Abstract
T5P γC-crystallin mutation is associated with Coppock-like cataract, one of the autosomal dominant congenital cataracts. It is not known why the abundant α-crystallin cannot prevent the mutation-related aggregation. Our previous studies indicate that the mutation changes conformation and reduces solubility and stability, but it is not known whether it is these events or the loss of interaction with other crystallins that causes the cataract. It is also not known whether the α-crystallin can protect T5P mutant as effectively from heat-induced aggregation as the wild-type (WT) γC-crystallin. To investigate the mechanism of interactions and chaperone function between αA- and γC-crystallin, human αA-crystallin and W9F mutant as well as WT γC-crystallin and T5P mutant were cloned. Interactions between αA- and γC-crystallin were studied with fluorescence resonance energy transfer (FRET), and chaperone activity was assessed by the suppression of heat-induced aggregation of substrate proteins. Conformational changes of substrate proteins were studied by spectroscopic measurements. The results indicate that the T5P mutant showed a slightly greater FRET than WT γC-crystallin with αA-crystallin, and αA-crystallin could effectively prevent both WT and T5P γC-crystallin from heat-induced aggregation. Spectroscopic measurements show that both αA-crystallin and γC-crystallin underwent only slight conformational change after chaperone binding. Together with previous results obtained with a two-hybrid system assay of interactions between αA- and γC-crystallin, the present FRET and chaperone results indicate that loss of interactions of T5P mutant with other crystallins may play a larger role than the protection afforded by chaperone-like activity in Coppock-like cataract.
Keywords: αA-crystallin, γC-crystallin, T5P γC-crystallin mutant, chaperone-like activity, congenital cataract, spectroscopy
Many human autosomal dominant congenital cataracts have been reported; the genotype is a single mutation in a specific crystallin or noncrystallin gene. Such mutated crystallin genes include CRYAA (Rl16C; Litt et al. 1998), CRYAB (R120G; Vicart et al. 1998; Berry et al. 2001), CRYBB2 (Q155*; Litt et al. 1997; Gill et al. 2000), and CRYGC (T5P; Heon et al. 1999; Santhiya et al. 2002). T5P γC-crystallin mutation is associated with Coppock-like cataract and has a phenotype of a dust-like opacity of the fetal nucleus (Heon et al. 1999; Santhiya et al. 2002). It is not known why the abundant α-crystallin cannot prevent the mutation-related aggregation. α-Crystallin has been found to function as a chaperone molecule; it can prevent both heat-induced aggregation of other crystallins (Horwitz 1992) and DTT reduction-induced aggregation of insulin (Farahbakhsh et al. 1995; Sun et al. 1997). The common mechanism is the binding of partially unfolded substrate proteins, thus preventing them from aggregating. Our previous studies indicate that the T5P mutation of γC-crystallin not only causes changes in conformation (partial unfolding), but also reduces solubility and stability (Fu and Liang 2002a). Therefore, it is reasonable to assume that α-crystallin will bind to T5P γC-crystallin. However, our previous two-hybrid assay did not detect increased interactions between α-crystallin and T5P γC-crystallin (Fu and Liang 2002b). It is not known whether conformational changes or loss of interactions with other crystallins causes this type of cataract. It is also not known whether α-crystallin is as effective in protecting the T5P mutant as the wild-type (WT) γC-crystallin from heat-induced aggregation. In the present study, we further investigated the interactions between αA-crystallin and T5PγC-crystallin with fluorescence resonance energy transfer (FRET) and chaperone binding with heat-treated T5P γC-crystallin. The results indicate that αA-crystallin binds only slightly stronger with T5P γC-crystallin than with WT γC-crystallin without heating, which may explain why T5P γC-crystallin aggregates in the congenital cataract.
Results
FRET for interaction between γC-crystallin and αA-crystallin
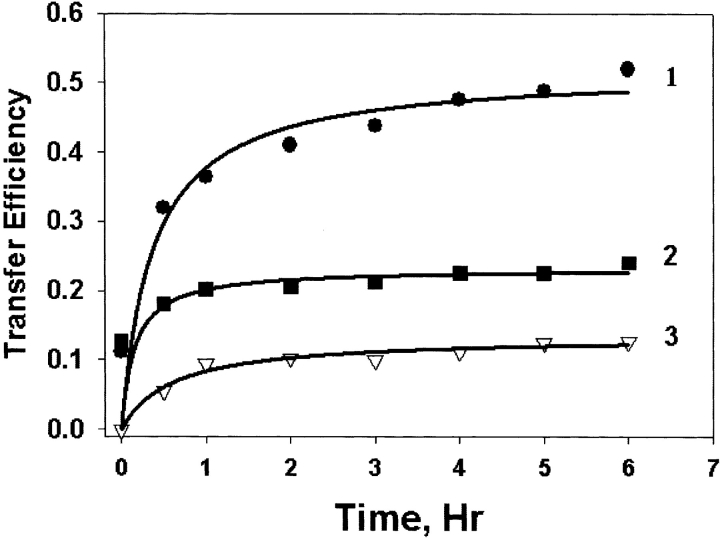
Interactions through either subunit exchange or other protein–protein interactions are shown in Figure 1 ▶. Compared with WT αA-crystallin, WT γC-crystallin shows much less interaction with MIANS-labeled W9F αA-crystallin, although the T5P mutant showed slightly higher levels of interaction. This is consistent with previous studies: FRET did not show subunit exchange between γ- and α-crystallin (Bova et al. 1997), and a two-hybrid system assay detected little CAT activity between γC- and αA-crystallin (Fu and Liang 2002c).
Figure 1.
Fluorescence resonance energy transfer (FRET) between MIANS-labeled W9F αA-crystallin and WT or T5P γC-crystallin. WT αA-crystallin was included for comparison. Curve 1, WT αA-crystallin; curve 2, T5P γC-crystallin; curve 3, WT γC-crystallin.
Chaperone-like activity
The chaperone-like activities of WT αA-crystallin and the W9F mutant are shown in Figure 2 ▶, A and B, respectively. Unfolding and aggregation of γC-crystallin induced by heat were successively suppressed by increasing the amounts of αA-crystallin. At a ratio of 1:1 (by weight), aggregation was completely suppressed. No appreciable difference was observed between the WT and W9F mutant, as was observed previously (Andley et al. 1996).
Figure 2.
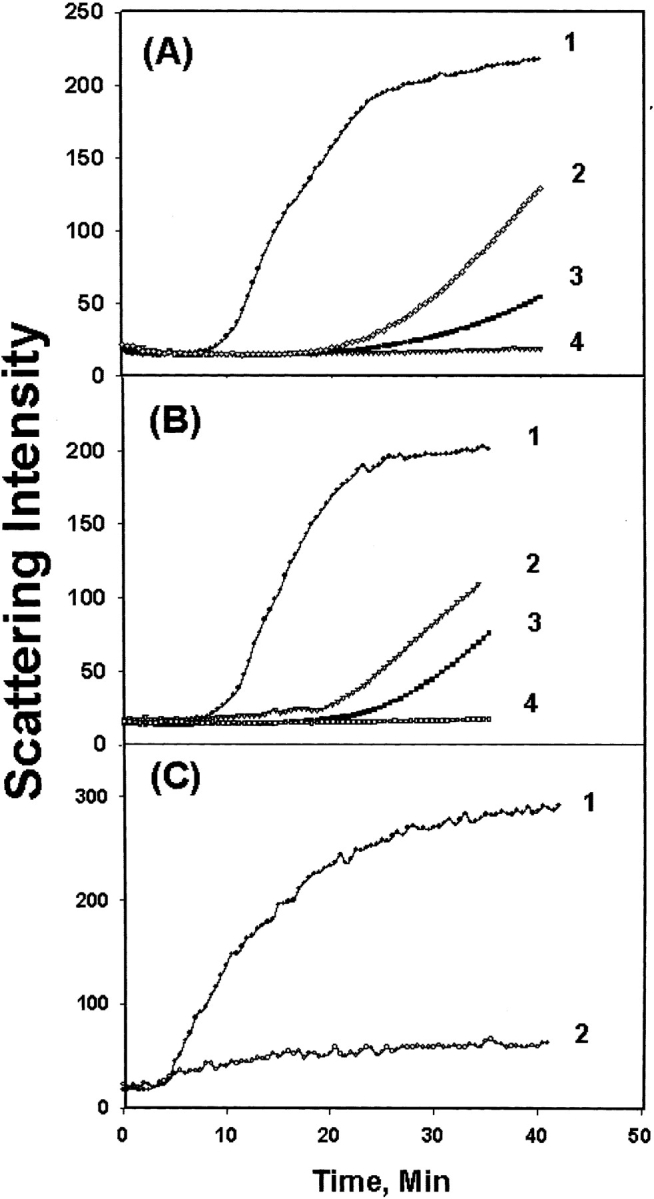
Chaperone-like activity of (A) WT αA-crystallin toward WT γC-crystallin; (B) W9F αA-crystallin toward WT γC-crystallin; (C) W9F αA-crystallin toward T5P γC-crystallin: Scattering was measured at 65°C with a fluorometer set at 400 nm for both emission and excitation wavelength. (A,B) Curve 1, γC-crystallin alone; curve 2, αA/γC ratio 1:4; curve 3, αA/γC ratio 1:2; curve 4, αA/γC ratio 1:1. (C) Curve 1, T5P γC-crystallin alone; curve 2, αA/T5PγC ratio 1:1.
The T5P γC-crystallin mutant aggregated faster than WT γC-crystallin (Fu and Liang 2002a), but αA-crystallin effectively suppressed the aggregation of T5P γC-crystallin at the same ratio, 1:1, for WT γC-crystallin (Fig. 2B,C ▶).
Thermal unfolding
Trp fluorescence is sensitive to Trp environmental changes either by heat or denaturant. Upon heating at 65°C, the Trp fluorescence intensity of both WT αA- and γC-crystallin initially decreases, but the intensity of γC-crystallin increases once unfolding occurs; the increase was suppressed by the presence of αA-crystallin (Fig. 3A ▶). The results are consistent with those of chaperone activity measurements.
Figure 3.
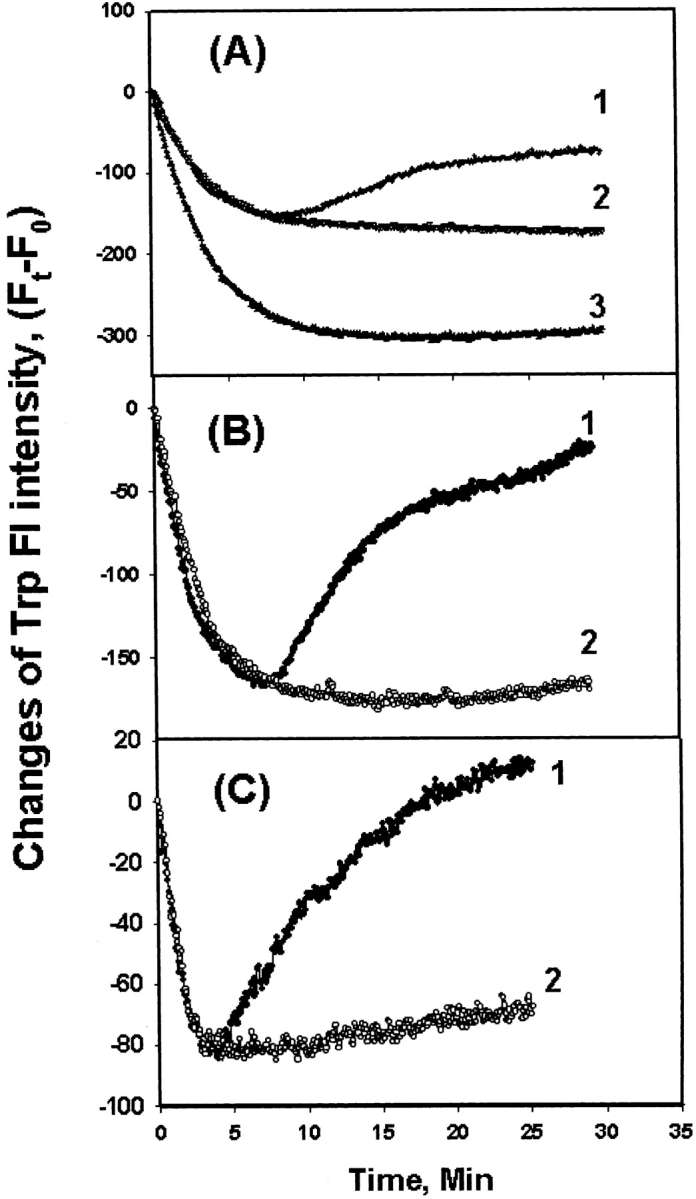
Time course change of Trp fluorescence intensity at 340 nm (λex = 295 nm) at 65°C. (A) Mixture of WT γC- and WT αA-crystallin; (B) mixture of WT γC-crystallin and W9F αA-crystallin; (C) mixture of T5P γC-crystallin and W9F αA-crystallin. Curves 1, 2, and 3 in A: WT γC-crystallin only, αA-crystallin only, and mixtures of WT γC-crystallin and WT αA-crystallin (1:1), respectively. Curves 1 and 2 in B and C control WT or T5P γC-crystallin and mixture of WT or T5P γC-crystallin and W9F αA-crystallin (1:1), respectively. The curves were plotted as (Ft−F0) vs. time, where Ft is the intensity at time t and F0 at time 0.
To measure the contribution to Trp fluorescence from γC-crystallin alone, the W9F αA-crystallin mutant was used again. The Trp-free W9F αA-crystallin mutant shows no Trp fluorescence (Sun et al. 1998). Results were similar to those using WT αA-crystallin, indicating that the increase in Trp fluorescence arose from aggregation of γC-crystallin (Fig. 3B ▶). The same changes were observed for T5P γC-crystallin, though at a faster rate, and aggregation was retarded by αA-crystallin (Fig. 3C ▶).
Conformational changes
Conformational changes, resulting from chaperone binding on either chaperone or substrate protein, were studied by Trp fluorescence and CD. The individual samples or mixtures, using W9F αA-crystallin and WT or T5P γC-crystallin as models, were heated at 65°C for 1 h and then cooled to room temperature. Preliminary data indicated that WT or T5P γC-crystallin solution, but not αA-crystallin, became cloudy when heated at this temperature, but the mixtures of γC- and αA-crystallin remained clear.
Trp fluorescence spectra of WT and T5P γC-crystallin are shown in Figure 4A ▶; the mutation shifted the emission maximum from 328–330 nm to 340–342 nm and increased the intensity. Trp fluorescence spectra in GdnHCl were included for comparison; increasing GdnHCl concentrations gradually shifted the maximum to longer wavelengths, and at 4M the proteins were completely denatured and displayed the emission maximum at 350–352 nm (Fig. 4A ▶, curves 2 and 4). In contrast, preheating of γC- or γA-crystallin individually increased Trp fluorescence intensity, but no shift of emission maximum was observed (Fig. 4B ▶). To reduce the cloudiness of the γC-crystallin solution, heating time was shortened to 20 min. Figure 4C ▶ shows the increases of Trp fluorescence, but no shift in wavelength by chaperone binding of W9F αA- with WT or T5P γC-crystallin. As the W9F αA-crystallin shows very little Trp fluorescence (Sun et al. 1998), the increases are due to partial unfolding of WT or T5P γC-crystallin.
Figure 4.
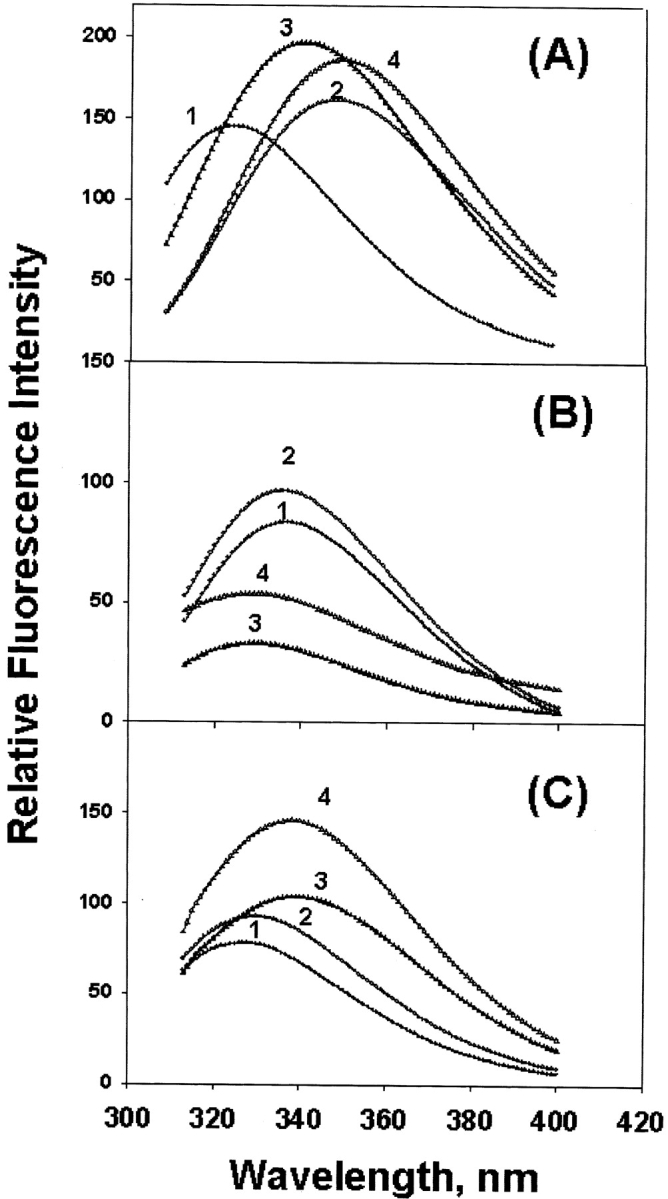
Trp fluorescence spectra of γC-crystallin samples. (A) Individual WT and T5P γC-crystallin control (curves 1 and 3) and in 4M GdnHCl (curves 2 and 4). (B) Individual WT αA- and WT γC-crystallin control (curves 1 and 3) and corresponding preheated samples (curves 2 and 4), and (C) control mixture of W9F αA- and WT or T5P γC-crystallin (curves 1 and 3) and corresponding preheated mixture samples (curves 2 and 4). Excitation wavelength is 295 nm; samples were preheated at 65°C for 1 h except for γC-crystallin, which was preheated at 65°C for 20 min.
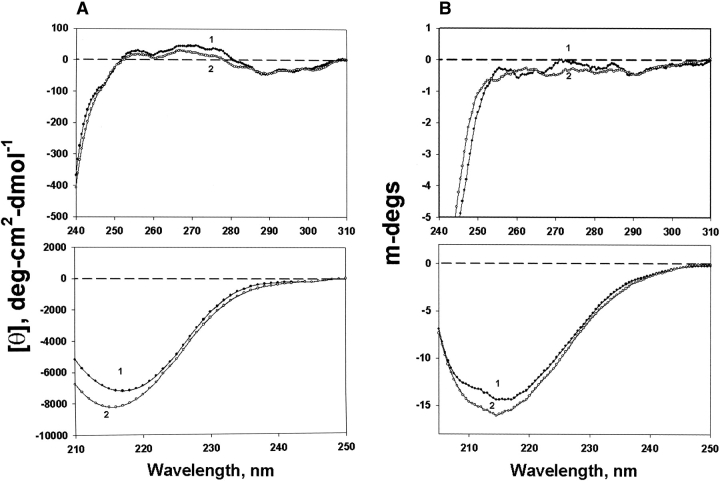
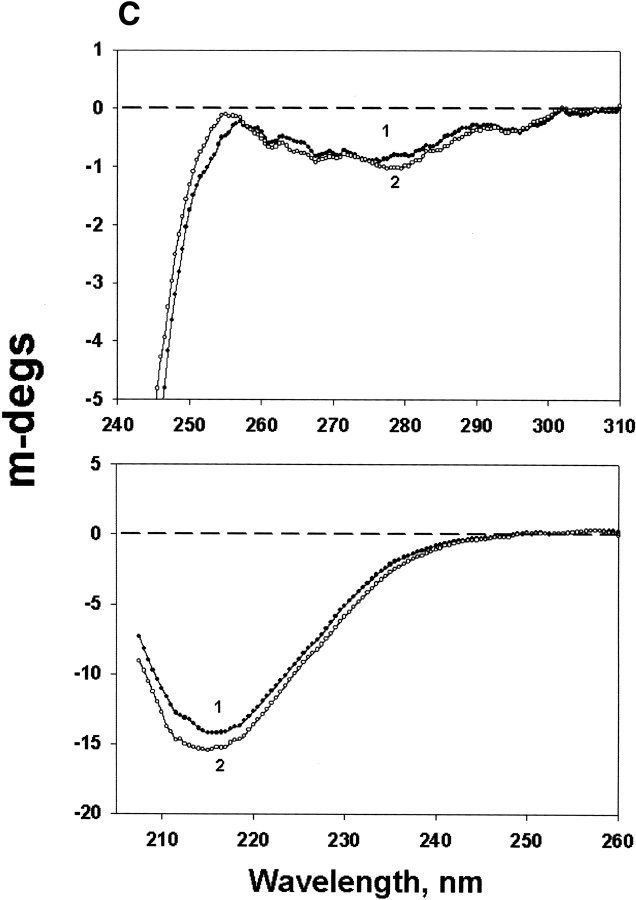
Figure 5A ▶ shows CD spectra of W9F αA- and WT γC-crystallin mixtures; the major change was decreased near-UV CD intensity in the region below 280 nm for the preheated samples. The far-UV CD reflects the secondary structure, and there are some changes for the preheated samples. The corresponding CD changes for W9F αA-and T5P γC-crystallin are shown in Figure 5B ▶. Little change was observed for preheated W9F αA-crystallin alone (Fig. 5C ▶).
Figure 5.
Circular dichroism of W9F αA- and γC-crystallin mixtures in near-UV region (upper panel) and far-UV region (lower panel). (A) W9FαA- and γC-crystallin; (B) W9F αA- and T5P γC-crystallin; (C) W9F αA-crystallin alone. Curve 1, controls; curve 2, preheated samples at 65°C for 1 h. Protein concentrations were 0.25 and 0.15 mg/mL for αA- and γC-crystallin, respectively. Cell path length was 10 mm and 1 mm for the measurements at near- and far-UV regions, respectively.
Complex formation between αA- and WT or T5P γC-crystallin was obvious from the elution profiles of fast performance liquid chromatography (FPLC) size exclusion chromatography (Fig. 6 ▶). Because preheating of WT αA-crystallin alone induced little high molecular weight (HMW) aggregation (Fig. 6C ▶), the changes of eluting profiles shown in Figure 6 ▶, A and B, were caused by aggregation of WT or T5P γC-crystallin. The results also show that αA-crystallin prevented aggregation of T5P mutant more effectively than aggregation of WT γC-crystallin.
Figure 6.
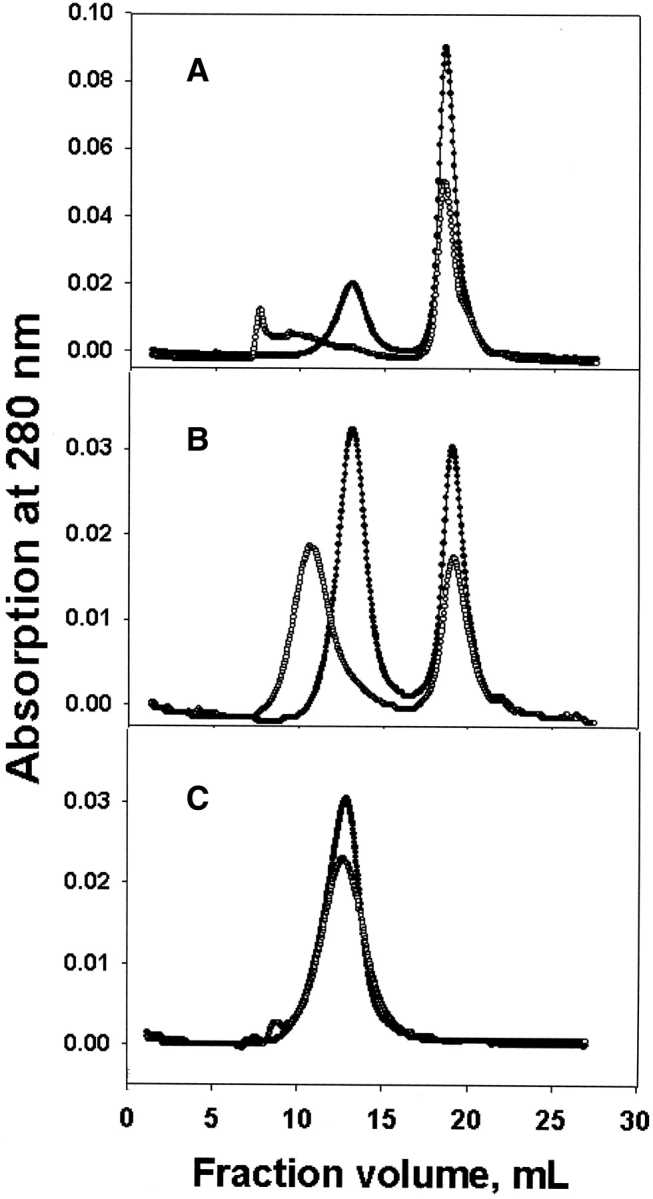
FPLC size exclusion chromatography of WT αA- and γC-crystallin samples. (A) αA- and WT γC-crystallin mixtures; (B) αA- and T5P γC-crystallin mixtures; (C) αA-crystallin alone. Controls, solid circles; preheated samples, open circles.
Discussion
We used W9F αA-crystallin in some experiments to take advantage of the fact that it lacks Trp residue, so that any change in Trp fluorescence would be attributed to substrate proteins (WT or T5P γC-crystallin). In other experiments, such as FPLC, we used WT αA-crystallin because absorption at 280 nm was very low for W9F αA-crystallin mutant. The W9F αA-crystallin mutant has been reported to be similar to WT αA-crystallin regarding some biophysical properties, such as size, conformation, and chaperone activity (Andley et al. 1996; Sun et al. 1998).
FRET results suggest that the extent of interactions between αA- and γC-crystallins was low, which agrees with the results of a previous FRET study (Bova et al. 1997) and two-hybrid system assays (Fu and Liang 2002b, c). The small extent of interactions between αA- and γC-crystallin may not be due to subunit exchanges, as were observed for the αA-αA or αA-αB interactions (Bova et al. 1997; Sun and Liang 1998; Sun et al. 1998). Rather, they are likely nonspecific hydrophobic interactions. The partial unfolding of T5P γC-crystallin exposed some hydrophobic surfaces and increased interactions with αA-crystallin. Our previous studies indicate that T5P γC-crystallin mutant has undergone a conformational change, possibly a partial unfolding (Fu and Liang 2002a). It is reasonable to expect that αA-crystallin interacts strongly with the partially unfolded T5P γC-crystallin. However, only slightly greater interactions were detected compared to WT γC-crystallin, indicating that interactions were not due to chaperone binding of αA-crystallin with a partially unfolded protein. αA-crystallin prevented heat-induced aggregation for T5P γC-crystallin as effectively as for WT γC-crystallin. Unfolding by mutation is different from heat-induced unfolding and cannot be recognized by chaperone. A difference in partial unfolding by the two mechanisms was revealed by Trp fluorescence; the GdnHCl-, but not heat-induced unfolding showed emission maximum shifting to longer wavelengths (Fig. 4A,B ▶). Another difference between the heat and GdnHCl treatments is that only the heat treatment induces aggregation. It appears that αA-crystallin chaperone recognizes only substrate proteins that are undergoing both partial unfolding and aggregation. The FPLC gel filtration results (Fig. 6 ▶) clearly indicate that αA-crystallin bound to aggregating γC-crystallin and prevented it from further aggregation or precipitation. The extent to which aggregation was suppressed was greater for T5P than for WT γC-crystallin, possibly because of greater interactions between αA- and T5P γC-crystallin than between αA- and WT γC-crystallin (Fig. 1 ▶).
The observed changes in Trp fluorescence of preheated mixtures of W9F αA- and γC-crystallin should arise from partial unfolding of γC-crystallin, because W9F αA-crystallin contributes little fluorescence (Sun et al. 1998). The partial unfolding is either rather mild or occurring in its early stages. This is consistent with a previous report (Das et al. 1996). However, this does not exclude the possibility of a conformational change in αA-crystallin due to chaperone binding. Heating WT αA-crystallin alone or in mixtures with γC-crystallin showed a similar increase of fluorescence intensity without shifting in wavelength, indicating little change in Trp environments due to chaperone binding in αA- or γC-crystallin. It has been reported that Trp emission maximum reflects the hydrophobicity surrounding the Trp residue (Andley et al. 1982; Lakowicz 1983). This is in agreement with near-UV CD data (Fig. 5A,B ▶), in which the negative vibronic bands at 290 nm from Trp transition were not changed.
The partial unfolding should expose hydrophobic surfaces and promote hydrophobic interactions and facilitate aggregation or insolubilization if the concentration of the partially unfolded proteins is sufficiently high. This was observed in cloning of T5P γC-crystallin, in which the majority of protein was expressed in exclusion bodies, and limited solubility was observed upon reconstitution of the denaturated protein (Fu and Liang 2002a). Together with the limited capability of α-crystallin to bind the T5P mutant, it is no surprise that the T5P mutation causes lens opacity as seen in the Coppock-like cataract.
Materials and methods
Cloning of αA-crystallin, W9F αA-crystallin mutant, and γC-crystallin
Preparations of the recombinant wild-type αA-crystallin, W9Fα-crystallin mutant, and wild-type γC-crystallin are described elsewhere (Sun et al. 1997, 1998; Fu and Liang 2002a). Samples stored at −20°C were thawed and treated with 20 mM DTT and then dialyzed against 50 mM phosphate buffer at pH 7.6.
Protein concentrations were determined by measuring absorption at 280 nm with the following absorbance: A0.1% = 0.724 for WT and 0.422 for W9F αA-crystallin, and 2.14 for both WT and T5P γC-crystallin, calculated based on the amount of aromatic amino acids (Mach et al. 1992).
Interaction study with FRET
Interactions between αA- and γC-crystallin or T5P mutant were studied by FRET, using W9F αA-crystallin labeled with MIANS [2–(4′-maleimidylanilino) naphthalene-6-sulfonate] as an acceptor and Trp in γC-crystallin as a donor. MIANS is a sulfhydryl (SH)-specific fluorescent probe. αA-crystallin has one Trp and was substituted with Phe in the W9F mutant. The emission spectrum of Trp (320–350 nm with 295 nm excitation) overlaps with the absorption spectrum of MIANS (excitation and emission maxima at 322 nm and 420 nm, respectively). When interaction occurs, a decrease in Trp emission is observed with a concomitant increase in MIANS emission (Sun et al. 1998). The efficiency (E) of energy transfer between donor probe (Trp) and acceptor probe (MIANS) was determined by measuring the fluorescence intensity of the donor both in the absence (Fd) and presence (Fda) of the acceptor according to the equation E = 1 − (Fda / Fd). The efficiency is a function of the inverse sixth power of the distance between donor and acceptor (Lakowicz 1983). As the critical distance for energy transfer is in the range 20–50 Å and the size of the α-crystallin aggregate is 150–200 Å, the energy transfer efficiency is a sensitive method for detection of intermolecular interactions.
Chaperone-like activity measurements
Chaperone-like activity was measured with the time-dependent scattering measurements using a Shimadzu fluorometer. The fluorometer was set at 400 nm for both excitation and emission wavelength (at a 90° angle), and an increase in sensitive Rayleigh scattering was recorded at 65°C. WT γC-crystallin samples with increasing concentrations of αA-crystallin or W9F αA-crystallin mutant were used. Temperature was controlled with a Lauda RC-6 water bath (Brinkmann Instruments).
Thermal stability measurements
Thermal stability was studied by time-dependent change of Trp emission intensity at a wavelength of 340 nm (excitation at 295 nm) at 65°C.
Spectroscopic measurements
Based on chaperone-like activity and thermal stability measurements, crystallin samples, either individually or in mixtures of γC-and αA-crystallin heated at 65°C for 1 h, should unfold γC-crystallin and induce chaperone binding. Therefore, to detect conformational changes due to chaperone binding, the samples were heated at 65°C for 1 h and then cooled to room temperature.
CD spectra were obtained with an Aviv Circular Dichroism Spectrometer (model 60 DS, Aviv Associates). Five scans were recorded, averaged, and followed by a polynomial-fitting program. The CD was expressed in millidegrees rather than molar ellipticity, because protein mixtures were measured.
Fluorescence was measured with a Shimadzu spectrofluorometer (model RF-5301PC, Shimadzu Instruments). Trp emission was scanned with an excitation wavelength at 295 nm.
FPLC size exclusion chromatography was performed with a Superose-6 column. Samples of controls or preheated mixtures of αA- and WT or T5P γC-crystallin were applied to the column and eluted at the rate of 0.5 mL/min.
Acknowledgments
This work was supported by grants from the NIH (EY05803 and EY13968) and the Massachusetts Lions Eye Research Fund. Technical help from Michael Liang is acknowledged.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Abbreviations
UV CD, ultraviolet circular dichroism
FRET, fluorescence resonance energy transfer
MIANS, 2–(4′-maleimidylanilino) naphthalene-6-sulfonate.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.04815104.
References
- Andley, U.P., Liang, J.N., and Chakrabarti, B. 1982. Spectroscopic studies of lens crystallins. II. Fluorescence probes for polar-apolar nature and sulfhydryl group accessibility. Biochemistry 21 1853–1857. [DOI] [PubMed] [Google Scholar]
- Andley, U.P., Mathur, S., Griest, T.A., and Petrash, J.M. 1996. Cloning, expression, and chaperone-like activity of human α-crystallin. J. Biol. Chem. 271 31973–31980. [DOI] [PubMed] [Google Scholar]
- Berry, V., Francis, P., Reddy, M.A., Collyer, D., Vithana, E., MacKay, I., Dawson, G., Carey, A.H., Moore, A., Bhattacharya, S.S., et al. 2001. αB crystallin gene (CRYAB) mutation causes dominant congenital posterior polar cataract in humans. Am. J. Hum. Genet. 69 1141–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bova, M.P., Ding, L.L., Horwitz, J., and Fung, B.K. 1997. Subunit exchange ofαA-crystallin. J. Biol. Chem. 272 29511–29517. [DOI] [PubMed] [Google Scholar]
- Das, K.P., Petrash, J.M., and Surewicz, W.K. 1996. Conformational properties of substrate proteins bound to a molecular chaperone α-crystallin. J. Biol. Chem. 271 10449–10452. [DOI] [PubMed] [Google Scholar]
- Farahbakhsh, Z.T., Huang, Q.-L., Ding, L.-L., Altenbach, C., Steinhoff, H.J., Horwitz, J., and Hubbell, W.L. 1995. Interaction of α-crystallin with spin-labeled peptides. Biochemistry 34 509–516. [DOI] [PubMed] [Google Scholar]
- Fu, L. and Liang, J.J.N. 2002a. Conformational change and destabilization of cataract γC-crystallin T5P mutant. FEBS Lett. 513 213–216. [DOI] [PubMed] [Google Scholar]
- ———. 2002b. Alteration of protein-protein interactions of cataract crystallin gene mutants. Invest. Ophthalmol. Vis. Sci. 44 1155–1159. [DOI] [PubMed] [Google Scholar]
- ———. 2002c. Detection of protein-protein interactions among lens crystallins in a mammalian two-hybrid system assay. J. Biol. Chem. 277 4255–4260. [DOI] [PubMed] [Google Scholar]
- Gill, D., Klose, R., Munier, F.L., McFadden, M., Priston, M., Billingsley, G., Ducrey, N., Schorderet, D.F., and Heon, E. 2000. Genetic heterogeneity of the Coppock-like cataract: A mutation in CRYBB2 on chromosome 22q11.2. Invest. Ophthalmol. Vis. Sci. 41 159–165. [PubMed] [Google Scholar]
- Heon, E., Priston, M., Schorderet, D.F., Billingsley, G.D., Girard, P.O., Lubsen, N., and Munier, F.L. 1999. The γ-crystallins and human cataracts: A puzzle made clearer. Am J. Hum. Genet. 65 1261–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz, J. 1992. α-Crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. 89 10449–10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz, J.R. 1983. Principles of fluorescence spectroscopy. Plenum Press, New York.
- Litt, M., Carrero-Valenzuela, R., LaMorticella, D.M., Schultz, D.W., Mitchell, T.N., Kramer, P., and Maumenee, I.H. 1997. Autosomal dominant cerulean cataract is associated with a chain termination mutation in the human β-crystallin gene CRYBB2. Hum. Mol. Genet. 6 665–668. [DOI] [PubMed] [Google Scholar]
- Litt, M., Kramer, P., LaMorticella, D.M., Murphey, W., Lovrien, E.W., and Weleber, R.G. 1998. Autosomal dominant congenital cataract associated with a missense mutation in the human α-crystallin gene CRYAA. Hum. Mol. Genet. 7 471–474. [DOI] [PubMed] [Google Scholar]
- Mach, H., Middaugh, C.R., and Lewis, R.V. 1992. Statistical determination of the average values of the extinction coefficients of tryptophan and tyrosine in native proteins. Anal. Biochem. 200 74–80. [DOI] [PubMed] [Google Scholar]
- Santhiya, S.T, Shyam Manohar, M., Rawlley, D., Vijayalakshmi, P., Namperumalsamy, P., Gopinath, P.M., Loster, J., and Graw, J. 2002. Novel mutations in the γ-crystallin genes cause autosomal dominant congenital cataracts. J. Med. Genet. 39 352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, T.-X. and Liang, J.J.N. 1998. Intermolecular exchange and stabilization of human lens recombinant αA- and αB-crystallins. J. Biol. Chem. 273 286–290. [DOI] [PubMed] [Google Scholar]
- Sun, T.-X., Das, B.K., and Liang, J.J.N. 1997. Conformational and functional differences between recombinant human lens αA- and αB-crystallin. J. Biol. Chem. 272 6220–6225. [DOI] [PubMed] [Google Scholar]
- Sun, T.-X., Akhtar, N.J., and Liang, J.J.N. 1998. Subunit exchange of lens α-crystallin: A fluorescence energy transfer study with the fluorescent labeled αA-crystallin mutant W9F as a probe. FEBS Lett. 430 401–404. [DOI] [PubMed] [Google Scholar]
- Vicart, P., Caron, A., Guicheney, P., Li, Z., Prevost, M.C., Faure, A., Chateau, D., Chapon, F., Tome, F., Dupret, J.M., et al. 1998. A missense mutation in the αB-crystallin chaperone gene causes a desmin-related myopathy. Nat. Genet. 20 92–95. [DOI] [PubMed] [Google Scholar]