Abstract
The α-D-phosphohexomutase superfamily is composed of four related enzymes that catalyze a reversible, intramolecular phosphoryl transfer on their sugar substrates. The enzymes in this superfamily play important and diverse roles in carbohydrate metabolism in organisms from bacteria to humans. Recent structural and mechanistic studies of one member of this superfamily, phosphomannomutase/phosphoglucomutase (PMM/PGM) from Pseudomonas aeruginosa, have provided new insights into enzyme mechanism and substrate recognition. Here we use sequence–sequence and sequence–structure comparisons via evolutionary trace analysis to examine 71 members of the α-D-phosphohexomutase superfamily. These analyses show that key residues in the active site, including many of those involved in substrate contacts in the P. aeruginosa PMM/PGM complexes, are conserved throughout the enzyme family. Several important regions show class-specific differences in sequence that appear to be correlated with differences in substrate specificity exhibited by subgroups of the family. In addition, we describe the translocation of a 20-residue segment containing the catalytic phosphoserine of phosphoacetylglucosamine mutase, which uniquely identifies members of this subgroup.
Keywords: phosphohexomutase, evolutionary trace, enzyme superfamily, carbohydrate metabolism
The α-D-phosphohexomutase enzyme superfamily is widespread and diverse. Two related and well-characterized proteins make up the majority of the family: the highly specific PGM, which only uses glucose as a substrate, and the less specific PMM/PGM, which can use either glucose or mannose. Two other enzymes, PNGM and PAGM, are also members of the superfamily, although to date these two proteins have been less extensively characterized. The α-D-phosphohexomutases play varied roles in carbohydrate metabolism and other biosynthetic pathways. PGM is best known for its role in providing substrates that enter the glycolytic pathway. The PMM/PGM proteins are primarily bacterial and participate in the biosynthesis of a variety of carbohydrates, such as lipopolysaccharide and alginate (Shankar et al. 1995; Rocchetta et al. 1999). PNGM and PAGM are involved in the biosynthesis of UDP-N-acetylglucosamine, which is an essential common precursor for bacterial cell wall components and is also required for the posttranslational N-acetylglucosamine modification of eukaryotic proteins (Jolly et al. 1999; Mio et al. 2000).
Despite differences in substrate specificity, the enzymes in this superfamily that have been characterized to date appear to use the same mechanism. They catalyze the reversible conversion of 1-phospho to 6-phosphosugars via a bisphosphorylated sugar intermediate. Active enzyme is phosphorylated at a conserved serine residue and binds one Mg2+ ion. The reaction mechanism entails two phosphoryl transfer reactions: first, from the enzyme to substrate, and second, from the reaction intermediate back to the enzyme. The initial phosphoryl transfer is from phosphoserine to bound substrate, creating the bisphosphorylated intermediate (e.g., glucose 1,6-bisphosphate). The intermediate must reorient and bind in an alternative position, allowing transfer of a phosphoryl group back to the protein, and thereby forming product and regenerating the active form of the enzyme (Fig. 1A ▶). Ray and coworkers have extensively characterized the mechanism of rabbit PGM (Ray et al. 1989, 1990, 1991). Other members of the family have also been studied mechanistically to varying degrees, including PMM/PGM from P. aeruginosa (Naught and Tipton 2001; Naught et al. 2003); PGM from maize (Manjunath et al. 1998), wheat (Davies et al. 2003), Arabidopsis (Periappuram et al. 2000), and A. xylinum (Brautaset et al. 1998, 2000); PNGM from Escherichia coli and P. aeruginosa (Jolly et al. 1999, 2000; Tavares et al. 2000); and PAGM from humans (Mio et al. 2000).
Figure 1.
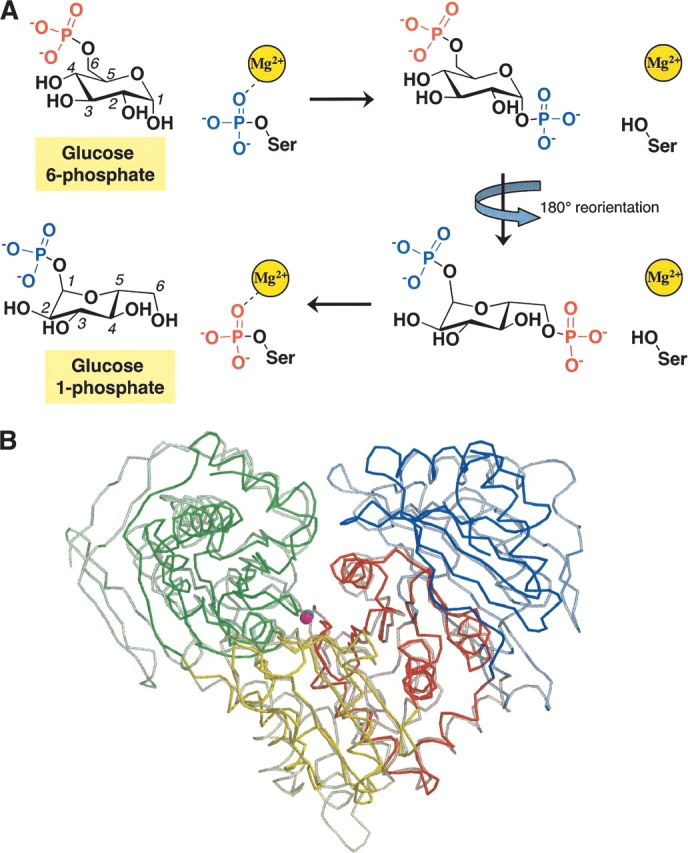
(A) Schematic of the intramolecular phosphoryl transfer reaction catalyzed by the α-D-phosphohexomutase family, illustrating the conversion of glucose 6-phosphate to glucose 1-phosphate. (B) A Cα-trace of superimposed P. aeruginosa PMM/PGM (solid colors) and rabbit PGM (semitransparent). Domains I–IV of each protein are shown in green, yellow, red, and blue, respectively, and correspond to residues 1–153, 154–256, 257–368, and 369–463 of PMM/PGM. The figure was made with MolScript (Esnouf 1997).
Representatives of both the PMM/PGM and PGM proteins have been characterized by X-ray crystallography (Liu et al. 1997; Regni et al. 2002). A comparison of the structure of PMM/PGM from the bacterium P. aeruginosa with that of PGM from rabbit (Fig. 1B ▶) shows that the two enzymes are quite similar in overall tertiary structure, with four domains and a large central active site cleft (Regni et al. 2002). In addition, recent high-resolution crystallographic analysis of P. aeruginosa PMM/PGM in complex with four substrates (glucose 1-phosphate, glucose 6-phosphate, mannose 1-phosphate, and mannose 6-phosphate) has shown that each of the four domains contains residues essential for catalysis and/or substrate recognition (Fig. 2 ▶; Regni et al. 2004). Domain I contains the catalytic phosphoserine residue that is necessary for phosphoryl transfer to and from the bisphosphorylated reaction intermediate. Domain II contains a metal-binding loop that coordinates the Mg+2 ion required for enzyme activity. Domain III contains the sugar-binding loop, which has key residues that enable the enzyme to recognize the two different binding orientations of its 1-and 6-phospho sugar substrates. Domain IV provides most of the residues that create a phosphate-binding site that determines the orientation of the incoming phosphosugar substrates.
Figure 2.
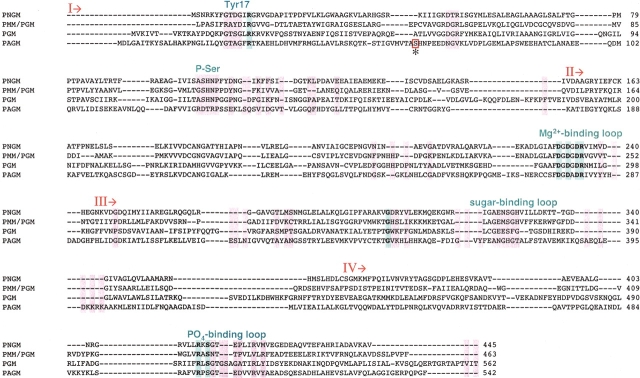
Multiple sequence alignment of representative sequences from the four subgroups of the α-D-phosphohexomutase family: PNGM from E. coli (gi:401561), PMM/PGM from P. aeruginosa (gi:113630; PDB code 1K35), PGM from rabbit (gi:548497; PDB code 3PMG), and human PAGM (gi:12643302). Invariant and nearly invariant residues are highlighted with bold text and blue shading; class-specific residues are highlighted in pink. The alternative location of the catalytic phosphoserine in PAGM is enclosed in a box and labeled with an asterisk. Important regions of the active site are indicated above the alignment, in blue text. Although only four sequences are shown, 68 members of the family were used to create the alignment and for the determination of invariant and class-specific residues. The four domains of the proteins are indicated by I, II, III, and IV.
In light of the new information provided by structural studies of P. aeruginosa PMM/PGM in complex with its substrates, we have conducted a phylogenetic and sequence-structure analysis of the α-D-phosphohexomutase superfamily via the ET method (Lichtarge et al. 1996). ET analysis is a convenient and powerful method for combining sequence relationships with three-dimensional structure and has been used successfully in several different systems to identify residues involved in function and important interactions, such as protein–protein contacts (Landgraf et al. 1999; Chakrabarti and Sowdhamini 2003; Lee et al. 2003; Madabushi et al. 2004; Zhu et al. 2004). Using this method, we find several regions of the α-D-phosphohexomutases that are highly conserved and clearly important for function in all family members, supporting the proposal that all of these enzymes use a common mechanism. In addition, we find a number of class-specific residues that distinguish the four enzyme subgroups in the family, and may be involved in their differing substrate specificities. In particular, we find that the PAGM proteins form a unique branch of the α-D-phosphohexomutase family because of a translocation of the catalytic phosphoserine residue. This work has implications for an understanding of enzyme mechanism and substrate recognition by the α-D-phosphohexomutase family and provides a basis for future functional studies of these related proteins.
Results
Sequence comparisons and phylogenetic analysis
Seventy-one members of the α-D-phosphohexomutase family were identified by PSI-BLAST (Altschul et al. 1997) in the SWISS-PROT database. These proteins are found in the three major domains of life—bacteria (26 organisms), archaea (1 organism), and eukarya—in 18 organisms including fungi, plants, and animals. The 71 sequences in the family vary in length from 444 to 632 residues: PGM and PAGM proteins are typically ~550 amino acids in length, whereas the PMM/PGMs and PNGMs are ~100 residues shorter. Sequence identities between P. aeruginosa PMM/PGM (the query sequence) and various proteins in the alignment range from 13% to 88%, with regions of homology extending over their entire length. Thus, all family members seem likely to share similar tertiary structures and the four-domain architecture first noted in rabbit PGM (Lin et al. 1986). For purposes of discussion, we have selected one protein in each of the four enzyme subgroups in the superfamily to serve as representative sequences. Because of their previous structural characterization, rabbit PGM and P. aeruginosa PMM/PGM (algC gene product) were chosen as the representatives for these two subgroups. In addition, we have selected E. coli PNGM (glmM gene product) and human PAGM (AGM1 gene product) as representative sequences for the other two subgroups. An alignment of these four protein sequences is given in Figure 2 ▶.
The phylogenetic tree shows that the α-D-phosphohexomutase superfamily has two major branches (Fig. 3 ▶). One of these is composed of the PAGM proteins (seven sequences, all eukaryotic); the other branch contains all of the remaining members of the family. Within this “other” branch, there are two major subgroups, one of which contains the PGM proteins (27), and the other of which has both the PMM/PGM and PNGM proteins (16 and 10 sequences, respectively). With three exceptions, the PGM proteins are eukaryotic in origin, whereas the PMM/PGM and PNGM sequences are exclusively from bacteria or archaea. (Two small groups of sequences, also within this branch of the family, are not discussed further here because none of these proteins have been characterized and their substrate specificities are unknown.) Overall, the four characterized enzymatic activities of the family correspond well to different branches of the phylogenetic tree, with the PAGM proteins being the most divergent branch of the family, and the PMM/PGM and PNGM proteins being the most similar to each other.
Figure 3.
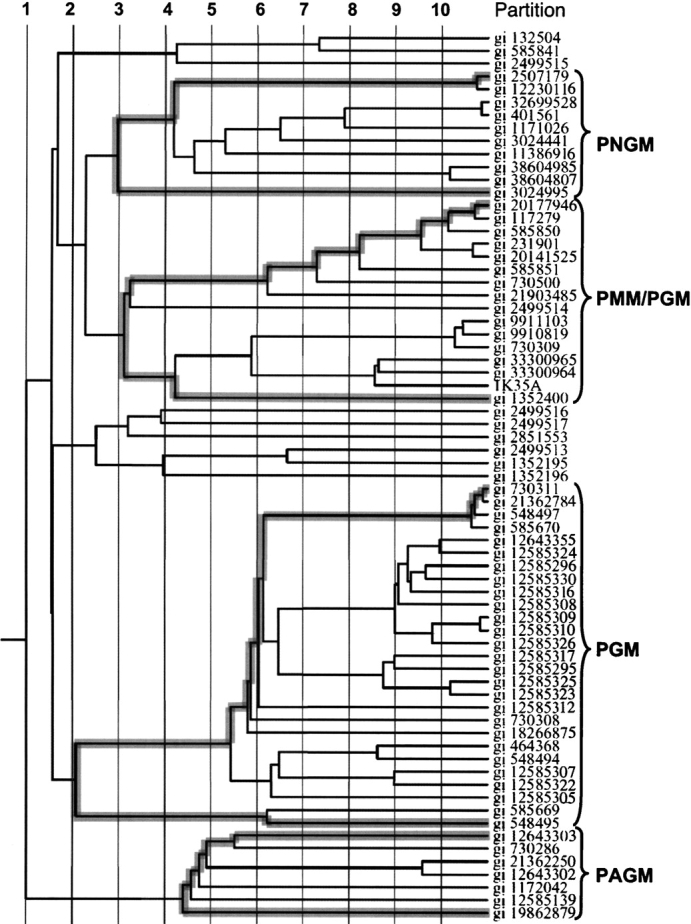
Phylogenetic tree of 69 members of the α-D-phosphohexomutase family as produced by the ET server (Lichtarge et al. 1996). Vertical lines define the 10 partitions. The sequence branches belonging to the four enzyme subgroups are highlighted with gray shading and identified with brackets.
Several proteins in the PGM subgroup have no known enzymatic activity, including one structurally characterized protein, parafusin (PDB code 1KFI; Muller et al. 2002). These proteins have been modeled elsewhere (Levin et al. 1999) and are not included in our discussion.
Invariant and highly conserved residues
To examine the structural context of the conserved regions of the α-D-phosphohexomutase family, we performed an ET of the superfamily, using P. aeruginosa PMM/PGM as the reference structure. The ET identifies six invariant residues in the family, which are dispersed widely throughout the sequence (Fig. 2 ▶). Four of these, Asp 242, Asp 244, Asp 246, and Arg 247 (residue numbers refer to P. aeruginosa PMM/PGM), are in domain II. The other two invariant residues (Gly 307 and Ser 423) are found in domains III and IV, respectively. We note here two additional residues (Arg 20 and Arg 421) that are conserved in all but one of the 71 family members and located in domains I and IV. Surprisingly, the phosphoserine residue (Ser 108) critical for catalysis is not invariant, even though it is found in every family member. Rather, it is categorized as a class-specific residue (see following) as a result of a different relative location of this residue in the PAGM subgroup of the family.
Mapping the position of the six invariant and two nearly invariant residues on the structure of PMM/PGM shows that they cluster in the active site of the enzyme (Fig. 4A ▶). The four residues in domain II help form the conserved metal-binding loop: Specifically, the three aspartates coordinate the Mg2+ ion, which is essential for catalysis. The two residues in domain IV, Arg 421 and Ser 423, participate in the phosphate-binding site, a key determinant of substrate recognition defined by the four PMM/PGM complexes, which functions to anchor substrates in the appropriate position for catalysis. Gly 307 is not directly involved in catalysis or substrate contacts; however, in the PMM/PGM complexes, its backbone amide participates in a hydrogen bond with the side chain of Glu 325, a residue critical for recognition of the three and four hydroxyls of the phosphosugar substrates. There is no clearly established role for Arg 20 in catalysis or recognition, although it presumably contributes to the positive electrostatic environment of the active site, which is important for activity (Naught et al. 2003). However, the high conservation of this residue in the family suggests that it may play a more critical role: One possibility for this would be to assist with the reorientation of the bisphosphorylated intermediate during catalysis. In the case of PMM/PGM, kinetic studies (P. Tipton, unpubl.) indicate that the intermediate remains associated with the enzyme during the reaction, despite its 180° reorientation in the active site, and that the flexible side chain of this residue would be well suited to assist with this task. Studies to address this possibility are currently under way with PMM/PGM.
Figure 4.

Close-up of the active site of P. aeruginosa PMM/PGM in complex with (A) glucose 1-phosphate (yellow) and (B) glucose 6-phosphate (green). The invariant (blue) and class-specific residues (pink) identified by the ET are highlighted. The bound metal ion is magenta. Residues that interact with the O2 position of these substrates (which occupies two distinct positions in the different complexes) are labeled in red. The figure was made with PyMol (DeLano 2002). (C) Schematic illustrating the translocation of the phosphoserine loop in the primary structure of human PAGM, relative to other members of the family. P. aeruginosa PMM/PGM is shown for comparison. The conserved phosphoserine loop is shown in pink. Regions of sequence that could be aligned by BLAST 2 Sequences (Tatusova and Madden 1999) are in blue (20% sequence identity overall).
Class-specific residues
Analysis of class-specific residues in the α-D-phosphohexomutases is a convenient and objective way to identify features that distinguish the four enzyme subgroups in the superfamily. Class specificity is determined by sequence conservation within defined subgroups of a protein family. The ET analyzes class specificity at multiple levels within the superfamily, called partitions (Fig. 3 ▶). Partition 1, for example, includes every sequence in the family and treats it as one large group. Higher partition levels represent analysis of successively smaller sequence groups as defined by branches on the phylogenetic tree, and thus discriminate between progressively more subtle differences in sequence. We focus our discussion on class-specific residues at an intermediate level (partition 6) and on clusters containing four or more class-specific residues within a stretch of 10 on the multiple sequence alignment (Fig. 2 ▶).
The most striking result of the ET analysis is the designation of the catalytic phosphoserine and nearby conserved residues as class specific (Fig. 4B ▶). This occurs because of a translocation of a 20-residue segment of the PAGM proteins, to a position ~50 residues earlier in the sequence relative to the other family members (Whitehouse et al. 1998). (The term translocation is used to refer to the exchange of positions of these residues within the protein sequence and should be distinguished from its alternative usage to describe chromosomal rearrangements.) Despite its two different locations in the two major branches of the family, the phosphoserine loop is still very highly conserved, as would be expected given its critical role in catalysis. The translocation of the phosphoserine residue is both restricted and common to all of the PAGM proteins, and clearly contributes to the phylogenetic placement of these proteins in a distinct branch of the family, separate from the other three enzymes.
The translocation of this loop in the PAGM subfamily may allow these enzymes to accommodate the acetyl substitution at the N2 of the substrate, which would occupy additional space in the active site relative to the other family members (see also “Substrate specificity” section). It is intriguing to speculate on the structural changes in the active site of PAGM that accompany this sequence rearrangement, because the catalytic phosphoserine residue must still interact with the metal-binding loop, which remains in its usual location in the sequence. The phosphoserine is still located in domain I of the protein but is apparently found on a different loop in the active site. The multiple sequence alignment (Fig. 2 ▶) shows that the phosphoserine of human PAGM corresponds to Pro 49 of PMM/PGM. This residue is not in the active site of PMM/PGM, although it is only six residues away from an active site loop (residues 55–61). However, the relatively poor sequence identity and gaps in this region of the multiple sequence alignment make it difficult to definitively predict where the PAGM serine will be found on a 3D model.
Aside from the phosphoserine, many of the class-specific residues identified by the ET surround the positions of the invariant/nearly invariant residues described earlier. Class-specific differences in these critical regions, such as near the phosphate-binding site, probably reflect relatively small local changes in the structural environment, rather than significant differences in function. For instance, the phosphate-binding site in the PMM/PGM complexes occurs at an interface between domains I and IV of the protein, which is formed via a conformational change of the protein on substrate binding. In PMM/PGM, a single residue in domain I, Tyr 17, contacts the phosphate group and also participates in a hydrogen bond with the side chain of Asn 424 (Fig. 4A ▶). The ET analysis shows that Tyr 17 is part of a cluster of class-specific residues found in an active site loop of domain I (Fig. 3 ▶). Because Tyr 17 is a class-specific residue, some class-specific differences in the phosphate-binding loop of domain IV would be expected to accommodate the differing interface with domain I. A potential role for Tyr 17 in substrate specificity is not established, but this side chain may be important for accommodating mannose-based substrates, because only the PMM/PGM enzymes have a tyrosine at this position.
Another significant cluster of class-specific residues in the α-D-phosphohexomutase superfamily is located in the sugar-binding loop. In PMM/PGM, two residues in this region (Glu 325 and Ser 327) make key contacts with different hydroxyls in the 1- and 6-phosphosugar substrates, thus accommodating their two different binding orientations in the active site. Differences between the sequences of the sugar-binding loops of PMM/PGM and PGM subgroups have been previously noted (Shankar et al. 1995); here we find that each of the four enzymes in the family has a unique “signature” in this region (Table 1). Except for the PAGM proteins, nearly all of the other family members have Glu and Ser in positions corresponding to the PMM/PGM residues, making it likely that these residues play similar roles in contacting the O3 and O4 hydroxyls of the phosphosugars. The sugar-binding loop of the PAGM proteins is rather distinct in sequence: The most likely counterparts to Glu 325 and Ser 327 appear to be Glu 381 and Asn 383 of human PAGM, which are shifted by two residues in the multiple sequence alignment but are part of a highly conserved block of residues in this enzyme family. Glu 381 could make a bidentate contact to the O3 and O4 hydroxyls of the substrate, in similar fashion to Glu 325 of PMM/PGM, and Asn 383 could make a contact analogous to that of Ser 327 with one of its side-chain oxygens.
Table 1.
Class-specific residues in the sugar-binding loop
| Enzyme | Consensus sequence | Partition | No.sequences | ||
| PNGM | 323 | -G - E - S g h | 329 | 3 | 9 |
| PMM | 323 | -- G E mS g H | 329 | 3 | 16 |
| PGM | 373 | -c G E E s f g | 380 | 2 | 27 |
| PAGM | 380 | FE A N G H G T | 387 | 2 | 7 |
Capital letters indicate class-specific residues; lowercase are highly conserved. Partition number indicates level in multiple sequence alignment at which the consensus sequence was derived.
Substrate specificity
Currently, it is unknown which residues in the active site are directly responsible for the differing substrate specificities of the four enzymes in the α-D-phosphohexomutase superfamily. It is worth noting that the structural differences between the various substrates used by the family members are all located at the 2 position of the sugar: an equatorial hydroxyl (glucose), an axial hydroxyl (mannose), an amine (glucosamine), or acetylated amine group (acetylglucosamine). The preponderance of class-specific residues in and around the sugar-binding loop, and the proximity of this region to the 2 position of the substrate, makes it tempting to speculate that this region may play a role. The PMM/PGM complexes support this to some extent. Contacts between PMM/PGM and the O2 hydroxyl of its mannose substrates are nonspecific (water-mediated), making them difficult to address via sequence analysis. However, three residues of PMM/PGM make direct contacts with the O2 hydroxyl of its glucose-based substrates, and all of these are categorized as class specific. Only one of these is made by a residue in the sugar-binding loop (His 329); the other two are elsewhere in the sequence (Lys 285 and Ser 108). In fact, these three residues are found in two different regions of the active site, as discussed following.
A consideration of residues potentially involved in substrate specificity must take into account the two different binding orientations of the 1-phospho and 6-phosphosugars, required by the common mechanism of the superfamily. A major consequence of this is that the O2 hydroxyls occupy two disparate positions in the active site (Fig. 4 ▶, cf. A and B). Therefore, two sets of residues must be considered, one for the 1-phospho substrates (Lys 285 and vicinity) and another for the 6-phospho substrates (Ser 108, His 329, and vicinity). In the first case, the ET shows a cluster of residues including and around Lys 285 as class specific in PMM/PGM (Fig. 2 ▶). The other family members also have class-specific residues in this region, but their class-specific nature only appears at relatively high partition levels within the subgroups, making a simple correspondence between sequence and enzyme specificity difficult to elucidate. In the second case, that of the 6-phosphosugars, Ser 108 is one of the residues involved in a contact with O2. The class-specific nature of Ser 108 was noted previously because of its sequence translocation in the PAGM subfamily. Figure 4B ▶ shows that a change in the position of the active site serine was likely necessary because of the close approach between this residue and O2 of the 6-phosphosugars, in order to accommodate the acetyl group of the substrate. His 329 also contacts the O2 of glucose 6-phosphate; however, Table 1 shows that there is no simple pattern regarding class specificity or residue identity at this position in the four enzyme subgroups.
In summary, the fact that a clear pattern for the sequence/structural basis of substrate specificity in the phosphohexomutase superfamily is not apparent, even using the sophisticated ET method, is likely due to a number of factors. First, as noted earlier, the 2 position of the substrate occupies very different locations depending on which form of the phosphosugar is bound (1 versus 6) and therefore involves several sets of residues at different positions in the active site. Second, the PMM/PGM complexes show that many residues in the active site change conformation depending on the identity of the bound ligand, and/or change the type of contact in which they participate (Regni et al. 2004). Lys 285, for instance, only contacts the O2 hydroxyl in one of the four enzyme–ligand complexes; in two other complexes, it exists in an alternate conformation and contacts a phosphate group of the substrate. Finally, an analysis of substrate specificity may be further complicated by the fact that, despite their unique metabolic roles, the four different enzyme subgroups appear to have overlapping substrate specificities. PNGM from P. aeruginosa, for example, has both PMM and PGM activities, in addition to its primary PNGM activity (Tavares et al. 2000). Because of these subtleties, direct structural characterization of multiple enzyme–substrate complexes will likely be necessary for a full understanding of substrate specificity in the superfamily.
Discussion
The phylogenetic analysis of the α-D-phosphohexomutase superfamily shows that these proteins span the major kingdoms of life and may in fact be present in all organisms (Whitehouse et al. 1998). On the basis of our current analysis, we find a fairly clear division of enzyme subgroups between eukaryotes (PGM and PAGM) and prokaryotes (PMM/PGM and PNGM), although this distribution will likely become more ambiguous as additional members of the family are identified. Indeed, one of the prokaryotic PGM proteins has been proposed to result from horizontal gene transfer between Agrobacterium tumefaciens and its eukaryotic host (Whitehouse et al. 1998). Nevertheless, the preponderance of PMM/PGM and PNGM proteins in bacteria suggests that these two enzymes have potential utility as markers for infection or as targets for inhibition (Lu et al. 1999; Regni et al. 2002). Such inhibitors would need to be specific enough to avoid affecting the essential eukaryotic homologs.
Our sequence comparisons of the family reveal a number of previously unnoted features that should be useful for annotations of new sequences, both as members of the superfamily and also for placing them into the most appropriate subgroup. The catalytic phosphoserine and metal-binding loops of the α-D-phosphohexomutases are well known for their high sequence conservation and are typically used to categorize new sequences as part of this enzyme family. However, our analysis reveals that other regions of the active site, such as the phosphate-binding site in domain IV, are also common to the entire enzyme family. In the P. aeruginosa PMM/PGM complexes, residues in this region were shown to make multiple interactions with the phosphate group of the substrate. These contacts are identical in all four complexes, regardless of whether a 1-phospho or 6-phosphosugar is bound. Their sequence conservation in the superfamily suggests that all of these proteins will recognize the 1- and 6-phospho forms of their sugar substrate in the same fashion, with contacts to the phosphate being the primary determinant of the binding orientation of the substrate. This finding strengthens the proposal that all four enzyme subgroups use similar catalytic mechanisms and strategies for substrate recognition. In addition, we find that one member of the α-D-phosphohexomutase family, PAGM, has undergone a dramatic relocation of the catalytic phosphoserine in its sequence. The alternative location of the catalytic phosphoserine and highly conserved surrounding residues in the PAGM proteins serves as a unique signature in the amino acid sequence that can be used to identify other proteins in this branch of the family.
The ability of the ET analysis to identify residues of functional importance in the α-D-phosphohexomutase superfamily is validated by previous characterization of family members, in particular structural studies of rabbit PGM and P. aeruginosa PMM/PGM, as well as by site-directed mutagenesis of other family members (Liu et al. 1997; Brautaset et al. 2000; Regni et al. 2002; Naught et al. 2003). The invariant and class-specific residues cluster in the active site of PMM/PGM and also correspond quite highly to residues involved in substrate contacts with that enzyme (Regni et al. 2004). This remarkable correlation with previous biochemical studies gives us confidence in predicting that the residues identified by the ET are critical for all members of the family. The ET analysis therefore provides a foundation for future studies of other family members, such as the design of site-directed mutants. This should be especially useful for the structurally uncharacterized members of the α-D-phosphohexomutase superfamily, PNGM and PAGM.
Materials and methods
Sequence alignment
Members of the α-D-phosphohexomutase family were identified by sequence homology using PSI-BLAST (Altschul et al. 1997). The input sequence for querying the SWISS-PROT database was gi:113630 (PMM/PGM from P. aeruginosa). Three iterations were required to reach convergence in PSI-BLAST; 71 related proteins were identified. Similar results were obtained when using other query sequences, including rabbit PGM (data not shown).
Evolutionary trace
Sixty-eight of the sequences identified by PSI-BLAST were aligned with CLUSTALW (Thompson et al. 1994; http://workbench.sdsc.edu/) and used as input into the ET analysis Web server (http://www-cryst.bioc.cam.ac.uk/~jiye/evoltrace/evoltrace.html) (Lichtarge et al. 1996). The query sequence (P26276) and two other sequences missing 50 or more residues relative to the other members of the family, Q15124 (human PGM5 or acuiculin) and Q48463 (ManB from K. pneumonia), were removed from the alignment prior to the ET. The input PDB file was 1K35, the 2.1 Å P. aeruginosa PMM/PGM crystal structure. Selenomethionine residues in this file were manually renamed to methionine so that they would be recognized in the sequence comparisons. The phyloge-netic tree in Figure 3 ▶ was produced by the ET server.
Acknowledgments
We thank Peter Tipton for a critical reading of the manuscript. This work was supported by NIH grant GM59653. C.A.R. is supported by NIH training grant GM08396-13.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Abbreviations
PGM, phosphoglucomutase
PMM/PGM, phosphomannomutase/phosphoglucomutase
PNGM, phosphoglucosamine mutase
PAGM, phosphoacetylglucosamine mutase
ET, evolutionary trace.
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.04801104.
References
- Altschul, S.F., Madden, T.L., Schaffer, A.A., Zhang, J., Zhang, Z., Miller, W., and Lipman, D.J. 1997. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 25 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brautaset, T., Petersen, S., and Valla, S. 1998. An experimental study on carbon flow in Escherichia coli as a function of kinetic properties and expression levels of the enzyme phosphoglucomutase. Biotechnol. Bioeng. 58 299–302. [PubMed] [Google Scholar]
- ———. 2000. In vitro determined kinetic properties of mutant phosphoglucomutases and their effects on sugar catabolism in Escherichia coli. Metab. Eng. 2 104–114. [DOI] [PubMed] [Google Scholar]
- Chakrabarti, S. and Sowdhamini, R. 2003. Functional sites and evolutionary connections of acylhomoserine lactone synthases. Protein Eng. 16 271–278. [DOI] [PubMed] [Google Scholar]
- Davies, E.J., Tetlow, I.J., Bowsher, C.G., and Emes, M.J. 2003. Molecular and biochemical characterization of cytosolic phosphoglucomutase in wheat endosperm (Triticum aestivum L. cv. Axona). J. Exp. Bot. 54 1351–1360. [DOI] [PubMed] [Google Scholar]
- DeLano, W.L. 2002. The PyMOL molecular graphics system. DeLano Scientific, San Carlos, CA.
- Esnouf, R.M. 1997. An extensively modified version of MolScript that includes greatly enhanced coloring capabilities. J. Mol. Graph. Model. 15 132–134. [DOI] [PubMed] [Google Scholar]
- Jolly, L., Ferrari, P., Blanot, D., Van Heijenoort, J., Fassy, F., and Mengin-Lecreulx, D. 1999. Reaction mechanism of phosphoglucosamine mutase from Escherichia coli. Eur. J. Biochem. 262 202–210. [DOI] [PubMed] [Google Scholar]
- Jolly, L., Pompeo, F., van Heijenoort, J., Fassy, F., and Mengin-Lecreulx, D. 2000. Autophosphorylation of phosphoglucosamine mutase from Escherichia coli. J. Bacteriol. 182 1280–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf, R., Fischer, D., and Eisenberg, D. 1999. Analysis of heregulin symmetry by weighted evolutionary tracing. Protein Eng. 12 943–951. [DOI] [PubMed] [Google Scholar]
- Lee, G.I., Ding, Z., Walker, J.C., and Van Doren, S.R. 2003. NMR structure of the forkhead-associated domain from the Arabidopsis receptor kinase-associated protein phosphatase. Proc. Natl. Acad. Sci. 100 11261–11266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, S., Almo, S.C., and Satir, B.H. 1999. Functional diversity of the phosphoglucomutase superfamily: Structural implications. Protein Eng. 12 737–746. [DOI] [PubMed] [Google Scholar]
- Lichtarge, O., Bourne, H.R., and Cohen, F.E. 1996. An evolutionary trace method defines binding surfaces common to protein families. J. Mol. Biol. 257 342–358. [DOI] [PubMed] [Google Scholar]
- Lin, Z., Konno, M., Abad-Zapatero, C., Wierenga, R., Murthy, M.R., Ray Jr., W.J., and Rossmann, M.G. 1986. The structure of rabbit muscle phosphoglucomutase at intermediate resolution. J. Biol. Chem. 261 264–274. [PubMed] [Google Scholar]
- Liu, Y., Ray, W., and Baranidharan, S. 1997. Structure of rabbit muscle phosphoglucomutase refined at 2.4 Å resolution. Acta Crystallogr. D 53 392–405. [DOI] [PubMed] [Google Scholar]
- Lu, J.J., Perng, C.L., Shyu, R.Y., Chen, C.H., Lou, Q., Chong, S.K., and Lee, C.H. 1999. Comparison of five PCR methods for detection of Helicobacter pylori DNA in gastric tissues. J. Clin. Microbiol. 37 772–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madabushi, S., Gross, A.K., Philippi, A., Meng, E.C., Wensel, T.G., and Lichtarge, O. 2004. Evolutionary trace of G protein-coupled receptors reveals clusters of residues that determine global and class-specific functions. J. Biol. Chem. 279 8126–8132. [DOI] [PubMed] [Google Scholar]
- Manjunath, S., Lee, C.H., VanWinkle, P., and Bailey-Serres, J. 1998. Molecular and biochemical characterization of cytosolic phosphoglucomutase in maize. Expression during development and in response to oxygen deprivation. Plant Physiol. 117 997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mio, T., Yamada-Okabe, T., Arisawa, M., and Yamada-Okabe, H. 2000. Functional cloning and mutational analysis of the human cDNA for phosphoacetylglucosamine mutase: Identification of the amino acid residues essential for the catalysis. Biochim. Biophys. Acta 1492 369–376. [DOI] [PubMed] [Google Scholar]
- Muller, S., Diederichs, K., Breed, J., Kissmehl, R., Hauser, K., Plattner, H., and Welte, W. 2002. Crystal structure analysis of the exocytosis-sensitive phosphoprotein, pp63/parafusin (phosphoglucomutase), from Paramecium reveals significant conformational variability. J. Mol. Biol. 315 141–153. [DOI] [PubMed] [Google Scholar]
- Naught, L.E. and Tipton, P.A. 2001. Kinetic mechanism and pH dependence of the kinetic parameters of Pseudomonas aeruginosa phosphomannomutase/phosphoglucomutase. Arch. Biochem. Biophys. 396 111–118. [DOI] [PubMed] [Google Scholar]
- Naught, L.E., Regni, C., Beamer, L.J., and Tipton, P.A. 2003. Roles of active site residues in P. aeruginosa phosphomannomutase/phosphoglucomutase. Biochemistry 42 9946–9951. [DOI] [PubMed] [Google Scholar]
- Periappuram, C., Steinhauer, L., Barton, D.L., Taylor, D.C., Chatson, B., and Zou, J. 2000. The plastidic phosphoglucomutase from Arabidopsis. A reversible enzyme reaction with an important role in metabolic control. Plant Physiol. 122 1193–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray Jr., W.J., Post, C.B., and Puvathingal, J.M. 1989. Comparison of rate constants for (PO3–) transfer by the Mg(II), Cd(II), and Li(I) forms of phosphoglucomutase. Biochemistry 28 559–569. [DOI] [PubMed] [Google Scholar]
- Ray Jr., W.J., Burgner 2nd, J.W., and Post, C.B. 1990. Characterization of vanadate-based transition-state-analogue complexes of phosphoglucomutase by spectral and NMR techniques. Biochemistry 29 2770–2778. [DOI] [PubMed] [Google Scholar]
- Ray Jr., W.J., Puvathingal, J.M., and Liu, Y.W. 1991. Formation of substrate and transition-state analogue complexes in crystals of phosphoglucomutase after removing the crystallization salt. Biochemistry 30 6875–6885. [DOI] [PubMed] [Google Scholar]
- Regni, C., Tipton, P.A., and Beamer, L.J. 2002. Crystal structure of PMM/PGM: An enzyme in the biosynthetic pathway of P. aeruginosa virulence factors. Structure 10 269–279. [DOI] [PubMed] [Google Scholar]
- Regni, C., Naught, L.E., Tipton, P.A., and Beamer, L.J. 2004. Structural basis of diverse substrate recognition by the enzyme PMM/PGM from P. aeruginosa. Structure 12 55–63. [DOI] [PubMed] [Google Scholar]
- Rocchetta, H.L., Burrows, L.L., and Lam, J.S. 1999. Genetics of O-antigen biosynthesis in Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 63 523–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar, S., Ye, R.W., Schlictman, D., and Chakrabarty, A.M. 1995. Exopolysaccharide alginate synthesis in Pseudomonas aeruginosa: Enzymology and regulation of gene expression. Adv. Enzymol. Relat. Areas Mol. Biol. 70 221–255. [DOI] [PubMed] [Google Scholar]
- Tatusova, T.A. and Madden, T.L. 1999. BLAST 2 Sequences, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol. Lett. 174 247–250. [DOI] [PubMed] [Google Scholar]
- Tavares, I.M., Jolly, L., Pompeo, F., Leitao, J.H., Fialho, A.M., Sa-Correia, I., and Mengin-Lecreulx, D. 2000. Identification of the Pseudomonas aeruginosa glmM gene, encoding phosphoglucosamine mutase. J. Bacteriol. 182 4453–4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, J.D., Higgins, D.G., and Gibson, T.J. 1994. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse, D.B., Tomkins, J., Lovegrove, J.U., Hopkinson, D.A., and McMillan, W.O. 1998. A phylogenetic approach to the identification of phosphoglucomutase genes. Mol. Biol. Evol. 15 456–462. [DOI] [PubMed] [Google Scholar]
- Zhu, S., Huys, I., Dyason, K., Verdonck, F., and Tytgat, J. 2004. Evolutionary trace analysis of scorpion toxins specific for K-channels. Proteins 54 361–370. [DOI] [PubMed] [Google Scholar]