased PnO GABA levels (Figure 2) and increased wakefulness (Figure 5) suggest that hypocretin-1 may promote wakefulness, in part, by increasing GABA levels in the PnO.
Dialysis delivery of hypocretin-1 to the PnO of anesthetized rat also has been shown to increase PnO acetylcholine release.34 Microinjection of cholinomimetics into the PnO of cat,70 rat,71 and mouse72 increases REM sleep, and the release of endogenous acetylcholine in the PnO is significantly increased during REM sleep (reviewed in2). Interestingly, microinjecting hypocretin-1 into cat PnO was shown to increase REM sleep,20 as would be predicted by the hypocretin-1-induced increase in acetylcholine release.34 By what mechanisms might PnO microinjection of hypocretin-1 in rat induce wakefulness (Figure 5) rather than REM sleep?
Differences in the dose of hypocretin-1 may account for reported differences in sleep responses between cat and rat following PnO microinjection of hypocretin-1. In cat, 25 to 125 pmol of hypocretin-1 increased REM sleep, whereas 1.25 to 12.5 pmol of hypocretin-1 had no effect on REM sleep.20 In rat, 10 pmol of hypocretin-1 increased wakefulness and decreased REM sleep (Figure 5). Larger doses of hypocretin-1 may be required to increase REM sleep in rat, as in cat.
Differences between species and between anatomic location of the microinjection sites may also play a role in different sleep-wake responses to hypocretin-1. For example, the magnitude of the REM sleep response to PnO microinjection of carbachol is significantly larger in cat than in rat (reviewed in73). The PnO microinjection sites from which hypocretin-1 increased REM sleep in cat20 are not homologous to the microinjection sites from which hypocretin-1 increased wakefulness in rat (Figure 5H). Anatomic site-dependent differences in REM-sleep enhancement caused by PnO microinjection of cholinomimetics are well documented for both cat73,74 and rat.71,75,76 Thus, species differences in REM sleep-regulating mechanisms as well as anatomic differences in microinjection site may play a role in different sleep responses to PnO microinjection of hypocretin-1 in cat and rat.
Finally, the behavioral state of the animal during microinjection of hypocretin-1 may be a determining factor in the sleep response. PnO microinjection of hypocretin-1 in cat triggered REM sleep when it was delivered during NREM sleep,20 and in rat increased wakefulness when it was delivered during wakefulness (Figure 5). These findings suggest that hypocretinergic regulation of arousal may change in a state-dependent manner. State-dependent effects of cholinomimetics have been described previously.77,78 In normal humans, administering the acetylcholine esterase inhibitor physostigmine during NREM sleep induces REM sleep, whereas administering the same dose of physostigmine at the onset of REM sleep induces wakefulness.77 In experimental animals, the effects on sleep and wakefulness of microinjecting cholinomimetics into the PnO also are dependent on the behavioral state of the animal at the time when the microinjection is made.78 The PnO is a component of the ascending reticular activating system,79 and hypocretin-1, similar to acetylcholine, increases cortical EEG activation. Thus, PnO administration of hypocretin-1 during NREM sleep may stimulate neuronal networks to produce the EEG-activated state of REM sleep, whereas administration of hypocretin-1 during wakefulness may prolong the EEG-activated state of wakefulness. This concept is supported by the finding that the probability of transitioning to REM sleep greatly increases if a drug is injected during NREM sleep rather than during wakefulness.20,78
Endogenous GABA in the PnO Promotes Wakefulness
Results from microinjection studies using 3 species have consistently led to the conclusion that GABAergic transmission in the PnO promotes wakefulness. PnO microinjection of the GABAA receptor agonist muscimol increases wakefulness in cat,9 rat,7 and mouse.6 Microinjecting the GABAA receptor antagonist bicuculline into the PnO increases REM sleep and decreases wakefulness in cat,9 rat,8 and mouse.10 The present study is the first to quantify the effects on sleep and wakefulness of microinjecting drugs into rat PnO that selectively alter GABAergic tone. Nipecotic acid increases GABA levels by blocking GABA uptake,22 and dialysis administration of nipecotic acid to rat PnO has been shown to significantly increase PnO GABA levels.21 3-MPA inhibits the GABA synthetic enzyme glutamic acid decarboxylase, and 3-MPA is known to decrease GABA levels in the striatum,22 hypothalamus,80 nucleus accumbens,81 and substantia nigra.82
PnO microinjection of nipecotic acid (Figure 6) significantly increased wakefulness and decreased sleep. Increasing wakefulness and decreasing sleep by selectively increasing GABA levels in the PnO is consistent with data showing that PnO microinjection of muscimol increases wakefulness and decreases sleep.6,7,9 The increase in wakefulness and decrease in sleep caused by nipecotic acid supports the interpretation that similar changes in sleep and wakefulness caused by PnO microinjection of hypocretin-1 (Figure 5) may be due, at least in part, to the hypocretin-1-induced increase in PnO GABA levels (Figure 2).
The present data show that microinjecting 3-MPA into rat PnO decreased wakefulness and increased sleep (Figure 7). There are measurable levels of GABA in rat PnO21 (Figure 1), and rat PnO contains neurons that are immunoreactive for the GABA synthetic enzyme glutamic acid decarboxylase.83 Therefore, it is reasonable to assume that 3-MPA decreases endogenous GABA levels in the PnO, as it does in other brain regions.22,80–82 The 3-MPA-induced decrease in wakefulness and increase in REM sleep are consistent with a report showing that bilateral microinjection of antisense oligonucleotides against glutamic acid decarboxylase decreases wakefulness and increases REM sleep in cat.9 PnO microinjection of the GABAA receptor antagonist bicuculline increases REM sleep without altering NREM sleep.8–10 The present finding that 3-MPA increased both REM sleep and NREM sleep suggests that decreasing GABAergic tone and blocking GABAA receptors may have different physiologic consequences.
Sleep or sedation are increased by systemic administration of GABAmimetic drugs in humans and animals, yet drugs that increase GABAergic transmission administered directly into specific brain regions can produce increases in wakefulness and decreases in sleep. In addition to the PnO, other brain regions where direct administration of GABAmimetics has been shown to increase wakefulness and decrease sleep include the midbrain reticular formation84 and the preoptic area/anterior hypothalamus.85 Microinjection of muscimol into the posterior hypothalamus, however, induces sleep with a short latency85 in a manner similar to systemic administration of GABAmimetics. Thus, route and brain site of drug administration are key for determining the sleep response to GABAA receptor agonists and benzodiazepines.
CONCLUSIONS
Hypocretinergic neurons project to all brain regions known to promote wakefulness,16 and the excitatory effects of hypocretin67 are manifest by causing increased release of classic arousal-promoting transmitters such as acetylcholine,19,34,86 norepinephrine,87 and serotonin.88 The inability to maintain wakefulness and normal skeletal muscle tone in narcoleptic patients is now appreciated to involve defects in hypocretinergic signaling (reviewed in11). The data presented here show, for the first time, that PnO administration of hypocretin-1 increases PnO GABA levels. The PnO is a component of the ascending reticular activating system79 and is a key part of the pathway mediating REM sleep-dependent skeletal muscle atonia.89,90 Remaining to be determined is how the absence of hypocretin alters neurotransmission in brain regions that promote wakefulness and muscle tone.
This study also demonstrated, for the first time, that PnO administration of hypocretin-1 increases wakefulness and decreases sleep, as does PnO administration of the GABA uptake inhibitor nipecotic acid. PnO administration of the GABA synthesis inhibitor 3-MPA was shown to increase sleep and decrease wakefulness. Hypocretin-1 and nipecotic acid increase extracellular GABA levels, and 3-MPA decreases extracellular GABA levels. Thus, the decrease in wakefulness caused by systemic administration of GABAmimetics is unlikely to be mediated at the level of the PnO. Taken together, the data are consistent with the possibility that hypocretin-1 increases wakefulness, in part, by increasing PnO GABA levels. The data provide novel support for the conclusion that increasing GABAergic tone in the PnO promotes wakefulness.
ACKNOWLEDGMENTS
Supported by National Institutes of Health grants MH45361, HL57120, HL40881, HL65272, and the Department of Anesthesiology. The authors thank S. Jiang, M.A. Norat, and K. Walther for expert assistance.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no conflicts of interest.
REFERENCES
- 1.Bateson AN. Further potential of the GABA receptor in the treatment of insomnia. Sleep Med. 2006;7S1:S3–9. [Google Scholar]
- 2.Lydic R, Baghdoyan HA. Sleep, anesthesiology, and the neurobiology of arousal state control. Anesthesiology. 2005;103:1268–95. doi: 10.1097/00000542-200512000-00024. [DOI] [PubMed] [Google Scholar]
- 3.Mashour GA, Forman SA. Mechanisms of general anesthesia: from molecules to mind. Best Prac Res Clin Anaesthesiol. 2005;19:349–64. doi: 10.1016/j.bpa.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;7:709–20. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 5.Drake CL, Roehrs T, Roth T. Insomnia causes, consequences, and therapeutics: an overview. Depress Anxiety. 2003;18:163–76. doi: 10.1002/da.10151. [DOI] [PubMed] [Google Scholar]
- 6.Flint RR, Lydic R, Baghdoyan HA. Microinjection of the GABAA receptor agonist muscimol into the pontine reticular nucleus, oral part (PnO) of C57BL/6J (B6) mouse increases wakefulness and decreases sleep. Sleep. 2007;30(Abstr Suppl) [Google Scholar]
- 7.Camacho-Arroyo I, Alvarado R, Manjarrez J, Tapia R. Microinjections of muscimol and bicuculline into the pontine reticular formation modify the sleep-waking cycle in the rat. Neurosci Lett. 1991;129:95–7. doi: 10.1016/0304-3940(91)90728-c. [DOI] [PubMed] [Google Scholar]
- 8.Sanford LD, Tang X, Xiao J, Ross RJ, Morrison AR. GABAergic regulation of REM sleep in reticularis pontis oralis and caudalis in rats. J Neurophysiol. 2003;90:938–45. doi: 10.1152/jn.00993.2002. [DOI] [PubMed] [Google Scholar]
- 9.Xi M-C, Morales FR, Chase MH. Evidence that wakefulness and REM sleep are controlled by a GABAergic pontine mechanism. J Neurophysiol. 1999;82:2015–9. doi: 10.1152/jn.1999.82.4.2015. [DOI] [PubMed] [Google Scholar]
- 10.Chang T, Vihtelic CM, Gold C, Lydic R, Baghdoyan HA. Microinjection of the GABAA receptor antagonist bicuculline into the pontine reticular formation of C57BL/6J mouse decreases wakefulness and increases sleep. Sleep. 2006;29(Abstr Suppl) [Google Scholar]
- 11.Nishino S, Sakurai T, editors. The Orexin/Hypocretin System Physiology and Pathophysiology. Totawa: Humana Press; 2006. [Google Scholar]
- 12.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 13.Lin L, Faraco J, Li R, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–76. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 14.Chemelli RM, Willie JT, Sinton CM, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–51. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 15.Nuñez A, Moreno-Balandrán ME, Rodrigo-Angulo ML, Garzón M, De Andrés I. Relationship between the perifornical hypothalamic area and oral pontine reticular nucleus in the rat. Possible implication of the hypocretinergic projection in the control of rapid eye movement sleep. Eur J Neurosci. 2006;24:2834–42. doi: 10.1111/j.1460-9568.2006.05159.x. [DOI] [PubMed] [Google Scholar]
- 16.Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J-H, Sampogna S, Morales FR, Chase MH. Distribution of hypocretin (orexin) immunoreactivity in the feline pons and medulla. Brain Res. 2004;995:205–17. doi: 10.1016/j.brainres.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Greco MA, Shiromani PJ. Hypocretin receptor protein and mRNA expression in the dorsolateral pons of rats. Mol Brain Res. 2001;88:176–82. doi: 10.1016/s0169-328x(01)00039-0. [DOI] [PubMed] [Google Scholar]
- 19.Bernard R, Lydic R, Baghdoyan HA. Hypocretin-1 causes G protein activation and increases ACh release in rat pons. Eur J Neurosci. 2003;18:1775–85. doi: 10.1046/j.1460-9568.2003.02905.x. [DOI] [PubMed] [Google Scholar]
- 20.Xi M-C, Fung SJ, Yamuy J, Morales FR, Chase MH. Induction of active (REM) sleep and motor inhibition by hypocretin in the nucleus pontis oralis of the cat. J Neurophysiol. 2002;87:2880–8. doi: 10.1152/jn.2002.87.6.2880. [DOI] [PubMed] [Google Scholar]
- 21.Watson CJ, Lydic R, Baghdoyan HA. Sleep and GABA levels in the oral part of rat pontine reticular formation are decreased by local and systemic administration of morphine. Neuroscience. 2007;144:375–86. doi: 10.1016/j.neuroscience.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kehr J, Ungerstedt U. Fast HPLC estimation of gamma-aminobutyric acid in microdialysis perfusates: effects of nipecotic and 3-mecaptopropionic acids. J Neurochem. 1988;51:1308–10. doi: 10.1111/j.1471-4159.1988.tb03101.x. [DOI] [PubMed] [Google Scholar]
- 23.Soto-Calderon H, Watson CJ, Lydic R, Baghdoyan HA. GABA levels in the pontine reticular nucleus, oral part (PnO) of anesthetized rat are increased by dialysis administration of hypocretin-1 (orexin A) Sleep. 2005;28(Abstr Suppl) [Google Scholar]
- 24.Watson CJ, Walther KJ, Lydic R, Baghdoyan HA. Microinjection of hypocretin-1 (hcrt) or nipecotic acid (NPA) into the pontine reticular nucleus, oral part (PnO) of Sprague-Dawley rat increases wakefulness (W) and decreases sleep. Society for Neuroscience Abstract Viewer/Itinerary Planner. Online 2006;Program No. 157.15.
- 25.Watson CJ, Lydic R, Baghdoyan HA. Microinjection of 3-mercaptopropionic acid (3MPA) into the pontine reticular nucleus, oral part (PnO) of Sprague-Dawley rat decreases wakefulness (W) and increases sleep. Society for Neuroscience Abstract Viewer/Itinerary Planner. Online 2007;Program No. 631.3.
- 26.Nitz D, Siegel JJ. GABA release in posterior hypothalamus across sleep-wake cycle. Am J Physiol. 1996;271:R1707–R12. doi: 10.1152/ajpregu.1996.271.6.R1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nitz D, Siegel JJ. GABA release in the dorsal raphe nucleus: role in the control of REM sleep. Am J Physiol. 1997;273:R451–R5. doi: 10.1152/ajpregu.1997.273.1.R451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nitz D, Siegel JJ. GABA release in the locus coeruleus as a function of sleep/wake state. Neuroscience. 1997;78:795–801. doi: 10.1016/s0306-4522(96)00549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marks GA, Kramer GL, Birabil CG. GABAergic mechanisms in the REM sleep induction zone of the rat. Sleep. 2003;26(Abstr Suppl) [Google Scholar]
- 30.Thakkar MM, Ta R, Ma Z, Winston S, Yunren B, McCarley RW. GABA release in the mPRF: role in the regulation of sleep-wakefulness. Sleep. 2004;27(Abstr Suppl) [Google Scholar]
- 31.Vanini G, Watson CJ, Soto-Calderon H, Lydic R, Baghdoyan HA. GABA levels in cat pontine reticular formation (PRF) and pedunculopontine tegmental nucleus (PPT) vary with arousal state. Society for Neuroscience Abstract Viewer/Itinerary Planner. Online 2006;Program No. 458.15.
- 32.Kiyashchenko LI, Mileykovskiy BY, Maidment N, et al. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–6. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida Y, Fujiki N, Nakajima T, et al. Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur J Neurosci. 2001;14:1075–81. doi: 10.1046/j.0953-816x.2001.01725.x. [DOI] [PubMed] [Google Scholar]
- 34.Bernard R, Lydic R, Baghdoyan HA. Hypocretin (orexin) receptor subtypes differentially enhance acetylcholine release and activate G protein subtypes in rat pontine reticular formation. J Pharmacol Exp Ther. 2006;317:163–71. doi: 10.1124/jpet.105.097071. [DOI] [PubMed] [Google Scholar]
- 35.Osman NI, Baghdoyan HA, Lydic R. Morphine inhibits acetylcholine release in rat prefrontal cortex when delivered systemically or by microdialysis to basal forebrain. Anesthesiology. 2005;103:779–87. doi: 10.1097/00000542-200510000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Skulsky EM, Osman NI, Baghdoyan HA, Lydic R. Microdialysis delivery of morphine to the hypoglossal nucleus of Wistar rat increases hypoglossal acetylcholine release. Sleep. 2007;30:566–73. doi: 10.1093/sleep/30.5.566. [DOI] [PubMed] [Google Scholar]
- 37.Watson CJ, Venton BJ, Kennedy RT. In vivo measurements of neurotransmitters using microdialysis sampling. Anal Chem. 2006;78:1391–9. doi: 10.1021/ac0693722. [DOI] [PubMed] [Google Scholar]
- 38.Timmerman W, Westerink BHC. Brain microdialysis of GABA and glutamate: What does it signify? Synapse. 1997;27:242–61. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 39.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed. New York: Academic Press; 1998. [Google Scholar]
- 40.Kehr J. In vitro recovery of peptides (Application Note #9) [Accessed on January 4, 2008];1991 Available at: http://www.microdialysis.se/public/dokument.php?art=42&parent01=5&parent02=&parent03=
- 41.Opp MR. Rat strain differences suggest a role for corticotropin-releasing hormone in modulating sleep. Physiol Behav. 1998;63:67–74. doi: 10.1016/s0031-9384(97)00390-9. [DOI] [PubMed] [Google Scholar]
- 42.Camacho-Arroyo I, Alvarado R, Tapia R. Release of acetylcholine and GABA, and activity of their synthesizing enzymes in the rat pontine reticular formation. Neurochem Res. 1991;16:837–41. doi: 10.1007/BF00965530. [DOI] [PubMed] [Google Scholar]
- 43.de la Roza C, Reinoso-Suárez F. Ultrastructural synaptic organization of axon terminals in the ventral part of the cat oral pontine reticular nucleus. J Comp Neurol. 2000;427:31–53. doi: 10.1002/1096-9861(20001106)427:1<31::aid-cne3>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 44.de la Roza C, Martínez-Mena J, Sánchez-Valle ME, Reinoso-Suárez F. Projections from the cat posterior lateral hypothalamus to the ventral part of the oral pontine reticular nucleus contain a GABAergic component. Brain Res. 2004;1020:118–29. doi: 10.1016/j.brainres.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 45.Marks GA, Birabil CG, Liang CL. Extra-reticular sources of GABA implicated in control of REM sleep and wakefulness. Society for Neuroscience Abstract Viewer/Itinerary Planner. Online 2006;Program No. 458.16.
- 46.Border BG, Kosinski RJ, Azizi SA, Mihailoff GA. Certain basilar pontine afferent systems are GABA-ergic: combined HRP and immunocytochemical studies in the rat. Brain Res Bull. 1986;17:169–79. doi: 10.1016/0361-9230(86)90113-9. [DOI] [PubMed] [Google Scholar]
- 47.de la Roza C, Reinoso-Suárez F. GABAergic structures in the ventral part of the oral pontine reticular nucleus: an ultrastructural immunogold analysis. Neuroscience. 2006;142:1183–93. doi: 10.1016/j.neuroscience.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 48.Mugnaini E, Ortel WH. Handbook of Chemical Neuroanatomy. Vol. 4. Amsterdam: Elsevier Science; 1985. An atlas of the distribution of GABAergic neurons and terminals. In: Bjorklund A, Hokfelt T, eds; pp. 436–608. [Google Scholar]
- 49.Del Arco A, Segovia G, Fuxe K, Mora F. Changes in dialysate concentrations of glutamate and GABA in the brain: an index of volume transmission mediated actions? J Neurochem. 2003;85:23–33. doi: 10.1046/j.1471-4159.2003.01692.x. [DOI] [PubMed] [Google Scholar]
- 50.Newman EA. New roles for astrocytes: regulation of synaptic transmission. Trends Neurosci. 2003;26:536–42. doi: 10.1016/S0166-2236(03)00237-6. [DOI] [PubMed] [Google Scholar]
- 51.Haydon PG. Glia: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–93. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 52.Bezzi P, Volterra A. A neuron-glia signalling network in the active brain. Curr Opin Neurobiol. 2001;11:387–94. doi: 10.1016/s0959-4388(00)00223-3. [DOI] [PubMed] [Google Scholar]
- 53.Wu Y, Wang W, Richerson GB. GABA transaminase inhibition induces spontaneous and enhances depolarization-evoked GABA efflux via reversal of the GABA transporter. J Neurocsci. 2001;21:2630–9. doi: 10.1523/JNEUROSCI.21-08-02630.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mody I. Distinguishing between GABAA receptors responsible for tonic and phasic conductances. Neurochem Res. 2001;26:907–13. doi: 10.1023/a:1012376215967. [DOI] [PubMed] [Google Scholar]
- 55.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–29. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 56.Semyanov A, Walker MC, Kullman DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–9. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 57.de Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 59.de Lecea L, Sutcliffe JG. Hypocretin as a wakefulness regulatory peptide. In: Nishino S, Sakurai T, editors. The Orexin/Hypocretin System Physiology and Pathophysiology. Totawa: Humana Press; 2006. pp. 143–53. [Google Scholar]
- 60.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–20. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mignot E, Taheri S, Nishino S. Sleeping with the hypothalamus: emerging therapeutic targets for sleep disorders. Nat Neurosci. 2002;5(Suppl):1071–5. doi: 10.1038/nn944. [DOI] [PubMed] [Google Scholar]
- 62.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–98. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prober DA, Rihel J, Onah AA, Sung R-J, Schier AF. Hypocretin/orexin overexpression induces an insomnia-like phenotype in zebrafish. J Neurosci. 2006;26:13400–10. doi: 10.1523/JNEUROSCI.4332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Siegel JM. Hypocretin/orexin and motor function. In: Nishino S, Sakurai T, editors. The Orexin/Hypocretin System Physiology and Pathophysiology. Totawa: Humana Press; 2006. pp. 209–17. [Google Scholar]
- 65.Takakusaki K, Takahashi K, Saitoh K, et al. Orexinergic projections to the cat midbrain mediate alternation of emotional behavioural states from locomotion to cataplexy. J Physiol. 2005;568:1003–20. doi: 10.1113/jphysiol.2005.085829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Torterolo P, Yamuy J, Sampogna S, Morales FR, Chase MH. Hypocretinergic neurons are primarily involved in activation of the somatomotor system. Sleep. 2002;26:25–8. [PubMed] [Google Scholar]
- 67.van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18:7962–71. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eriksson KS, Sergeeva OA, Selbach O, Haas HL. Orexin (hypocretin)/dynorphin neurons control GABAergic inputs to tuberomammillary neurons. Eur J Neurosci. 2004;19:1278–84. doi: 10.1111/j.1460-9568.2004.03243.x. [DOI] [PubMed] [Google Scholar]
- 69.Zhang J, Sampogna S, Morales F, Chase M. Multiple neurotransmitter systems control the activity of GABAergic neurons in the NPO. Sleep. 2006;29(Abstr Suppl) [Google Scholar]
- 70.Baghdoyan HA, Lydic R. M2 muscarinic receptor subtype in the feline medial pontine reticular formation modulates the amount of rapid eye movement sleep. Sleep. 1999;22:835–47. doi: 10.1093/sleep/22.7.835. [DOI] [PubMed] [Google Scholar]
- 71.Bourgin P, Escourrou P, Gaultier C, Adrien J. Induction of rapid eye movement sleep by carbachol infusion into the pontine reticular formation. Neuroreport. 1995;6:532–6. doi: 10.1097/00001756-199502000-00031. [DOI] [PubMed] [Google Scholar]
- 72.Lydic R, Douglas CL, Baghdoyan HA. Microinjection of neostigmine into the pontine reticular formation of C57BL/6J mouse enhances rapid eye movement sleep and depresses breathing. Sleep. 2002;25:835–41. doi: 10.1093/sleep/25.8.835. [DOI] [PubMed] [Google Scholar]
- 73.Baghdoyan HA. Cholinergic mechanisms regulating REM sleep. In: Schwartz WJ, editor. Sleep Science: Integrating Basic Research and Clinical Practice. Basel: Karger; 1997. pp. 88–116. [Google Scholar]
- 74.Baghdoyan HA, Lydic R. Neurotransmitters and neuromodulators regulating sleep. In: Bazil C, Malow B, Sammaritano M, editors. Sleep and Epilepsy: The Clinical Spectrum. New York: Elsevier Science; 2002. pp. 17–44. [Google Scholar]
- 75.Deurveilher S, Hars B, Hennevin E. Pontine microinjection of carbachol does not reliably enhance paradoxical sleep in rats. Sleep. 1997;20:593–607. [PubMed] [Google Scholar]
- 76.Gnadt JW, Pegram GV. Cholinergic brainstem mechanisms of REM sleep in the rat. Brain Res. 1986;384:29–41. doi: 10.1016/0006-8993(86)91216-3. [DOI] [PubMed] [Google Scholar]
- 77.Sitaram N, Wyatt RJ, Dawson S, Gillin C. REM sleep induction by physostigmine infusion during sleep. Science. 1976;191:1281–3. doi: 10.1126/science.176724. [DOI] [PubMed] [Google Scholar]
- 78.López-Rodrígez F, Kohlmeier K, Morales FR, Chase MH. State dependency of the effects of microinjection of cholinergic drugs into the nucleus pontis oralis. Brain Res. 1994;649:271–81. doi: 10.1016/0006-8993(94)91074-x. [DOI] [PubMed] [Google Scholar]
- 79.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1:455–73. [PubMed] [Google Scholar]
- 80.Herbison AE, Heavens RP, Dyer RG. Endogenous release of γ-aminobutyric acid from the medial preoptic area measured by microdialysis in the anesthetized rat. J Neurochem. 1990;55:1617–23. doi: 10.1111/j.1471-4159.1990.tb04947.x. [DOI] [PubMed] [Google Scholar]
- 81.Smith S, Sharp T. An investigation of the origin of extracellular GABA in rat nucleus accumbens measured in vivo by microdialysis. J Neural Transm Gen Sect. 1994;97:161–71. doi: 10.1007/BF01277951. [DOI] [PubMed] [Google Scholar]
- 82.Timmerman W, Zwaveling J, Westerink BHC. Characterization of extracellular GABA in the substantia nigra reticulata by means of brain microdialysis. Naunyn-Schmiedeberg's Arch Pharmacol. 1992;345:661–5. doi: 10.1007/BF00164580. [DOI] [PubMed] [Google Scholar]
- 83.Boissard R, Fort P, Gervasoni D, Barbagli B, Luppi P-H. Localization of the GABAergic and non-GABAergic neurons projecting to the sublaterodorsal nucleus and potentially gating paradoxical sleep onset. Eur J Neurosci. 2003;18:1627–39. doi: 10.1046/j.1460-9568.2003.02861.x. [DOI] [PubMed] [Google Scholar]
- 84.Tsuchiya T, Fukushima H. Effects of benzodiazepines on PGO firings and multiple unit activity in the midbrain reticular formation in cats. Electroencephalogr Clin Neurophysiol. 1977;43:700–6. doi: 10.1016/0013-4694(77)90085-2. [DOI] [PubMed] [Google Scholar]
- 85.Lin J-S, Sakai K, Vanni-Mercier G, Jouvet M. A critical role of the posterior hypothalamus in the mechanisms of wakefulness determined by microinjection of muscimol in freely moving cats. Brain Res. 1989;479:225–40. doi: 10.1016/0006-8993(89)91623-5. [DOI] [PubMed] [Google Scholar]
- 86.Fadel J, Pasumarthi R, Reznikov LR. Stimulation of cortical acetylcholine release by orexin A. Neuroscience. 2005;130:541–7. doi: 10.1016/j.neuroscience.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 87.Walling SG, Nutt DJ, Lalies MD, Harley CW. Orexin-A infusion in the locus ceruleus triggers norepinephrine (NE) release and NE-induced long-term potentiation in the dentate gyrus. J Neurosci. 2004;24:7421–6. doi: 10.1523/JNEUROSCI.1587-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tao R, McKenna JT, Thakkar MM, Winston S, Strecker RE, McCarley RW. Differential effect of orexins (hypocretins) on serotonin release in the dorsal and median raphe nucleus of freely behaving rats. Neuroscience. 2006;141:1101–5. doi: 10.1016/j.neuroscience.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 89.Lai YY, Siegel JM. Medullary regions mediating atonia. J Neurosci. 1988;8:4790–6. doi: 10.1523/JNEUROSCI.08-12-04790.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lai YY, Siegel JM. Muscle atonia in REM sleep. In: Mallick BN, Inoué S, editors. Rapid Eye Movement Sleep. New Delhi: Narosa Publishing House; 1999. pp. 69–90. [Google Scholar]