Abstract
Real-time reverse transcriptase polymerase chain reaction (RT-PCR) is becoming a widely used method to quantify cytokines from cells, tissues, or tissue biopsies. The method allows for the direct detection of PCR product during the exponential phase of the reaction, combining amplification and detection in a single step. Using TaqMan chemistry (Applied Biosystems, Foster City, CA) and the ABI Prism 7700 Sequence Detection System (Applied Biosystems), we validated a large panel of murine and human cytokines, as well as other factors playing a role in the immune system, such as chemokines and apoptotic markers. Although the method allows fast, sensitive, and accurate quantification, different control assays are necessary for the method to be reliable. By construction of complementary DNA (cDNA) plasmid clones, standard curves are generated that allow direct quantification of every unknown sample. Furthermore, the choice of a reliable housekeeping gene is very important. Finally, co-amplification of contaminating genomic DNA is avoided by designing sets of primers located in different exons or on intron–exon junctions. In conclusion, the real-time RT-PCR technique is very accurate and sensitive, allows high throughput, and can be performed on very small samples. The development of real-time RT-PCR has resulted in an exponential increase in its use over the last couple of years, and the method has undoubtedly become the standard for quantifying cytokine patterns, clarifying many functional properties of immune cells and their associated diseases.
Keywords: , real-time RT-PCR, TaqMan, cytokine
Many cellular functions are regulated by changes in gene expression. Thus, quantification of transcription levels of genes plays a central role in the understanding of gene function and of abnormal alterations in regulation that may result in a disease state. The innovation of the real-time polymerase chain reaction (PCR) technique played a crucial role in molecular medicine and clinical diagnostics. Examples are the quantitation of relative gene expression (as described herein), detection of minimal residual disease,1 cancer diagnostics,2 pathogen detection,3 and quantitation of viral load.4 Other applications include detection of genetically modified organisms in food samples,5 measurements of DNA or transgene copy number, and allelic discrimination.6
In the field of immunology, many studies have shown that chronic inflammatory disorders, autoimmune diseases, and transplant rejections are closely associated with specific changes in the balance between pro- and anti-inflammatory cytokines in the affected cells or tissues. Quantification of the cytokines involved is essential for gaining more insight into the immune processes involved. Because tissue samples available for analysis are often too small to allow quantification of cytokines at the protein level, analysis of messenger RNA (mRNA) is widely used to investigate the cytokine levels at sites of immune infiltration or inflammation. In interpreting the results, however, one should always take into account that a discrepancy may exist between mRNA and protein levels. Although post-transcriptional or post-translational modifications may play a role in the target genes of interest, different publications have described a good correlation between cytokine mRNA levels quantified by real-time PCR and protein levels quantified by enzyme-linked immunosorbent assay.7, 8
Real-time quantitative reverse transcriptase PCR (RT-PCR), which is the latest innovation in the field of PCR technology, provides a sensitive, reproducible, and accurate method for determining mRNA cytokine levels in tissues or cells. The method is based on the detection of a fluorescent signal produced and monitored during the amplification process, without the need for post-PCR processing.9 The method has been available for more than 5 years and has seen an exponential increase in use over the last 2 years.
PRINCIPLE OF REAL-TIME RT-PCR
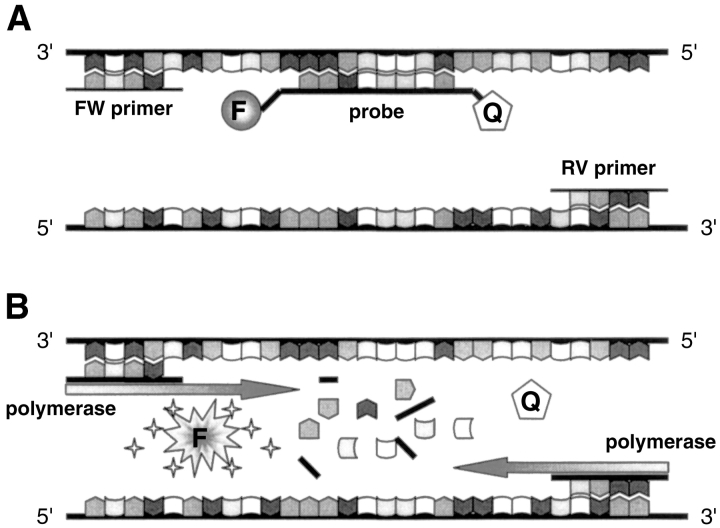
The discovery of the real-time PCR technique as it is used today was made possible by two important findings. First, the Taq polymerase has, apart from its polymerase activity, a 5′–3′ exonuclease activity.10 Second, dual-labeled fluorogenic oligonucleotide probes have been created which emit a fluorescent signal only upon cleavage, based on the principle of fluorescence resonance energy transfer.11 In the TaqMan assay (Applied Biosystems, Foster City, CA), which was the first real-time PCR assay developed, these two principles are combined. In this system a probe, the so-called TaqMan probe, is designed to anneal to the target sequence between the classical forward and reverse primers (Fig. 1 ). The probe is dually labeled, with a reporter fluorochrome (e.g., 6-carboxyfluorescein, or FAM) at one end and a quencher dye (e.g., 6-carboxy-tetramethyl-rhodamine, or TAMRA) at the 3′ end. Importantly, in its intact form, the fluorescence emission of the reporter dye will be absorbed by the quencher dye. The probe has a melting temperature (m) approximately 10°C higher than the m of the primers, in order to anneal to the amplicon during the extension phase of the PCR process (which is performed at 60°C). Consequently, the probe will be degraded during the extension phase by the 5′–3′ exonuclease activity of the Taq polymerase. This will result in an increase in reporter fluorescence emission because reporter and quencher are separated. The amount of fluorescence released is directly proportional to the amount of product generated in each PCR cycle and thus can be applied as a quantitative measure of PCR product formation.
FIGURE 1.
Schematic representation of the TaqMan principle. A: Primers and probe anneal to the target gene. Fluorescence emission does not occur because the probe is still intact. B: During the extension phase of the PCR reaction, the probe is cleaved by the 5′–3′ exonuclease activity of the Taq polymerase, allowing fluorescence emission. FW, forward; RV, reverse; F, fluorophore; Q, quencher dye.
As the technology has become more commonly used, other sophisticated chemistries have been developed to directly measure PCR product accumulation by fluorescence emission. Examples include molecular beacons, Scorpions, hybridization probes, and minor groove binder (MGB) probes (e.g., Eclipse, TaqMan MGB). Other new technologies include ResonSense probes, light-up probes, and Hy-Beacon probes.12, 13 Finally, the use of double-stranded DNA minor groove binding dyes, such as SYBR Green I, is a cheaper, widely used alternative, where the need for an expensive probe can be avoided.
One can choose among a diversity of competing instrumentations that have recently been launched on the market.12, 13 All of them run the PCR reaction as a closed tube and measure product accumulation in real time during the course of PCR amplification. Differences between the instrumentations are the sample format (tubes, microplates, strip tubes, capillaries, etc.), the maximum sample number (ranging from 16 to 384), the length of a run (ranging from 30 min to 2 h), the light source (halogen or laser), the fluorescence wavelength detection, the possibility of performing single or multiplex (i.e., measuring different fluorescence emissions simultaneously) PCR reactions, the availability of melting curve analysis, and finally the price. Some of the instruments are designed primarily for high-throughput applications, whereas others allow more flexibility. Therefore, the choice for an instrument will depend on the specific applications to be performed.
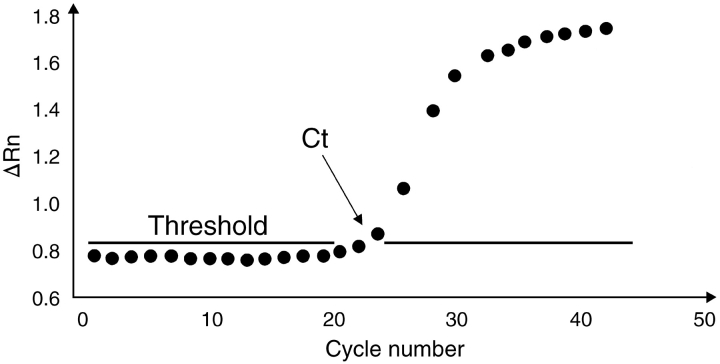
With any of the developed chemistries on any of the developed instrumentations, a software package is provided that measures the increase in fluorescence emission in real time, during the course of the reaction. This increase in fluorescence emission is directly related to the increase in target amplification. In the ABI Prism 7700 Sequence Detection System (SDS) (Applied Biosystems), for example, the software calculates a ▵Rn using the equation ▵Rn = Rn+ − Rn−, with Rn+ being the fluorescence emission of the product at each time point and Rn− being the fluorescence emission of the baseline.9 The ▵Rn values are plotted against cycle number, resulting in amplification plots for each sample (Fig. 2 ). Threshold values (Ct) are then determined as the cycle number at which the fluorescence emission (▵Rn) exceeds a chosen threshold, which is usually 10 times the standard deviation of the baseline (this threshold level can, however, be changed manually if desired). Software will plot Ct values of unknown samples on the standard curve to determine the starting copy numbers of the unknown samples. Alternatively, the Ct values can be used as a direct quantitative measurement.
FIGURE 2.
PCR amplification plot. Fluorescence emission is measured continuously during the PCR reaction and ▵Rn (increase in fluorescence emission, from which the background fluorescence signal is subtracted) is plotted against cycle number. The threshold cycle (Ct) is the cycle at which the fluorescence exceeds a chosen threshold.
VALIDATION OF THE REAL-TIME PCR SYSTEM FOR CYTOKINE ANALYSIS
Because the research in our laboratory mainly focuses on the pathophysiology of autoimmune diseases such as type 1 diabetes,14– 16 we were very interested in obtaining a high-throughput and reliable system for quantifying cytokine levels in immune cells, particularly in immune and invaded tissues. Therefore, we began optimizing a real-time RT-PCR system for a panel of murine cytokines about 5 years ago. In that time, the list of murine cytokines and other factors playing a role in the immune system that we validated using the real-time RT PCR system has grown continuously, we have recently expanded our studies to the human system.
We use the ABI Prism 7700 SDS in combination with TaqMan chemistry, using a specific set of primers and an internal fluorogenic probe for each target of interest. In designing these primer sets, special care was taken to avoid co-amplification of contaminating genomic DNA. With the objective of requiring an absolute quantification of transcription, we constructed cDNA plasmid standards for each target gene of interest.
RNA EXTRACTION AND cDNA SYNTHESIS
Total RNA has been extracted from many different tissues or cells. Isolated tissues are either used immediately or snap frozen in liquid nitrogen and stored at −80°C until use. Two different methods for total RNA extraction from tissues (such as spleen, lymph node, heart, brain, spinal cord, intestine, thymus, lung, pancreas) have been tested and used: TRIzol reagent (Life Technologies, Gaithersburg, MD) and, more recently, the SV Total RNA Isolation System from Promega (Madison, WI). Although extracting total RNA using the SV Total RNA Extraction Kit is somewhat more complicated and takes longer, it results in highly pure, DNA-free RNA, because the protocol includes a DNAse treatment. Moreover, the components of the kit are less toxic to the investigator. For pancreatic tissue, the TRIzol method does not give reliable results, probably because of the high endogenous RNAse concentrations in this tissue.
For extraction of total RNA from small amounts (up to 106 cells) of cultured cells, peritoneal murine macrophages, human peripheral blood mononuclear cells, and pancreatic β-cells the High Pure RNA Isolation Kit from Roche Diagnostics (Indianapolis, IN) is used. This kit combines RNA extraction and DNAse treatment in a spin column system. For extraction of total RNA from a larger amount of cells, both the RNeasy Mini Kit (Qiagen, Chatsworth, CA) (up to 107 cells) and the TRIzol Liquid Suspension reagent (Life Technologies) are used. Both methods result in pure RNA, although no DNAse treatment is included. The concentration of purified total RNA is determined spectrophotometrically at 260 nm.
Target RNA is reverse transcribed using the Superscript II reverse transcriptase enzyme (Life Technologies). In a first step, 5 μM Oligo(dT)16 is added to 0.5–1 μg of total RNA and annealed at 70°C for 10 min. Then, 100 U Superscript II reverse transcriptase is added in the presence of 50 mM Tris-HCl, pH 8.3, 75 mM KCl, 3 mM MgCl2, and 5 mM unlabeled deoxynucleotides (dNTPs) and incubated at 42°C for 80 min. For each experiment, RT-minus controls (i.e., RNA samples that are treated similarly but without addition of Superscript II RT enzyme) are included to provide a negative control for subsequent PCR reactions. For PCR amplification, a maximum of 1 μL of cDNA is used per 50 μL PCR. Larger amounts are avoided because they may significantly inhibit PCR amplification. To minimize variations in reverse transcriptase efficiency, all samples from a single experiment are reverse transcribed simultaneously.
PRIMER AND PROBE DESIGN
With the ABI Prism 7700 SDS instrumentation, Primer Express software is provided to design the primers and fluorogenic probes. The primary object of this program is to design sets of primers and internal probes that can be run under universal thermal cycling conditions (15 s at 94°C and 1 min at 60°C). Thus, the default parameters of the software are set to be very narrow. Most important are the melting temperature (m) of the primers and probe, and the amplicon length. The m of the primers should be 58–60°C, whereas the m of the probe must be at least 10°C higher (thus approximately 68–70°C), in order to anneal to the target during the extension phase of the PCR reaction. For amplicon lengths the rule is “the shorter the more efficient”; default parameters for amplicon lengths are set between 50 and 150 bp.
To avoid co-amplification of contaminating genomic DNA, primers are (where possible) designed on different exons or intron–exon boundaries. With the Primer Express software, these primer sets must be searched manually.
For specific applications where no primer sets can be found using these stringent parameters, the parameters can, of course, be changed manually. In this case, the first parameter we change is amplicon length; in our experience, amplicon lengths up to 250 bp have been found to amplify efficiently.
At present, large panels of murine cytokines (Table 1 ), murine chemokines and other immune related factors (Table 2 ), human cytokines (Table 3 ), and apoptotic markers (Table 4 ) have been validated. In each table the possible co-amplification of genomic DNA is indicated (last column).
TABLE 1.
Primer and Probe Sequences for Murine Cytokinesa
| Sequence (5′–3′) | Length (bp)b | Accessionc | gDNAd | ||
| IL-1-β | FW | CAACCAACAAGTGATATTCTCCATG | 152 | M15131 | − |
| RV | GATCCACACTCTCCAGCTGCA | X04964 | |||
| TP | CTGTGTAATGAAAGACGGCACACCCACC | ||||
| IL-2 | FW | CCTGAGCAGGATGGAGAATTACA | 141 | X01772 | − |
| RV | TCCAGAACATGCCGCAGAG | M16760 | |||
| TP | CCCAAGCAGGCCACAGAATTGAAAG | AF195956 | |||
| IL-4 | FW | ACAGGAGAAGGGACGCCAT | 95 | M25892 | − |
| RV | GAAGCCCTACAGACGAGCTCA | X05253 | |||
| TP | TCCTCACAGCAACGAAGAACACCACA | ||||
| IL-5 | FW | AGCACAGTGGTGAAAGAGACCTT | 117 | X06270 | − |
| RV | TCCAATGCATAGCTGGTGATTT | X06271 | |||
| TP | CTGTTGACAAGCAATGAGACGATGAGG | ||||
| IL-6 | FW | GAGGATACCACTCCCAACAGACC | 141 | X54542 | − |
| RV | AAGTGCATCATCGTTGTTCATACA | M20572 | |||
| TP | CAGAATTGCCATTGCACAACTCTTTTCTCA | ||||
| IL-7 | FW | ATTATGGGTGGTGAGAGCCG | 257 | X07962 | − |
| RV | GTTCATTATTCGGGCAATTACTATCA | M29054 | |||
| TP | CCTCCCGCAGACCATGTTCCATGT | ||||
| IL-10 | FW | GGTTGCCAAGCCTTATCGGA | 191 | M37897 | − |
| RV | ACCTGCTCCACTGCCTTGCT | M84340 | |||
| TP | TGAGGCGCTGTCATCGATTTCTCCC | ||||
| IL-12 p40 | FW | GGAAGCACGGCAGCAGAATA | 180 | M86671 | − |
| RV | AACTTGAGGGAGAAGTAGGAATGG | S82420-6 | |||
| TP | CATCATCAAACCAGACCCGCCCAA | ||||
| IL-13 | FW | GGAGCTGAGCAACATCACACA | 142 | M23504 | − |
| RV | GGTCCTGTAGATGGCATTGCA | L13028 | |||
| TP | CGGGTTCTGTGTAGCCCTGGATTCC | ||||
| IL-15 | FW | CATCCATCTCGTGCTACTTGTGTT | 126 | U14332 | − |
| RV | CATCTATCCAGTTGGCCTCTGT | AB006745 | |||
| TP | AGGGAGACCTACACTGACACAGCCCAAAA | ||||
| IL-17 | FW | GCTCCAGAAGGCCCTCAGA | 142 | NM_010552 | − |
| RV | AGCTTTCCCTCCGCATTGA | U35108 | |||
| TP | CTCTCCACCGCAATGAAGACCCTGA | ||||
| IL-18 | FW | CAGGCCTGACATCTTCTGCAA | 105 | NM_008360 | − |
| RV | TCTGACATGGCAGCCATTGT | AJ002364 | |||
| TP | CTCCAGCATCAGGACAAAGAAAGCCG | ||||
| IFN-γ | FW | TCAAGTGGCATAGATGTGGAAGAA | 92 | K00083 | − |
| RV | TGGCTCTGCAGGATTTTCATG | M74466 | |||
| TP | TCACCATCCTTTTGCCAGTTCCTCCAG | M28381 | |||
| TNF-α | FW | CATCTTCTCAAAATTCGAGTGACAA | 175 | M13049 | − |
| RV | TGGGAGTAGACAAGGTACAACCC | Y00467 | |||
| TP | CACGTCGTAGCAAACCACCAAGTGGA | ||||
| TGF-β1 | FW | TGACGTCACTGGAGTTGTACGG | 170 | M13177 | − |
| RV | GGTTCATGTCATGGATGGTGC | L42460 | |||
| TP | TTCAGCGCTCACTGCTCTTGTGACAG | L42459 | |||
| MIC-1 | FW | CCAACCAGAGCCGAGAGGA | 113 | NM_011819 | − |
| RV | GTTGACGCGGAGTAGCAGCT | AJ011967-8 | |||
| TP | CCGGATACTCAGTCCAGAGGTGAGATTGG | ||||
| GM-CSF | FW | GCCATCAAAGAAGCCCTGAA | 114 | X02333 | − |
| RV | GCGGGTCTGCACACATGTTA | X03020 | |||
| TP | ACATGCCTGTCACATTGAATGAAGAGGTAGAAG |
FW, forward primer; RV, reverse primer; TP, TaqMan probe dual-labeled with 5′FAM and 3′TAMRA; IL, interleukin; IFN-γ, interferon-gamma; TNFα, tumor necrosis factor alpha; TGF-β, transforming growth factor-beta; iNOS, inducible nitric oxide synthetase; MIC-1, macrophage inhibiting cytokine-1; GM-CSF, granulocyte–macrophage colony-stimulating factor.
aAdapted from Giulietti et al.12 with permission from the publisher (Elsevier Science USA, New York, NY).
bAmplicon length in base pairs.
cGenbank accession number of cDNA and corresponding gene available online at http://www.ncbi.nlm.nih.gov/
dPCR amplification on genomic DNA.
TABLE 2.
Primer and Probe Sequences for Murine Chemokines and Other Immune-Related Factorsa
| Sequence (5′–3′) | Length (bp)b | Accessionc | gDNAd | ||
| MCP-1 | FW | CTTCTGGGCCTGCTGTTCA | 126 | L13763 | − |
| RV | CCAGCCTACTCATTGGGATCA | U12470 | |||
| TP | CTCAGCCAGATGCAGTTAACGCCCC | ||||
| Fractalkine | FW | GGGTGGCCATGTTTGCTTAC | 140 | U92565 | + |
| RV | CAGGCAAGCAGCTCACACTG | ||||
| TP | TCCCCCGTAGCTGTGGCAGTAACTCAT | ||||
| MIP3α | FW | CCAGGCAGAAGCAAGCAACT | 96 | AJ222694 | + |
| RV | TCGGCCATCTGTCTTGTGAA | AB015137 | |||
| TP | TGTTGCCTCTCGTACATACAGACGCCA | ||||
| IP10 | FW | GCCGTCATTTTCTGCCTCAT | 127 | AF227743 | − |
| RV | GCTTCCCTATGGCCCTCATT | M33266 | |||
| TP | TCTCGCAAGGACGGTCCGCTG | L07417 | |||
| CD40 | FW | GTCATCTGTGGTTTAAAGTCCCG | 91 | M83312 | + |
| RV | AGAGAAACACCCCGAAAATGG | M94129 | |||
| TP | AGCCCTGCTGGTCATTCCTGTCGTG | ||||
| CD40 ligand | FW | CTCAAACTCTGAACAGTGCGCT | 88 | X65453 | + |
| RV | GGCAGGTCCTAACTGACTTGCT | ||||
| TPd | AGGGAAGACTGCCAGCATCAGCCCT | ||||
| iNOS | FW | CAGCTGGGCTGTACAAACCTT | 95 | U43428 | − |
| RV | CATTGGAAGTGAAGCGTTTCG | L23806 | |||
| TP | CGGGCAGCCTGTGAGACCTTTGA | ||||
| PAF-AH | FW | CCTGCAAGCTGGAATTCTCC | 123 | U34277 | − |
| RV | CCCATTAGATGCCAAGCCAA | ||||
| TP | TTCTCATGGTCTCGGAGCCTTCAGGAC | ||||
| PAF-R | FW | CAACGAGGGCGACTGGATT | 97 | D50872 | + |
| RV | GACACCCAAAAAGGCCACACT | ||||
| TP | TCCTGTGCAACGTGGCTGGCTG | ||||
| IL1Ra | FW | CTGGGAAAAGACCCTGCAAG | 91 | NM_031167 | − |
| RV | CCAGCAATGAGCTGGTTGTTT | L32833 | |||
| TP | TGCAAGCCTTCAGAATCTGGGATACTAACCA | ||||
| PGS2 | FW | TGGTGCCTGGTCTGATGATG | 159 | M64291 | − |
| RV | GTGGTAACCGCTCAGGTGTTG | D28235 | |||
| TP | CCACCATCTGGCTTCGGGAGCA | ||||
| ICAM 1 | FW | CCGCAGGTCCAATTCACACT | 143 | X52264 | − |
| RV | TCCAGCCGAGGACCATACAG | X15372 | |||
| TP | CAGCTCGGAGGATCACAAACGAAGCT | M90546 | |||
| TNF-Rp55 | FW | GCTGACCCTCTGCTCTACGAA | 132 | X57796 | + |
| RV | GCCATCCACCACAGCATACA | M88067 | |||
| TP | CTGTTCAGAAATGGGAAGACTCCGCCC | M76655 |
FW, forward primer; RV, reverse primer; TP, TaqMan probe dual-labeled with 5′FAM and 3′TAMRA; MCP-1, monocyte chemoattractant protein 1; MIP3α, macrophage inflammatory protein 3 alpha; IP10, interferon gamma inducible protein; iNOS, inducible nitric oxide synthetase; PAF-AH, platelet activating factor acetyl hydrolase; PAF-R, platelet activating factor receptor; IL1-Ra, interleukin 1 receptor antagonist; PGS2, prostaglandin synthetase 2; ICAM 1, intercellular adhesion molecule 1; TNF-Rp55, TNF-receptor.
aAdapted from Giulietti et al.12 with permission from the publisher (Elsevier Science USA, New York, NY).
bAmplicon length in base pairs.
cGenbank accession number of cDNA and corresponding gene available online at http://www.ncbi.nlm.nih.gov/
dPCR amplification on genomic DNA.
TABLE 3.
Primer and Probe Sequences for Human Cytokinesa
| Sequence (5′–3′) | Length (bp)b | Accessionc | gDNAd | ||
| IL-1α | FW | CGCCAATGACTCAGAGGAAGA | 120 | X02531 | − |
| RV | AGGGCGTCATTCAGGATGAA | X03833 | |||
| TP | AGCACCTTTTAGCTTCCTGAGCAATGTGAAA | ||||
| IL-2 | FW | AACTCACCAGGATGCTCACATTTA | 148 | NM_000586 | − |
| RV | TCCCTGGGTCTTAAGTGAAAGTTT | J00264 | |||
| TP | TTTTACATGCCCAAGAAGGCCACAGAACT | ||||
| IL-4 | FW | CCACGGACACAAGTGCGATA | 149 | M13982 | − |
| RV | CCCTGCAGAAGGTTTCCTTCT | M23442 | |||
| TP | TCTGTGCACCGAGTTGACCGTAACAGAC | ||||
| IL-10 | FW | GTGATGCCCCAAGCTGAGA | 138 | AF043333 | − |
| RV | CACGGCCTTGCTCTTGTTTT | U16720 | |||
| TP | CCAAGACCCAGACATCAAGGCGCA | ||||
| IL-12p40 | FW | TGGAGTGCCAGGAGGACAGT | 147 | AF180563 | − |
| RV | TCTTGGGTGGGTCAGGTTTG | AY008847 | |||
| TP | ATGGTGGATGCCGTTCACAAGCTCAA | ||||
| IL-15 | FW | GGAGGCATCGTGGATGGAT | 143 | NM_000585 | − |
| RV | AACACAAGTAGCACTGGATGGAAA | X91233 | |||
| TP | CTGCTGGAAACCCCTTGCCATAGCC | ||||
| IFN-γ | FW | TCAGCTCTGCATCGTTTTGG | 120 | X01992 | − |
| RV | GTTCCATTATCCGCTACATCTGAA | J00219 | |||
| TP | TTGGCTGTTACTGCCAGGACCCATATGT | ||||
| TGF-β | FW | CAGCAACAATTCCTGGCGATA | 136 | NM_000660 | − |
| RV | AAGGCGAAAGCCCTCAATTT | Y00112 | |||
| TP | CTGCTGGCACCCAGCGACTCG | ||||
| TNF-α | FW | TCTTCTCGAACCCCGAGTGA | 151 | M10988 | − |
| RV | CCTCTGATGGCACCACCAG | X02910 | |||
| TP | TAGCCCATGTTGTAGCAAACCCTCAAGCT | X02159 |
FW, forward primer; RV, reverse primer; TP, TaqMan probe dual-labeled with 5′FAM and 3′TAMRA; IL, interleukin; IFN-γ, interferon-gamma; TGF-β, transforming growth factor-beta; TNF-α, tumor necrosis factor-alpha.
aAdapted from Giulietti et al.12 with permission from the publisher (Elsevier Science USA, New York, NY).
bAmplicon length in base pairs.
cGenbank accession number of cDNA and corresponding gene available online at http://www.ncbi.nlm.nih.gov/
dPCR amplification on genomic DNA.
TABLE 4.
Primer and Probe Sequences for Murine Apoptosis Related Factors
| Sequence (5′–3′) | Length (bp)a | Accessionb | gDNAc | ||
| Fas | FW | CTGCGATGAAGAGCATGGTTT | 208 | M83649 | − |
| RV | CCATAGGCGATTTCTGGGAC | ||||
| TP | TGCGATTCTCCTGGCTGTGAACACTG | ||||
| Fas-ligand | FW | AAGAAGGACCACAACACAAATCTG | 234 | U58995 | − |
| RV | CCCTGTTAAATGGGCCACACT | ||||
| TP | TGCAGAAGGAACTGGCAGAACTCCG | ||||
| Bcl-2β | FW | CTTAGAAAATACAGCATTGCGGAG | 194 | M16506 | + |
| RV | GGATGTGCTTTGCATTCTTGG | ||||
| TP | TTCCTGCATCTCATGCCAACGGG | ||||
| Bcl-xL | FW | CACTGTGCGTGGAAAGCGTA | 127 | U51278 | + |
| RV | AAAGTGTCCCAGCCGCC | U78030-1 | |||
| TP | CAAGGAGATGCAGGTATTGGTGAGTCGG | ||||
| Bax-α | FW | GTTTCATCCAGGATCGAGCAG | 238 | L22472 | + |
| RV | CCCCAGTTGAAGTTGCCATC | ||||
| TP | AGCTGAGCGAGTGTCTCCGGCG | ||||
| Bcl-6 | FW | ATGTACAGCCATCTCCCGCT | 134 | U41465 | + |
| RV | TTAGGGACTTGCCTGGCACT | ||||
| TP | AATGCCTGTGGCCAACCCTTTTCC | ||||
| Bid | FW | TGGCAGTGCTTGGAGCTACA | 140 | BC002031 | − |
| RV | CCTCCAGCTCTTGGCGAGTA | AC006404 | |||
| TP | TGAGGTCAGCAACGGTTCCGGC | ||||
| c-FLIP | FW | GCAACCCAGACACTGCACAA | 148 | U97076 | + |
| RV | CGTCTCCTGCCTTGCTTCAG | ||||
| TP | AGAAGCCCTCCAGCTCATCCTCTGTGT | ||||
| c-jun | FW | CCTGTCCCCTATCGACATGG | 93 | J04115 | + |
| RV | CTTTTCCGGCACTTGGAGG | X12740 | |||
| TP | TCCTCATGCGCTTCCTCTCTGCCT | ||||
| c-myc | FW | TGAGCCCCTAGTGCTGCAT | 137 | AF076523 | − |
| RV | AGCCCGACTCCGACCTCTT | L00038-9 | |||
| TP | CTTCTTGCTCTTCTTCAGAGTCGCTGCTG | ||||
| c-fos | FW | CTCCTTCTCCAGCATGGGC | 81 | − | |
| RV | GGGATAAAGTTGGCACTAGAGACG | J00370 | |||
| TP | TCAACACACAGGACTTTTGCGCAGATCT |
FW, forward primer; RV, reverse primer; TP, TaqMan probe dual-labeled with 5′FAM and 3′TAMRA; FLIP, Flice inhibitory protein.
aAmplicon length in base pairs.
bGenbank accession number of cDNA and corresponding gene available online at http://www.ncbi.nlm.nih.gov/
cPCR amplification on genomic DNA.
PCR AMPLIFICATION
PCR amplifications are performed on the ABI Prism 7700 SDS, using 96-well microtiter plates. They are performed in a total volume of 25 μL, containing 0.5 μL cDNA sample, 1× buffer A (50 mM KCl, 10 mM Tris-HCl, pH 8.3, 10 mM EDTA, 60 nM Passive Reference 1), 200 μM dNTPs, 3–9 mM MgCl2, 100–900 nM of each primer, 0.625 U AmpliTaqGold (Applied Biosystems), and 100 nM TaqMan probe (Eurogentec, Liege, Belgium). For each target gene, different MgCl2 (3–9 mM) and primer (100, 300, and 900 nM) concentrations are tested to optimize the PCR amplification, and all other components are maintained constant. PCR amplifications are always performed in duplicate or triplicate wells, using the universal temperature cycles: 10 min at 94°C, followed by 35–45 two-temperature cycles (15 s at 94°C and 1 min at 60°C).
The PCR reactions performed on the ABI Prism 7700 SDS have an absolute requirement for a reference dye (ROX, which is included in buffer A). Initially, a buffer containing this reference dye could only be purchased from Applied Biosystems as part of a complete kit, resulting in high expenses. Recently, however, the necessary buffer has become available in different formulations or can be purchased from other companies. Furthermore, competing companies are also providing dual-labeled fluorogenic probes. Wider availability of reagents should result in lower costs for performing real-time PCR amplifications.
QUANTIFICATION
To quantify the results obtained by real-time RT-PCR, we use the standard curve method. With this method, quantification results in absolute copy numbers per cell, total RNA concentration, or total tissue. For constructing a standard curve, either RNA or DNA can be used. In our laboratory, we routinely use cDNA plasmid standards. The advantage of using cDNA plasmids is that, once they are constructed, by cloning the target PCR fragment into a suitable plasmid vector, they can be easily prepared in very large amounts. Therefore, numerous experiments can be performed using the same dilutions of one standard, minimizing interassay variation.
To construct cDNA plasmid standards, total RNA is extracted from a tissue that abundantly expresses the target gene. The target gene of interest is amplified by classical qualitative RT-PCR, using the same primers as for real-time RT-PCR. The amplified target is purified on silica columns (e.g., the QIAquick PCR purification kit; Qiagen), ligated into the pGEM-Teasy plasmid (Promega), and transformed into DH5α-competent cells (Life Technologies). Finally, plasmid DNA is extracted using silica cartridges (Nucleobond AX plasmid purification; Macherey-Nagel, Düren, Germany), and cDNA plasmid concentrations are measured spectrophotometrically (Pharmacia, Uppsala, Sweden). The corresponding copy number is calculated using the equation 1 μg of 1000-bp DNA × 9.1 × 1011 molecules.
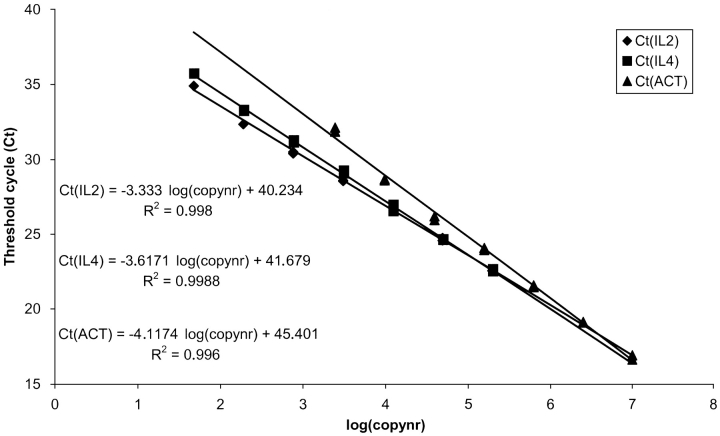
As an alternative to the standard curve method, the comparative Ct method is often used.17, 18 An advantage using this method is that no standards need to be constructed, and all 96 wells can be applied for unknown samples (except for the calibrator samples). A disadvantage, however, is that the efficiencies of target and housekeeping gene must be identical. If not, the results will not be reliable. As shown in Figure 3 , this is not always the case. The differences in slope and -intercept for the cytokine standard curves compared with the actin standard curve indicate a difference in amplification efficiency and sensitivity, respectively.
FIGURE 3.
Standard curves for IL2, IL4, and β-actin (ACT). Threshold cycle values are plotted against input cDNA copy number. The differences in slope and y-intercept for the cytokine standard curves compared with the actin standard curve indicate a difference in amplification efficiency and sensitivity, respectively. If the amplification efficiencies were identical, the three standard curves should have the same slope and should be superimposed.
NORMALIZATION
In each quantitative RT-PCR method, specific errors will be introduced due to minor differences in starting amount of RNA or differences in efficiency of cDNA synthesis and PCR amplification. Therefore, a reliable quantitative RT-PCR method requires correction for these experimental variations. At present this is most often performed by normalization to a housekeeping gene. Finding a suitable housekeeping gene (i.e., a gene that is constant during the experimental conditions) is, however, not always straightforward. Therefore, different housekeeping genes should be tested in each experimental setup to find the most suitable one (not influenced by the experimental treatment). In the search for the “ideal” reference gene, a kit (Applied Biosystems) can be purchased with a range of housekeeping genes, to test which one is the most suitable in a particular setup.
Many recent articles have discussed the problem of housekeeping genes, and it turns out that the housekeeping genes most commonly used are subject to variation in numerous experimental conditions, bringing into question the reliability of the results obtained. In particular, the use of GAPDH and of β-actin has been severely criticized.19– 21 Ribosomal RNA (rRNA) may be a more valuable alternative, because its expression is less likely to vary under conditions that change the levels of mRNA expression. Drawbacks of using rRNA, however, are that it cannot be used for quantification of samples when starting from mRNA, or when oligo d(T)16 is used for cDNA synthesis. Another drawback is its very abundant expression relative to mRNA expression levels of target genes.
Other alternative genes have been proposed for normalization in specified conditions. For instance, for expression profiling of T helper cell differentiation,22 the search for a suitable housekeeping gene was performed using a microarray approach. Although this method may find a reliable reference gene, it is a rather sophisticated approach and cannot be performed in every laboratory. Moreover, the search must be repeated for each individual experimental setup.
An alternative to normalization by a housekeeping gene could be normalization to an irrelevant exogenously added reference gene.23 In this case, an unrelated purified RNA is added to the tissue or cells before RNA extraction. Another method that has been proposed is to normalize to the input cell number or total RNA concentration (when working with tissues).19 Because none of the methods used today is completely satisfactory, the search for a more universal method in normalizing the results continues.
CONCLUDING REMARKS
Real-time PCR, as described in this review, is a revolutionary technique and is becoming the standard method for quantifying cytokine mRNA levels from organs, cells, or cell cultures. Compared with previously used endpoint PCR assays, the technique is very fast, accurate, and sensitive, and it has a decreased potential for PCR contamination. Evidently, the assay described herein for quantifying cytokine gene expression can be easily extrapolated to other classes of mRNA. Overall, the technique has enabled scientists to gain a better insight into many immunological mechanisms and diseases in a fast and relatively automated way.
Acknowledgments
This work was supported by grants from the Flemish Research Foundation (Fonds voor Wetenschappelijk Onderzoek [FWO]) and by a postdoctoral FWO fellowship for C.M.
REFERENCES
- 1.Li A, Forestier E, Rosenquist R, Roos G. Minimal residual disease quantification in childhood acute lymphoblastic leukemia by real-time polymerase chain reaction using the SYBR Green dye. Exp Hematol 2002;30:1170–1177. [DOI] [PubMed] [Google Scholar]
- 2.Bernard PS, Wittwer CT. Real-time PCR technology for cancer diagnostics. Clin Chem 2002;48:1178–1185. [PubMed] [Google Scholar]
- 3.Norton DM. Polymerase chain reaction-based methods for detection of Listeria monocytogenes: toward real-time screening for food and environmental samples. J AOAC Int 2002;85:505–515. [PubMed] [Google Scholar]
- 4.Niesters HG. Quantitation of viral load using real-time amplification techniques. Methods 2001;25:419–429. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed FE. Detection of genetically modified organisms in foods. Trends Biotechnol 2002;20:215–223. [DOI] [PubMed] [Google Scholar]
- 6.Sevall JS. Rapid allelic discrimination from real-time DNA amplification. Methods 2001;25:452–455. [DOI] [PubMed] [Google Scholar]
- 7.Hein J, Schellenberg U, Bein G, Hackstein H. Quantification of murine IFN-γ mRNA and protein expression: impact of real-time kinetic RT-PCR using SYBR Green I dye. Scand J Immunol 2001;54:285–291. [DOI] [PubMed] [Google Scholar]
- 8.Blaschke V, Reich K, Blaschke S, Zipprich S, Neumaan C. Rapid quantitation of proinflammatory and chemoattractant cytokine expression in small tissue samples and monocyte-derived dendritic cells: validation of a new real-time RT-PCR technology. J Immunol Methods 2000;246:79–90. [DOI] [PubMed] [Google Scholar]
- 9.Heid CA, Stevens J, Livak JK, Williams PM. Real time quantitative PCR. Genome Res 1996;6:986–994. [DOI] [PubMed] [Google Scholar]
- 10.Holland PM, Abramson RD, Watson R, Gelfand DH. Detection of specific polymerase chain reaction product by utilizing the 5′→3′ exonuclease activity of Theramus aquaticus DNA polymerase. Proc Natl Acad Sci 1991;88:7276–7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardullo RA, Agrawal S, Flores C, Zamecnick PC, Wolf DE. Detection of nucleic acid hybridization by nonradiative fluorescence resonance energy transfer. Proc Natl Acad Sci 1988;85:8790–8794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods 2001;25:386–401. [DOI] [PubMed] [Google Scholar]
- 13.Eurogentec. Quantitative and Qualitative PCR Technology [review and catalog]. 2002. Available online at www.eurogentec.com.
- 14.Mathieu C, Laureys J, Sobis H, Vandeputte M, Waer M, Bouillon R. 1,25-Dihydroxyvitamin D3 prevents insulitis in NOD mice. Diabetes 1992;41:1491–1495. [DOI] [PubMed] [Google Scholar]
- 15.Casteels KM, Mathieu C, Waer M, et al. Prevention of type 1 diabetes in NOD mice by late intervention with nonhypercalcemic analogs of 1,25-dihydroxyvitamin D3 in combination with a short induction course of cyclosporin A. Endocrinology 1998;139:95–102. [DOI] [PubMed] [Google Scholar]
- 16.Overbergh L, Decallonne B, Waer M, et al. 1α,25-Dihydroxyvitamin D3 induces an autoantigen-specific T-helper 1/T-helper 2 immune shift in NOD mice immunized with GAD65 (p524–543). Diabetes 2000;49:1301–1307. [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−▵▵ Ct method. Methods 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 18.Lehman U, Kreipe H. Real-time PCR analysis of DNA and RNA extracted from formalin-fixed and paraffin-embedded biopsies. Methods 2001;25:409–418. [DOI] [PubMed] [Google Scholar]
- 19.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 2000;25:169–193. [DOI] [PubMed] [Google Scholar]
- 20.Schmittgen TD, Zakrajsek BA. Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J Biochem Biophys Methods 2000;46:69–81. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki T, Higgins PJ, Crawford DR. Control selection for RNA quantitation. Biotechniques 2000;29:332–337. [DOI] [PubMed] [Google Scholar]
- 22.Hamalainen HK, Tubman JC, Vikman S, et al. Identification and validation of endogenous reference genes for expression profiling of T helper cell differentiation by quantitative real-time RT-PCR. Anal Biochem 2001; 299:63–70. [DOI] [PubMed] [Google Scholar]
- 23.Shibata M, Hariya T, Hatao M, Ashikaga T, Ichikawa H. Quantitative polymerase chain reaction using an external control mRNA for determination of gene expression in a heterogeneous cell population. Toxicol Sci 1999; 49:290–296. [DOI] [PubMed] [Google Scholar]