Abstract
Mice, homozygous for disrupted ganglioside GM2/GD2 synthase (EC 2.4.1.94) gene and lacking all complex gangliosides, do not display any major neurologic abnormalities. Further examination of these mutant mice, however, revealed that the males were sterile and aspermatogenic. In the seminiferous tubules of the mutant mice, a number of multinuclear giant cells and vacuolated Sertoli cells were observed. The levels of testosterone in the serum of these mice were very low, although testosterone production equaled that produced in wild-type mice. Testosterone was found to be accumulated in interstitial Leydig cells, and intratesticularly injected testosterone was poorly drained in seminiferous fluid in the mutant mice. These results suggested that complex gangliosides are essential in the transport of testosterone to the seminiferous tubules and bloodstream from Leydig cells. Our results provide insights into roles of gangliosides in vivo.
Gangliosides are present most abundantly in the brain of vertebrates (1–3), and their composition in the brain is well preserved among species. However, the biological functions of gangliosides are not fully understood, despite numerous studies conducted to date (4). In these studies, the gangliosides were added to the medium of cultured cells or injected into experimental animals (5). Despite interesting findings in the phenotypic changes of cells after ganglioside addition and in the therapeutic effects of ganglioside injection on pathological damage in experimental animals, the role of gangliosides in vivo has yet to be elucidated.
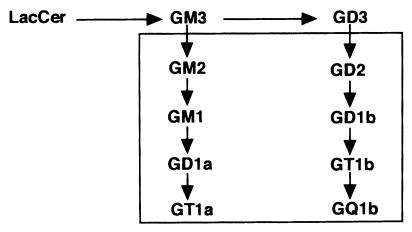
β1,4-N-Acetylgalactosaminyltransferase (β1,4GalNAc-T, GM2/GD2 synthase; EC 2.4.1.94) is a key enzyme in the biosynthesis of complex gangliosides (belonging to the sialoganglio series) (Fig. 1), since all complex gangliosides are synthesized through GM2 or GD2, direct products of the enzyme. [Ganglioside nomenclature is based on that of Svennerholm (6).] Moreover, glycosphingolipids belonging to the asialoganglio series are also synthesized via the action of this enzyme (7). Therefore, it has been expected that disruption of the β1,4GalNAc-T gene would produce serious defects in the nervous system.
Figure 1.
Proposed pathway of ganglioside synthesis. The box encloses gangliosides, whose biosynthesis is blocked in β1,4GalNAc-T-deficient mice. LacCer, lactosylceramide .
Recently, we have established knockout mouse lines of the β1,4GalNAc-T gene, and mice with a disrupted β1,4GalNAc-T allele lacked all complex ganglio-series gangliosides (8) (see Fig. 1). Surprisingly, no obvious abnormalities were found in the histological architecture of the brain or in gross behavior in the mutant mice. On the other hand, male mice lacking complex gangliosides showed serious defects in the testes. There have been only a few studies on ganglioside expression in the testis and sperm (9). The amount of gangliosides in the normal testis is almost comparable to that in other tissues, except in the nervous system (10). Nevertheless, a lack of complex gangliosides in the testis resulted in serious damage.
In the present study, we examined ganglioside function in the spermatogenesis and elucidated aspects of their function in vivo that have not been proposed in past studies.
MATERIALS AND METHODS
Generation of β1,4GalNAc-T Gene-Deficient Mice.
Constructs for the homologous recombination of the β1,4GalNAc-T gene and alteration of ganglioside synthesis in mutant mice were described in a previous report (8). The predicted changes in the ganglioside composition of the mutant mice are shown in Fig. 1.
Histological and Immunohistological Analysis.
Weights of testes were examined sequentially and presented average weights from five mice in each group. Smears of semen and testicular sections were fixed in 10% formalin and Bouins’ solution, respectively and were stained with hematoxylin and eosin. To analyze the distribution of gangliosides, frozen sections were prepared, as described (11). mAb R24 was kindly provided by L. J. Old of Sloan–Kettering Cancer Center, New York, and was used at 200 times dilution of acites fluid followed by fluorescein isothiocyanate-conjugated anti-mouse IgG (heavy and light; Cappel). mAbs specific for GD1a (mAb 92–22), GT1b (mAb 549), and GD1b (mAb 370) were generated in our laboratory (12). They were also used at 200 times dilution of ascites fluid, followed by fluorescein isothiocyanate-conjugated anti-mouse IgM (Zymed).
Extraction and Analysis of Gangliosides.
Glycolipids were extracted as described (13). Nongerm cells of testis were primarily cultured from 2- to 3-week-old mice after hypotonic treatment, as described (14). On day 4, [14C]glucosamine was added to the culture medium (100 μCi/ml) and was incubated for 3 days. On day 7, adherent cells were scraped, and glycolipids were extracted. After TLC, the plate was analyzed with a BAS 2000 image analyzer (Fuji). Neuraminidase treatment was performed as described (11).
In Situ Hybridization.
In situ hybridization analysis was performed as described (15), by using mouse β1,4GalNAc-T riboprobes.
Cholera Toxin Staining.
Cholera toxin staining was performed, as described previously (11). As controls of GM1 expression, brains from wild-type and mutant mice were used.
Northern Blot Analysis.
RNA was extracted from testis of mice at various periods after birth, by using acid phenol (16). Northern blot analysis was performed with [α-32P]dCTP-labeled mouse β1,4GalNAc-T cDNA (pTm3–5), or glyceraldehyde-3-phosphate dehydrogenase cDNA probes, as described previously (17).
Hormone Determination.
Follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone were measured by radioimmunoassay (FSH and LH, Amersham; testosterone, Japan DPC Corp., Tokyo), by using samples from at least eight mice. The human chorionic gonadotrophin stimulation in vitro was done as described (18). Medium from each culture was assayed for testosterone content by radioimmunossay. The total amount of testosterone produced is an average of five cultures.
Intratesticular Injection of Testosterone.
The intratesticular injection of [14C]testosterone was performed as described (19). The injected testosterone is reported to enter the lymph space, overspread in the testis, and penetrate into the seminiferous tubules. At each time point indicated, the mice were sacrificed, and epididymis and vas deferens were removed en bloc. The fluid was collected by squeezing (≈5–12 μl), and radioactivity was measured with a liquid scintillation counter.
Gene Expression in the Testis.
Gene expression in the testis was analyzed by reverse transcription-PCR and Southern blot analysis. Genes analyzed were CREM τ (20), inhibin α (21), androgen-binding protein (18), androgen receptor (22), FSH receptor (23), protamine 1 and 2 (24), and glyceraldehyde-3-phosphate dehydrogenase (control). Southern blotting was performed as described (25).
Binding of Testosterone to Glycolipids.
The testosterone binding to gangliosides was assayed as follows: gangliosides separated on TLC plates were transferred onto nylon membrane, as described (26). After blocking with 2% BSA/PBS, the membrane was incubated in PBS with 1% BSA, 1 mM CaCl2, and 10 mM MgCl2 containing 10 μM [14C]testosterone for 20 h at 4°C with or without unlabeled testosterone (200 μM), cortisol (200 μM), or estriol (200 μM). After washing with PBS three times at room temperature, the membrane was analyzed with a BAS 2000 image analyzer.
RESULTS
Sterility and Aspermatogenesis in Mice Lacking Complex Gangliosides.
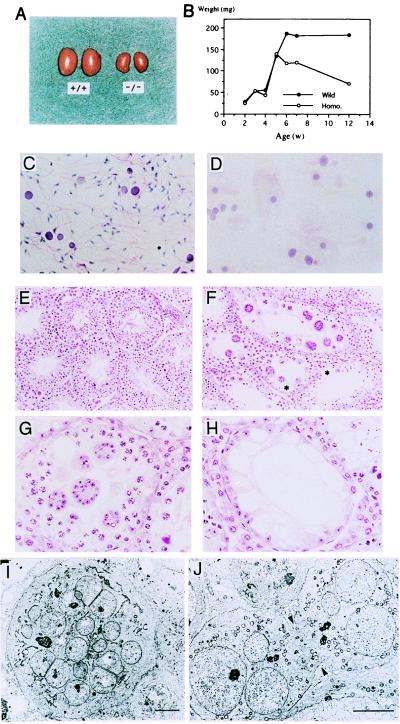
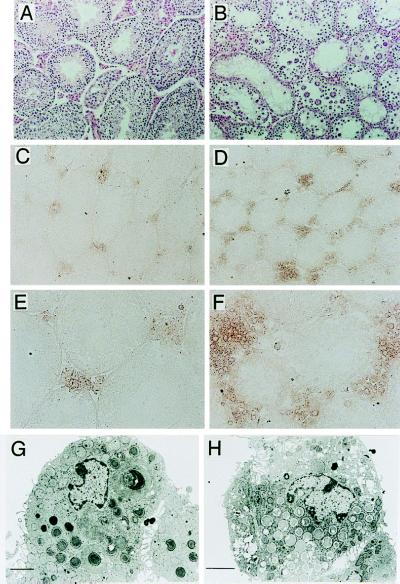
Homozygous mutant male mice were sterile, whereas female mutant mice were fertile. As shown in Fig. 2A, the testicular weight of mutant mice stopped increasing at 4 to 6 weeks of age, and their testes gradually became atrophic thereafter (Fig. 2B). Seminiferous fluid isolated from the vas deferens of 8-week-old mutant males showed a complete absence of mature sperm (Fig. 2 C and D). Light microscopic observation of mutant testis revealed multinuclear giant cells composed of round spermatids. There were no elongating, mature spermatids (Fig. 2F). Abnormal intracellular vacuolation was also found in mutant Sertoli cells (Fig. 2 F and H). High magnification of the specimen showed giant cells of various sizes and loss of germ cells (Fig. 2G). Electron micrography demonstrated that the giant cells contained numerous nuclei of spermatids (Fig. 2I) and the intercellular bridges connecting each round spermatid were prematurely opened, resulting in the formation of multinuclear giant cells (Fig. 2J).
Figure 2.
Male sterility in β1,4GalNAc-T- deficient mice. Morphology and growth of the testis of wild-type and mutant mice. (A) Eight-week-old wild-type (+/+) and mutant (−/−) testes. (B) Changes in testicular weight in wild-type and mutant mice. (C and D) Smear of seminiferous fluid from wild-type (C) and mutant (D) mice. (Hematoxylin/eosin; ×280.) (E and F) Histopathology of testis from 10-week-old wild-type (E) and mutant (F) mice. (Hematoxylin/eosin; ×140.) (G and H) High magnification of mutant mice testis. (Hematoxylin/eosin; ×280.) Note the diffuse vacuoles in Sertoli cells (in H and at asterisks in F). (I and J) Electron micrographs of multinuclear giant cells. A giant cell (I) and an unseparated prematurely opened intercellular bridge (arrows) (J) are shown. (Bars indicate 5 μm).
Gene Expression of βl,4GalNAc-T in Mouse Testis.
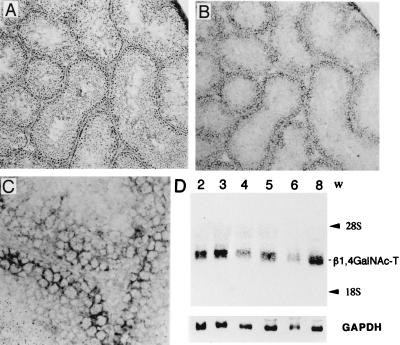
In situ hybridization analysis indicated that the β1,4GalNAc-T gene was expressed in seminiferous epithelium, including Sertoli cells, but not in interstitial tissue (Fig. 3 A–C). On the other hand, Northern blotting revealed that β1,4GalNAc-T mRNA was expressed in wild-type mouse testis from 2 weeks after birth and the expression level did not alter until 8 weeks (Fig. 3D).
Figure 3.
Expression of β1,4GalNAc-T gene in testis. (A–C) In situ hybridization of β1,4GalNAc-T gene in wild-type testis. (A) Hematoxylin/eosin staining of the testis from a wild-type mouse ×140. (B) With digoxigenin-labeled RNA probe, in situ hybridization was performed as described in Materials and Methods. (×140. (C) High magnification (×560) of B. (D) Total RNA was extracted from testes of ≈2- to 8-week-old wild-type mice and 20 μg each was applied for Northern blotting. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA was used to hybridize the same membrane as a control.
Ganglioside Expression in Mouse Testis.
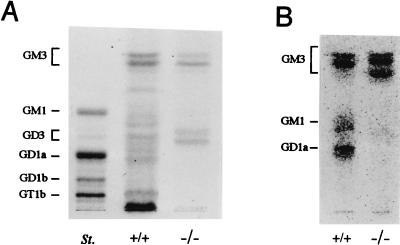
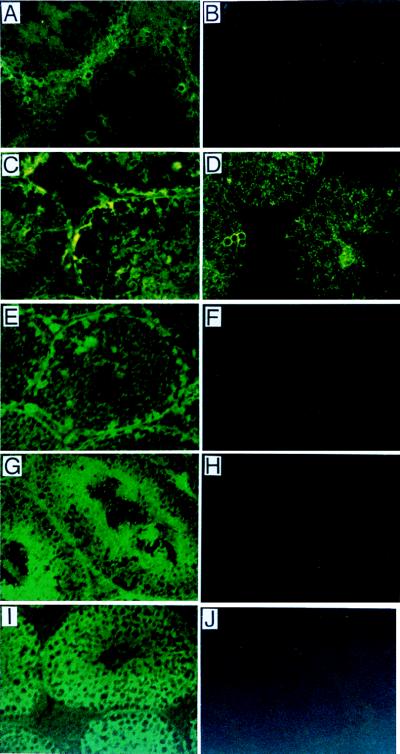
As shown in Fig. 4A, GM3, GM1, GD1a, and GT1b were major gangliosides in the whole testis of wild type, whereas only GM3 and GD3 were detected in mutant testis. Primarily cultured nongerm cells (mainly Sertoli cells) produced GM3, GM1, and GD1a in the wild type but only GM3 in the mutant, when analyzed by metabolic labeling with [14C]glucosamine (Fig. 4B). Thus, nongerm cells—i.e., Leydig cells, peritubular myoid cells, fibroblasts, and Sertoli cells — appeared to express mainly “a” series gangliosides. Consequently, the expression of “b” series gangliosides, such as GD3, GD1b, and GT1b, is ascribable to germ cells. In the binding assay with fluorescein isothiocyanate-conjugated cholera toxin, which bound specifically to GM1, positive staining was found mainly in interstitial tissue and weakly in seminiferous tubules (Fig. 5A). After neuraminidase treatment, which converts complex gangliosides to GM1, stronger staining was observed in the base of seminiferous tubules, possibly in Sertoli cells (Fig. 5C). At the same time, enhanced staining was seen in germ cells, which may reflect GT1b in these cells. Indeed, GD3 distribution was completely restricted to the inside of seminiferous tubules in mutant testis (Fig. 5D). Furthermore, immunohistostaining with mAbs specific for GD1a, GT1b, or GD1b revealed that GT1b was expressed only in the intermediate layer of germ cells (Fig. 5G), GD1b was in all germ cells (Fig. 5I), and GD1a was just at the edge of the seminiferous tubules, possibly in Sertoli cells (Fig. 5E). These findings corresponded well with the results of TLC of the extracted gangliosides. Thus, gangliosides are differentially distributed in wild-type mice, i.e., GT1b and GD1b mainly in germ cells, GD1a in Sertoli cells, and GM1 in Leydig cells.
Figure 4.
Expression of gangliosides in testis. (A) TLC of acidic glycosphingolipids from testis. St., bovine brain ganglioside mixture as a standard. Acidic glycolipids were separated by TLC with a solvent (chloroform/methanol/0.2% CaCl2, 55:45:10). Resorcinol spray was used for detection. (B) Profiles of acidic glycosphingolipids in nongerm cells. Nongerm cells from testis were cultured primarily after elimination of germ cells, then metabolically labeled with [14C]glucosamine and analyzed for acidic glycolipids by TLC. Solvent for TLC was as in A.
Figure 5.
Distribution of gangliosides in testis. (A–C) Cholera toxin staining. (A) Staining in wild-type testis. (B) No staining of mutant testis. (C) Staining after neuraminidase treatment of wild-type testis for detection of the complex gangliosides. (D) GD3 expression in mutant testis revealed by anti-GD3 mAb R24. Testis of wild-type mice did not show definite staining (data not shown). (E–J) Immunohistostaining with mAbs specific for GD1a (E, F), GT1b (G, H), and GD1b (I, J). Samples from wild-type (E, G, I) and mutant testis (F, H, J) were compared.
Hormone Abnormalities in the Mutant Mice.
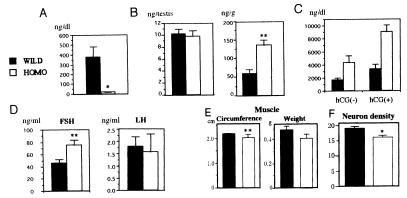
The serum testosterone level was markedly low in the mutant: ≈5% of wild type (Fig. 6A). Accordingly, the serum FSH level was elevated, while the serum LH level was not significantly altered in the mutant mice (Fig. 6D). Testosterone, however, was normally detectable in the mutant testicular homogenates, even more abundantly than in wild-type mice, when corrected as concentration per weight of testis (Fig. 6B). Furthermore, testicular cells of the mutant mice released more testosterone than wild type both with and without human chorionic gonadotrophin stimulation in vitro (Fig. 6C). Size and weight of gastrocnemius muscle and neuron density at the preoptic area were analyzed to examine the effects of lowered serum testosterone levels in the mutant mice. Homozygotes showed less developed muscles and lowered density of neurons (Fig. 6 E and F).
Figure 6.
Hormonal changes and their effects in β1,4GalNAc-T mutant mice. Black bars represent wild type and white bars homozygous mutant (A–F). Ten- to 12-week-old mice were analyzed. (A) Testosterone levels in serum. Note marked reduction in serum testosterone level in the mutant (21.0 ± 4.10 ng/dl) in contrast to the wild type (386.63 ± 111.90 ng/dl). ∗, P < 0.005. (B) Testosterone in testis. In testis, the amounts of total testosterone are comparable, and the amount per weight is somewhat higher in the mutant. ∗∗, P < 0.05. (C) Production of testosterone in cultured testicular cells with or without human chorionic gonadotrophin stimulation in vitro. (D) Serum FSH and LH levels in wild-type and mutant mice. (E) Comparison of muscle size. Circumferences of the gastrocnemius muscles and weights of parts between the knee joints and Achilles tendon were measured. ∗∗, P <0.05. (F) Neuron density at the preoptic area was examined by serial cross-sections, as described (34). Ordinate is unit of pixels. ∗, <0.005.
Distribution of Testosterone in the Testis.
By using anti-testosterone antibody (Fig. 7), testosterone was found to be accumulated to a much greater extent in interstitial Leydig cells of the mutant mice (Fig. 7 D and F) than those of wild type (Fig. 7 C and E). These results suggested a defect in testosterone transport from Leydig cells into serum and possibly into seminiferous tubules. In electron micrographs, Leydig cells containing a lot of granules with low density were frequently seen in the mutant testis (Fig. 7H), supporting the results of immunohistochemistry.
Figure 7.
Testosterone production in the interstitial cells of testis. (A and B) Hematoxylin/eosin staining of testis from wild-type (A) and mutant (B) mice. (C–F) Immunohistochemistry for testosterone with polyclonal antibody (Zymed) in wild-type (C and E) and mutant (D and F) testis. (×140 for C and D, ×280 for E and F.) (G and H) Electron micrograph of Leydig cells of wild-type (G) and mutant (H) mice. (Bars indicate 2 μm.)
Gene expression in Mouse Testis.
Expression levels of various genes relevant to the regulation of spermatogenesis in mouse testis were analyzed by reverse-transcription-PCR. No particular gene showed a definite reduction that could be considered to cause the aspermatogenesis.
Testosterone Binding to Gangliosides.
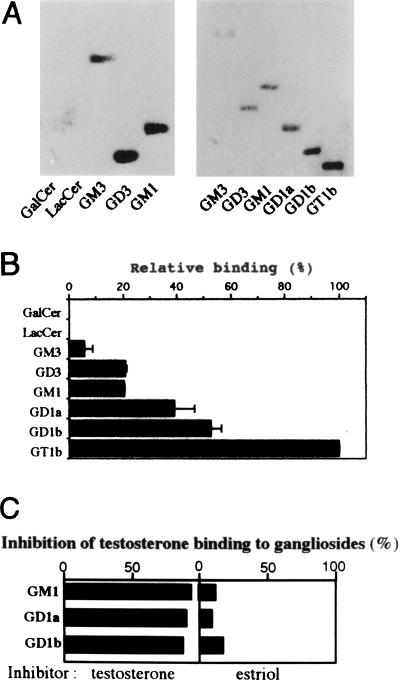
We then examined the interaction between gangliosides and testosterone in vitro. Gangliosides bound testosterone at various intensities on the membrane; among the gangliosides expressed in the testis, the order was GT1b > GD1a > GM1 = GD3 > GM3 (Fig. 8 A and B). The binding was specific, since it was inhibited by testosterone but not by other steroids, such as estriol and cortisol (Fig. 8C).
Figure 8.
Ganglioside–testosterone interaction. [14C]testosterone was applied to a poly(vinylidene difluoride) membrane on which individual glycolipids had been blotted. Binding and washing were performed as described in Materials and Methods. (A) Each lane contained 1.6 (left) and 0.4 (right) nmol of glycolipids. (B) Relative binding of testosterone to 0.4 nmol of each ganglioside is given as a percentage of the GT1b value (an average of three experiments). (C) Specific inhibition of testosterone binding to gangliosides by unlabeled testosterone. [14C]testosterone was applied to a poly(vinylidene difluoride) membrane, as in A, with individual inhibitors. Similar experiments with wide range of gangliosides were repeated 3 times, and a representative result only for testosterone and estriol is presented as percent inhibition.
Testosterone Transport in the Mutant Mice.
To directly observe the testosterone transport in the testis, excreted [14C]testosterone was examined in semen after intratesticular injection . As expected, the permeability of [14C]testosterone from interstitial tissue to seminiferous tubules was markedly diminished in the mutant mice (Fig. 9A), compared to the secretion pattern in wild-type mice. When ganglioside mixture was coinjected with testosterone in the mutant mice, secretion of [14C]testosterone markedly improved (Fig. 9B) at both 5 and 30 min after injection.
Figure 9.
(A) Transport of intratesticularly injected [14C]testosterone into semen. The radioactivity in semen after injection was measured at the time points indicated. These experiments were repeated at least three times and showed essentially the same results. A representative result is given. Black bars, wild type; white bars, mutant. (B) Improvement of [14C]testosterone excretion in the presence of gangliosides in the mutant mice. [14C]testosterone was injected as in A, with or without gangliosides (10 μg), then cpm in semen was examined at 5 and 30 min after injection. ∗∗, P < 0.05. (C) A scheme to show the essential role of complex gangliosides in testosterone transport from Leydig cells in testis. T, testosterone. An X superimposed on an arrow represents disrupted transport.
DISCUSSION
Complex gangliosides have been thought to be co-receptors of peptide hormones such as FSH and LH, or modulaters of the receptor function (4, 27). In particular, GM1 is considered a likely co-receptor of these hormones, since cholera toxin B subunit, which is a specific ligand of GM1, and those peptide hormones share some homology in amino acid sequences (28). Therefore, alteration of FSH signaling through the FSH receptor on Sertoli cells seemed to occur in the mutant mice. However, cultured Sertoli cells from mutant mice responded to FSH stimulation as well as did those from wild type, both in cAMP elevation and in Ca2+ influx (data not shown). Thus, disrupted hormonal signaling because of a lack of complex gangliosides seems not to be a mechanism for abnormal changes in the testes of the mutant mice.
Extensive examination of the structures and functions of testicular tissues revealed multinuclear giant cells in the testis and marked depression of serum testosterone levels as the most striking changes in the mutant mice. The reason for normal levels of serum LH in the mutant mice is not known now but seems to be because of the disruption of a feedback regulatory system based on the lack of complex gangliosides. The multinuclear giant cells seen in the mutant mice can be associated with other factors (29), such as trauma, aging (30), and cytochalasin D treatment (31). Sertoli cells generally undergo vacuolation on interruption of spermatogenesis (32). It is therefore difficult to specify the cause of aspermatogenesis on the basis of these morphological findings. Matikainen et al. (33) showed that the completion of meiosis and spermiogenesis supported by FSH depended on androgens from Leydig cells in hypophysectomized rats. Taking these results together, it is likely that the testosterone produced in the Leydig cells did not reach the Sertoli cells in seminiferous tubules or the bloodstream because of the disruption of the hormone transport pathway as shown in Fig. 9C, resulting in the degeneration of immature round spermatids and spermatocytes and in the formation of multinuclear giant cells. The suppressed drainage of testosterone injected into testes of mutant mice seemed to reflect the disordered transport of testosterone. The improved secretion of [14C]testosterone after co-injection with gangliosides (Fig. 9B) strongly supported this interpretation. The disrupted distribution of testosterone in the mutant mice also affects sufficient development of other tissues, such as muscles, and in a restricted part of the brain, (Fig. 6 E and F) which was previously reported as a sexually dimorphic nucleus in the preoptic area in rats (34).
Abnormal findings in the testis appear from 4 weeks of age, when the growth of gonads and sex maturation progress under hormonal control in normal mice. These results also support that the lack of testosterone flow is the main cause of aspermatogenesis in the mutant mice. However, some seminiferous tubules in mutant mice showed complete lack of germ cells (Fig. 2H). This change is more serious than that with simple absence of testosterone. Lack of complex gangliosides might bring about unknown testicular deterioration as well as the lack of testosterone in seminiferous tubules.
Testicular feminized mutant mice, which lack a functional androgen receptor, show external female phenotypes and Leydig cell dysfunction (35). Some features based on the testosterone defect were, therefore, similar between testicular feminized mutant mice and our mutant mice. However, the latter could fully synthesize testosterone, and a low-level leakage of the hormone seemed to attenuate the severity of symptoms caused by testosterone deficiency.
The specific binding of testosterone to gangliosides and the differential distribution of gangliosides in testis mentioned above suggest that gangliosides constitute an affinity gradient that may contribute to testosterone transport in testis. The fact that testosterone bound more strongly to gangliosides containing more sialic acids also appears to support this idea, since gangliosides distributed in the pathway of testosterone transport with an increasing number of sialic acids (Fig. 8B).
Androgen-binding protein (ABP) is a major androgen carrier protein secreted from Sertoli cells in rat, human, and rabbit (32). Mice, however, were reported to produce little testicular ABP (18). We were also unable to detect the presence of ABP or other molecules that bind specifically to testosterone. Thus, results with the gene knockout mice suggest that gangliosides substitute for ABP in mouse testis. However, the mechanisms of secretion from Leydig cells and transport into seminiferous tubules of testosterone are not yet fully understood, and ABP is not responsible for these processes. Precise molecular mechanisms for these steps remain to be investigated.
Consequently, it is conceivable that the insufficiency of testosterone transport results in the defects in spermatogenesis in the mutant mice. Moreover, intratesticular injection of the ganglioside mixture and testosterone, but neither of them alone, could cause partial restoration of spermatogenesis (data not shown), indicating that these two compounds need to coexist at some particular developmental stage of testis for sound spermatogenesis. However, it is not clear which particular structure among the lost complex gangliosides is essential to spermatogenesis. It also remains to be elucidated which cell lineage suffers the most functional damage on depletion of complex gangliosides. The generation of mutant mice in which other glycosyltransferase genes responsible for the synthesis of more restricted species of gangliosides are knocked out is awaited.
Acknowledgments
We thank Dr. Y. Nishimune, Osaka University, Osaka, Japan, and Dr. K. O. Lloyd, Memorial Sloan–Kettering Cancer Center, New York, for helpful discussions. This study was supported by a Grant-in-Aid for Scientific Research on Priority Areas and by a Grant-in-Aid for Core of Excellence Research from the Ministry of Education, Science and Culture of Japan.
ABBREVIATIONS
- β1
4GalNAc-T, β1,4-N-acetylgalactosaminyltransferase
- FSH
follicle-stimulating hormone
- LH
lutenizing hormone
- ABP
androgen-binding protein
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1. Suzuki K. J Neurochem. 1965;12:969–979. doi: 10.1111/j.1471-4159.1965.tb10256.x. [DOI] [PubMed] [Google Scholar]
- 2.Ledeen R, Yu R K. Methods Enzymol. 1982;83:139–191. doi: 10.1016/0076-6879(82)83012-7. [DOI] [PubMed] [Google Scholar]
- 3.Wiegandt H. In: Glycolipids. Wiegandt H, editor. Amsterdam: Elsevier; 1985. pp. 199–260. [Google Scholar]
- 4.Hakomori S. J Biol Chem. 1990;265:18713–18716. [PubMed] [Google Scholar]
- 5.Schengrund C L. Brain Res Bull. 1990;24:131–141. doi: 10.1016/0361-9230(90)90297-d. [DOI] [PubMed] [Google Scholar]
- 6.Svennerholm L. J Neurochem. 1963;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- 7.Yamashiro S, Haraguchi M, Furukawa K, Takamiya K, Yamamoto A, Nagata Y, Lloyd K O, Shiku H, Furukawa K. J Biol Chem. 1995;270:6149–6155. doi: 10.1074/jbc.270.11.6149. [DOI] [PubMed] [Google Scholar]
- 8.Takamiya K, Yamamoto A, Furukawa K, Yamashiro S, Shin M, Okada M, Fukumoto S, Haraguchi M, Takeda N, Fujimura K, et al. Proc Natl Acad Sci USA. 1996;93:10662–10667. doi: 10.1073/pnas.93.20.10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gore P J, Singh S P, Brooks D D. Biochim Biophys Acta. 1986;876:36–47. doi: 10.1016/0005-2760(86)90315-2. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura K, Hashimoto Y, Yamakawa T, Suzuki A. J Biochem (Tokyo) 1988;103:201–208. doi: 10.1093/oxfordjournals.jbchem.a122232. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto A, Yamashiro S, Takamiya K, Atsuta M, Shiku H, Furukawa K. J Neurochem. 1995;65:2417–2424. doi: 10.1046/j.1471-4159.1995.65062417.x. [DOI] [PubMed] [Google Scholar]
- 12.Miyazaki H, Fukumoto S, Okada M, Hasegawa T, Furukawa K, Furukawa K. J Biol Chem. 1997;272:24794–24799. doi: 10.1074/jbc.272.40.24794. [DOI] [PubMed] [Google Scholar]
- 13.Furukawa K, Clausen H, Hakomori S, Sakamoto J, Look K, Lundblad A, Mattes M J, Lloyd K O. Biochemistry. 1985;24:7820–7826. doi: 10.1021/bi00347a047. [DOI] [PubMed] [Google Scholar]
- 14.Cameron D F, Muffly K E. J Cell Sci. 1991;100:623–633. doi: 10.1242/jcs.100.3.623. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto A, Atsuta M, Hamatani K. Cell Biochem Funct. 1992;10:71–77. doi: 10.1002/cbf.290100202. [DOI] [PubMed] [Google Scholar]
- 16.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 17.Nagata Y, Yamashiro S, Yodoi J, Lloyd K O, Shiku H, Furukawa K. J Biol Chem. 1992;267:12082–12089. [PubMed] [Google Scholar]
- 18.Wang Y M, Sullivan P M, Petrusz P, Yarbrough W, Joseph D R. Mol Cell Endocrinol. 1989;63:85–92. doi: 10.1016/0303-7207(89)90084-1. [DOI] [PubMed] [Google Scholar]
- 19.Russell L D, Saxena N K, Weber J E. Gamete Res. 1987;17:43–56. doi: 10.1002/mrd.1120170106. [DOI] [PubMed] [Google Scholar]
- 20.Foulkes N S, Mellstrom B, Benusigli E, Sassone-Corsi P. Nature (London) 1992;355:80–84. doi: 10.1038/355080a0. [DOI] [PubMed] [Google Scholar]
- 21.Tone S, Katoh Y, Fujimoto H, Togashi S, Yanazawa M, Kato Y, Higashinakagawa T. Differentiation. 1990;44:62–68. doi: 10.1111/j.1432-0436.1990.tb00537.x. [DOI] [PubMed] [Google Scholar]
- 22.He W W, Fischer L M, Sun S, Bilhartz D L, Zhu X P, Young C Y, Kelley D B, Tindall D J. Biochem Biophys Res Commun. 1990;171:697–704. doi: 10.1016/0006-291x(90)91202-4. [DOI] [PubMed] [Google Scholar]
- 23.Sprengel R, Braun T, Nikolics K, Segaloff D L. J Mol Endocrinol. 1990;4:525–530. doi: 10.1210/mend-4-4-525. [DOI] [PubMed] [Google Scholar]
- 24.Johnson P A, Peschon J J, Yelick P C, Palmiter R D, Hecht N D. Biochim Biophys Acta. 1988;950:45–53. doi: 10.1016/0167-4781(88)90071-1. [DOI] [PubMed] [Google Scholar]
- 25.Furukawa K, Furukawa K, Shiku H. FEBS Lett. 1991;295:141–145. doi: 10.1016/0014-5793(91)81404-v. [DOI] [PubMed] [Google Scholar]
- 26.Taki T, Handa S, Ishikawa D. Anal Biochem. 1994;221:312–316. doi: 10.1006/abio.1994.1418. [DOI] [PubMed] [Google Scholar]
- 27.Fishman P H, Brady R O. Science. 1976;194:906–915. doi: 10.1126/science.185697. [DOI] [PubMed] [Google Scholar]
- 28.Ledley F D, Mullin B R, Lee G, Aloj S M, Fishman P H, Hunt L T, Dayhoff M O, Kohn L D. Biochem Biophys Res Commun. 1976;69:852–859. doi: 10.1016/0006-291x(76)90452-6. [DOI] [PubMed] [Google Scholar]
- 29.Holstein A F, Eckmann C. Andrologia. 1986;18:5–16. doi: 10.1111/j.1439-0272.1986.tb01729.x. [DOI] [PubMed] [Google Scholar]
- 30.Nistal M, Codesal J, Paniagua R. Arch Androl. 1986;16:125–129. doi: 10.3109/01485018608986931. [DOI] [PubMed] [Google Scholar]
- 31.Weber J E, Turner T T, Tung K S K, Russel L D. Am J Anat. 1988;182:130–147. doi: 10.1002/aja.1001820204. [DOI] [PubMed] [Google Scholar]
- 32.Bardin C W, Cheng C Y, Musto N A, Gunsalus G L. In: The Physiology of Reproduction. Knobil E, Neill J, editors. New York: Raven; 1988. pp. 933–974. [Google Scholar]
- 33.Matikainen T, Toppari J, Vihko K K, Huhtaniemi I. J Endocrinol. 1994;141:449–457. doi: 10.1677/joe.0.1410449. [DOI] [PubMed] [Google Scholar]
- 34.Gorski R A, Harlan R E, Jacobson C D, Shryne J E, Southam A M. J Comp Neurol. 1980;193:529–539. doi: 10.1002/cne.901930214. [DOI] [PubMed] [Google Scholar]
- 35.Murphy L, Jeffcoate I A, O’Shaughnessy P J. Endocrinology. 1994;135:1372–1377. doi: 10.1210/endo.135.4.7925099. [DOI] [PubMed] [Google Scholar]