Abstract
The pyridoxal 5′-phosphate-dependent enzymes tyrosine phenol-lyase and tryptophan indole-lyase were encapsulated in wet nanoporous silica gels, a powerful method to selectively stabilize tertiary and quaternary protein conformations and to develop bioreactors and biosensors. A comparison of the enzyme reactivity in silica gels and in solution was carried out by determining equilibrium and kinetic parameters, exploiting the distinct spectral properties of catalytic intermediates and reaction products. The encapsulated enzymes exhibit altered distributions of ketoenamine and enolimine tautomers, increased values of inhibitors dissociation constants, slow attaining of steady-state in the presence of substrate and substrate analogs, modified steady-state distribution of catalytic intermediates, and a sixfold–eightfold decrease of specific activities. This behavior can be rationalized by a reduced conformational flexibility for the encapsulated enzymes and a selective stabilization of either the open (inactive) or the closed (active) form of the enzymes. Despite very similar structures and catalytic mechanisms, the influence of encapsulation is more pronounced for tyrosine phenol-lyase than tryptophan indole-lyase. This finding indicates that subtle structural and dynamic differences can lead to distinct interactions of the protein with the gel matrix.
Keywords: protein immobilization, pyridoxal 5′-phosphate, catalysis, silica gels, conformational selection
The development of new materials in which biomolecules are integrated in either inorganic or organic matrices is a current biotechnological challenge. A particularly powerful approach, pioneered almost 30 years ago (Johnson and Whateley 1971), but only recently fully exploited (Gill and Ballesteros 2000; Gill 2001; Mozzarelli and Bettati 2001; Jin and Brennan 2002; Bettati et al. 2003) is protein encapsulation via the silica sol-gel method (Brinker and Scherer 1990). Silica gel encapsulation usually maintains the biological activity of proteins (Ellerby et al. 1992) and generally increases their stability (Eggers and Valentine 2001). Therefore, by making use of a large variety of composite biomaterials (Gill and Ballesteros 2000; Bettati et al. 2003), several systems have been developed tailored to the production of (1) bioreactors for the synthesis of specific compounds, (2) biosensors for the detection of analytes, (3) photoaddressable electronic devices, (4) nanocapsules for the controlled release of protein drugs, and (5) biocompatible surface coating. Silica gel encapsulation has also recently been used to trap and characterize conformational states of monomeric and allosteric proteins, as myoglobin (Gottfried et al. 1999; Abbruzzetti et al. 2001), green fluorescent protein (Chirico et al. 2002), hemoglobin (Shibayama and Saigo 1995; Bettati and Mozzarelli 1997; Juszczak and Friedman 1999; Bruno et al. 2001), fructose-1-6-bisphosphatase (McIninch and Kantrowitz 2001), and aspartate transcarbamoylase (West and Kantrowitz 2003). Several studies on encapsulated enzymes comparing their catalytic efficiency with respect to the soluble form found a significant reduction (Gill and Ballesteros 1998; Jin and Brennan 2002; Besanger et al. 2003; Bettati et al. 2003). However, a careful examination of the influence of silica gel entrapment on the individual steps of an enzyme-catalyzed reaction has not yet been attempted.
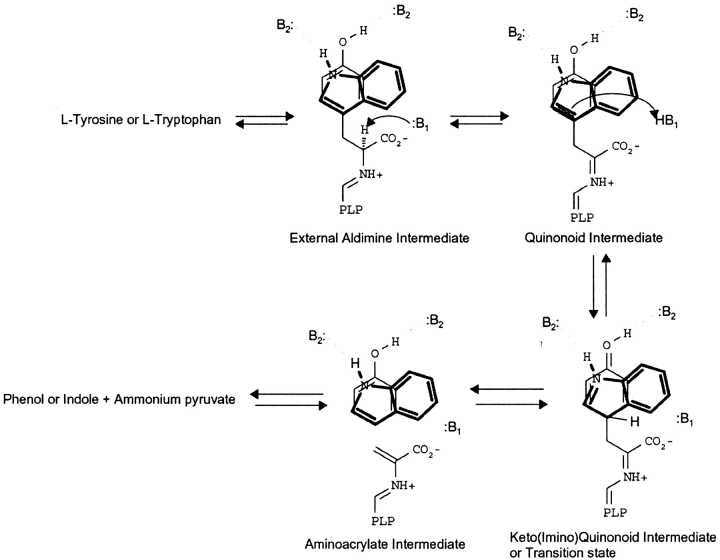
Tyrosine phenol-lyase (TPL) and tryptophan indole-lyase (Trpase) are pyridoxal 5′-phosphate-dependent enzymes that catalyze the β-elimination reaction of L-tyrosine and L-tryptophan, respectively, to form pyruvate, ammonium, and either phenol or indole. TPL and TRPase follow very similar catalytic mechanisms (see Scheme 1) with formation of intermediates absorbing at distinct wavelengths: the external aldimine at 420 nm, a quinonoid species at 505 nm, and the α-aminoacrylate Schiff base at 340 nm. Both enzymes exhibit a broad substrate specificity, acting on a wide range of amino acids with suitable leaving groups on the β-carbon, S-(o-nitrophenyl)-L-cysteine (Suelter et al. 1976), S-(alkyl)-L-cysteine (Kumagai et al. 1970), β-chloro-L-alanine (Chen and Phillips 1993), L-serine (Kumagai et al. 1970), and O-acetyl-L-serine (Phillips 1987). TPL is inhibited by several amino acids and amino acid analogs, including L-alanine, L-phenylalanine (Chen and Phillips 1993), and L-methionine (Chen et al. 1995a), whereas Trpase is inhibited by oxindolyl-L-alanine (Kiick and Phillips 1988). The reaction with these inhibitors forms an equilibrating mixture of external aldimine and quinonoid species. The three-dimensional structures of TPL from Citrobacter freundii (Antson et al. 1993) and Trpase from Proteus vulgaris (Isupov et al. 1998) were solved, revealing a close arrangement of active site residues. However, despite a high structural and functional similarity, in vivo TPL and Trpase are extremely specific for their respective physiological substrates. The molecular basis of this behavior has been investigated by site-directed mutagenesis (Phillips et al. 2003). The transition between open (inactive) and closed (active) states of these enzymes accompanies substrate binding and the catalytic cycle (Demidkina et al. 2002; Phillips et al. 2003), as also observed in other PLP-dependent enzymes belonging to the α- (Schirch et al. 1991; McPhalen et al. 1992) and β-functional family (Schneider et al. 1998; Burkhard et al. 1999).
In the present study, TPL and Trpase were encapsulated in wet nanoporous silica gels using tetramethyl orthosilicate as a precursor (Ellerby et al. 1992), and, by taking advantage of the distinct spectral properties of the PLP-enzyme complexes, a comparison of both thermodynamic and kinetic properties of these enzymes in solution and in silica gels was carried out.
Results
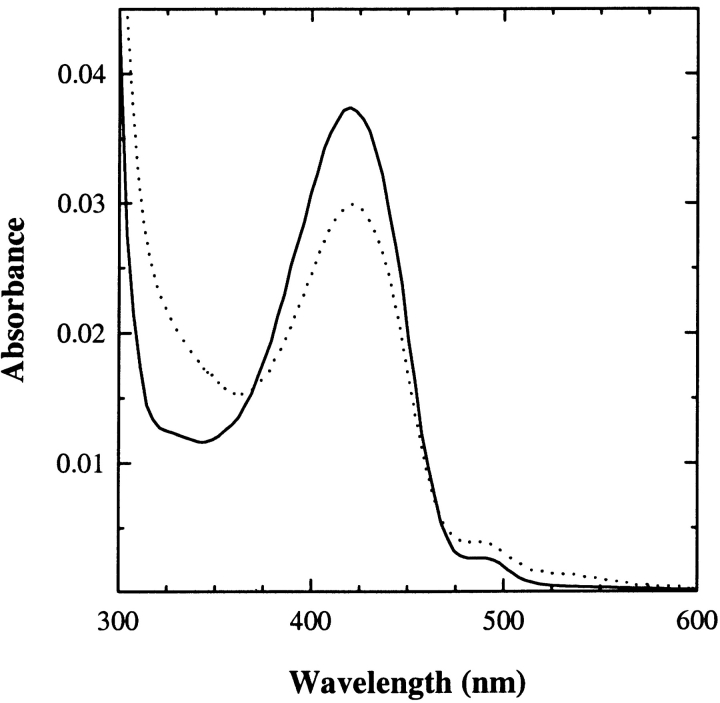
Tyrosine phenol-lyase from Citrobacter freundii
The absorption spectrum of TPL-doped silica gels shows two major bands at 420 and 330 nm, as in solution (Fig. 1 ▶). These bands have been attributed to the ketoenamine and enolimine tautomers of the internal aldimine, respectively (Bazhulina et al. 2000), the ketoenamine tautomer being more favored in a polar active site environment (Faeder and Hammes 1971). The absorbance ratio at 278 and 420 nm is 9.6 in solution and 11.8 in the gel. The relatively modest decrease of the absorbance at 420 nm in the gel is paralleled by a quantitatively comparable increase of the band around 340 nm. This result indicates that no significant denaturation of TPL took place upon encapsulation, whereas the tautomer distribution in the gel is altered, with the ketoenamine tautomer being less populated than in solution. The origin and the functional relevance of these changes were addressed by characterizing the reactivity of TPL-doped gels towards inhibitors and substrate analogs. Titrations of TPL-doped silica gels with L-phenylalanine (Fig. 2A ▶), L-methionine (Fig. 2B ▶), and L-alanine (Fig. 2C ▶) lead to the formation of a sharp band around 500 nm and to the concomitant decrease of the absorption band of the internal aldimine at 420 nm. The shape and position of the 500 nm band are similar to those attributed to the quinonoid intermediate in solution (Chen and Phillips 1993), thus indicating that the silica matrix does not prevent the formation of this key catalytic species. However, the amount of quinonoid species formed in the gel with respect to solution is about twofold less, as indicated by the ratios of absorbance at 420 and 500 nm (data not shown), suggesting a redistribution of catalytic intermediates. The dissociation constants of inhibitors for TPL gels are 1.2–6.6-fold higher than those determined in solution (Table 1), indicating that binding of ligands requires a moderately increased energetic cost, likely due to constraints imposed by the silica matrix on protein dynamics.
Figure 1.
Absorption spectrum of the internal aldimine of TPL-doped silica gels (solid line) and TPL in solution (dotted line), 50 mM potassium phosphate (pH 7.0) at 25°C. Spectra were normalized for the absorption intensity at 280 nm.
Figure 2.
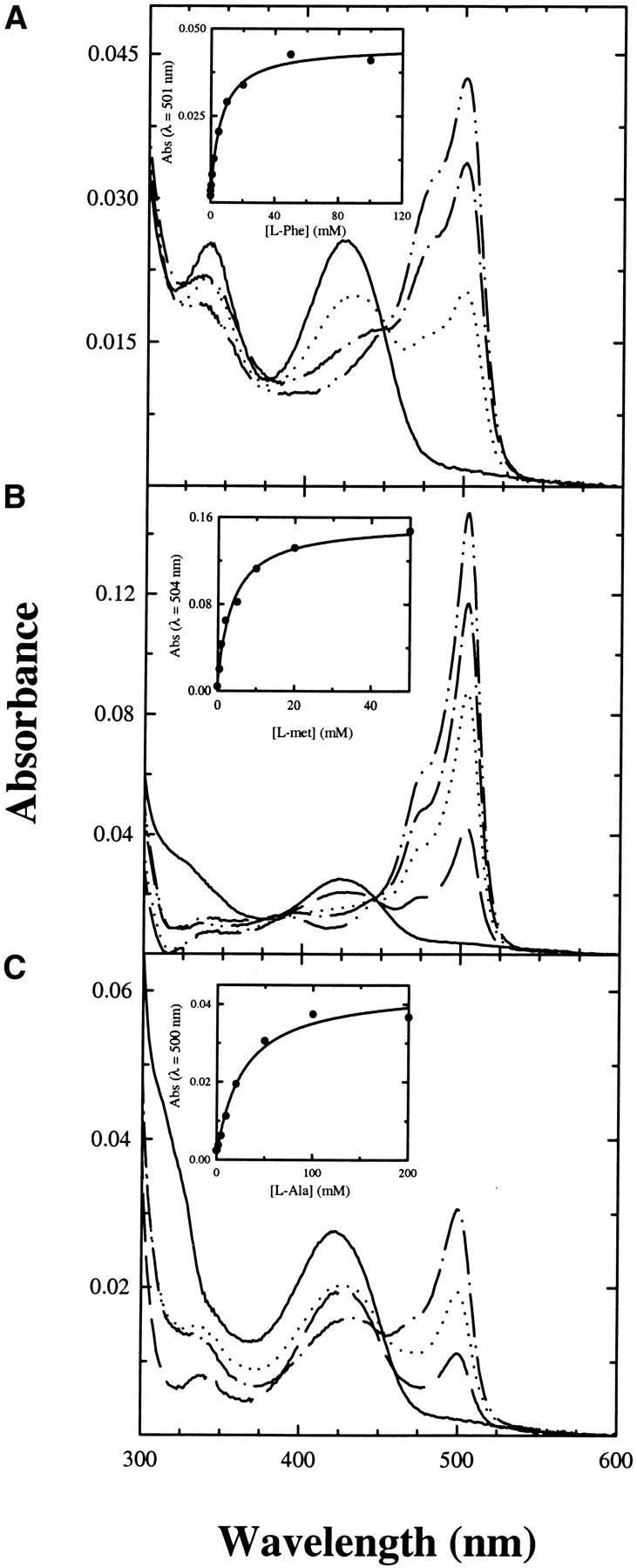
Absorption spectra of TPL-doped gels in the presence of increasing concentrations of L-phenylalanine (A), L-methionine (B), and L-alanine (C), 50 mM potassium phosphate (pH 7.5) at 25°C. (Insets) Absorbance change of the quinonoid band as a function of inhibitor concentration. Data points were fitted to a single binding isotherm (solid line).
Table 1.
Dissociation constants for ligands of Citrobacter freundii TPL and Proteus vulgaris Trpase in silica gels and in solution
| TPL-doped gels | Soluble TPL | Trpase-doped gels | Soluble Trpase | |
| KdissL-phe (mM) | 6.26 ± 0.70 | 1.26 ± 0.20 | ||
| KdissL-met (mM) | 3.83 ± 0.74 | 0.58 ± 0.03 | ||
| KdissL-ala (mM) | 26.83 ± 5.63 | 23.34 ± 0.49 | ||
| KdissOIA (mM) | 0.020 ± 0.003 | 0.014 ± 0.001 |
The catalytic competence of TPL-doped silica gels in the β-elimination reaction was evaluated by investigating the reaction with the substrate analogs S-Me-Cys and 3-F-Tyr. The reaction with S-Me-Cys leads to the slow disappearance of the internal aldimine, absorbing at 420 nm, and the concomitant formation of a quinonoid species absorbing at 500 nm (Fig. 3A ▶). The amount of quinonoid species at plateau is less than that observed in solution (Fig. 3C ▶), indicating that encapsulation has altered the steady-state distribution of intermediates. The rate of the reaction, monitored at either 500 or 420 nm, is significantly lower (Fig. 3A ▶, inset) than that observed in solution, where the steady-state is reached within the mixing time. This might be due both to a reduction in protein flexibility, and a limitation to the diffusion of the reagents. The average pore size of the silica matrix is 4–5 nm, allowing free movement of low-molecular-weight species within the gel (Bettati et al. 2003). However, a gel thickness of the order of 0.5 mm can slow down the equilibration of the enzyme sites with the external medium. The same reaction kinetics, monitored at 340 nm, the absorption peak of the enolimine tautomer of the aminoacrylate species (Kallen et al. 1985), exhibits a linear increase of absorption intensity (Fig. 3A ▶, inset). As suggested by the lack of a clear isosbestic point in the spectra collected at different times (data not shown), more than one species is concomitantly forming. On the basis of the reaction mechanism (Scheme 1), the quinonoid species is in equilibrium with the aminoacrylate Schiff base. Furthermore, the aminoacrylate is constantly converted to the final products pyruvate and ammonia, the former species absorbing at 318 nm. The amount of pyruvate produced as a function of time was evaluated via the lactate dehydrogenase assay. At the time by which the absorbance at 500 nm reaches the maximal value (30 min; see Fig. 3A ▶, inset), the spectrum of TPL gels reacted with S-Me-cys, corrected for pyruvate absorption (Fig. 3A ▶), indicates that the aminoacrylate intermediate is the predominant species, whereas in solution the predominant species is the quinonoid intermediate (Fig. 3C ▶). When 4-OH-pyr, an uncompetitive inhibitor that in solution stabilizes the aminoacrylate species (Fig. 3C ▶), is concomitantly added with S-Me-Cys to TPL-doped gels (Fig. 3B ▶), the peak at about 340 nm is higher and the amount of quinonoid species is lower. The spectrum obtained after subtraction of pyruvate absorption indicates that 4-OH-pyr leads to an increase of the steady-state concentration of the aminoacrylate intermediate (Fig. 3B ▶), as in solution (Fig. 3C ▶). Similar results were obtained in the reaction of TPL-doped silica gels with saturating concentrations of the substrate analog 3-F-Tyr in the absence and presence of 4-OH-pyr (data not shown).
Figure 3.
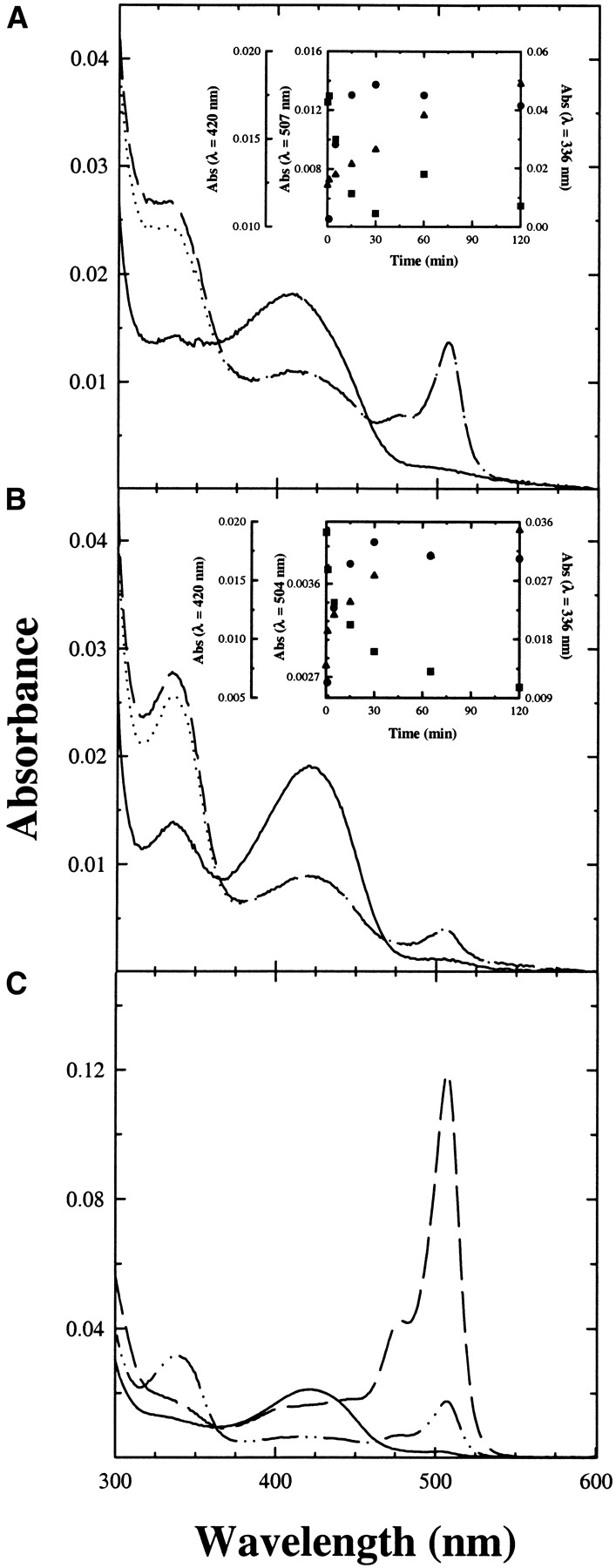
Reactivity of TPL-doped gel and TPL in solution with S-Me-Cys. Absorption spectra of TPL-doped gels were recorded in a solution containing 50 mM potassium phosphate (pH 7.5) at 25°C, (A) in the absence (solid line) and presence of 20 mM S-Me-Cys after 30 min of reaction (dashed line) and after subtraction of pyruvate absorbance (dotted line), (B) in the absence (solid line) and presence of 20 mM S-Me-Cys and 5 mM 4-OH-pyr, after 30 min of reaction (dashed line) and after subtraction of pyruvate absorbance (dotted line). Pyruvate concentration was evaluated by a coupled lactate dehydrogenase assay (see Materials and Methods). Insets: Absorbance changes as a function of time at λ = 420 nm (▪), 507 nm (•, A), 504 nm (•, B), and 336 nm (▴). (C) Absorption spectra of soluble TPL in 50 mM potassium phosphate (pH 7.5) at 25°C in the absence (solid line), and presence of 20 mM S-Me-Cys (dashed line) and 20 mM S-Me-Cys and 5 mM 4-OH-pyr (dashed/dotted line). No subtraction for pyruvate absorbance was carried out because spectra were recorded immediately upon mixing.
Tryptophan indole-lyase from Proteus vulgaris
The absorption spectrum of Trpase encapsulated in silica gels and in solution (Fig. 4 ▶) exhibits a major band at 422 nm, attributed to the ketoenamine tautomer of the internal aldimine, and minor bands at about 340 and 490 nm. The latter absorbing species is attributed to tightly bound ligands present in cell extracts (Zakormirdina et al. 2002). Moreover, the ratio of absorption intensity at 422 and 340 nm has been observed to vary as a function of aging for both the soluble and the encapsulated enzyme. Differently from TPL, Trpase exhibits a pH-dependent change of absorbance at 422 and 340 nm. For other PLP-dependent enzymes, such as aspartate aminotransferase and 1-aminocylopropane-1-carboxylate synthase, the species favored at high pH is attributed to the deprotonated coenzyme (Eliot and Kirsch 2002). On the contrary, for Trpase, it has been proposed to be a substituted aldamine (Metzler et al. 1991; Ikushiro et al. 1998). However, it should be noted that the pH-dependent spectral changes do not show a perfect isosbestic point, suggesting the possible presence of different equilibria, including a pH-dependent distribution of the ketoenamine-enolimine tautomers. For the Escherichia coli Trpase, the apparent pKa of the transition was found to be 7.6 (Metzler et al. 1991; Ikushiro et al. 1998) and was attributed to an ionizable residue of the protein (Metzler et al. 1991; Ikushiro et al. 1998). In order to determine whether the observed differences between soluble and encapsulated P. vulgaris enzyme were due to altered pH dependence, a titration was carried out in both physical states (Fig. 5 ▶). The pH dependence of the enzyme is described by a single pKa of 8.00 ± 0.17 in silica gels (Fig. 5A ▶, inset) and 8.18 ± 0.04 in solution (Fig. 5B ▶, inset). Such close values do not explain the difference in the distribution of species absorbing at 422 and 340 nm between enzyme-doped silica gels and solution. Thus, the increase in the absorption intensity at 422 nm should be ascribed to a stabilization of the ketoenamine tautomer in silica gel with respect to solution, an opposite behavior with respect to that observed for TPL. Stabilization of the ketoenamine tautomer of Trpase was also observed in the crystalline enzyme (Isupov et al. 1998). Control experiments carried out on E. coli Trpase-doped gels (data not shown) showed a stabilization of the enolimine tautomer with respect to solution (Metzler et al. 1991; Ikushiro et al. 1998) and a pH dependence of the internal aldimine with a pKa of 7.8. The decrease in the absorption at 422 nm as a function of pH is not associated with any increase in the absorption at 340 nm, in sharp contrast with that observed in solution (Metzler et al. 1991; Ikushiro et al. 1998).
Figure 4.
Absorption spectrum of the internal aldimine of Trpase-doped silica gels (solid line) and Trpase in solution (dotted line), 50 mM potassium phosphate (pH 7.0) at 25°C. Spectra were normalized for the absorption intensity at 280 nm.
Figure 5.
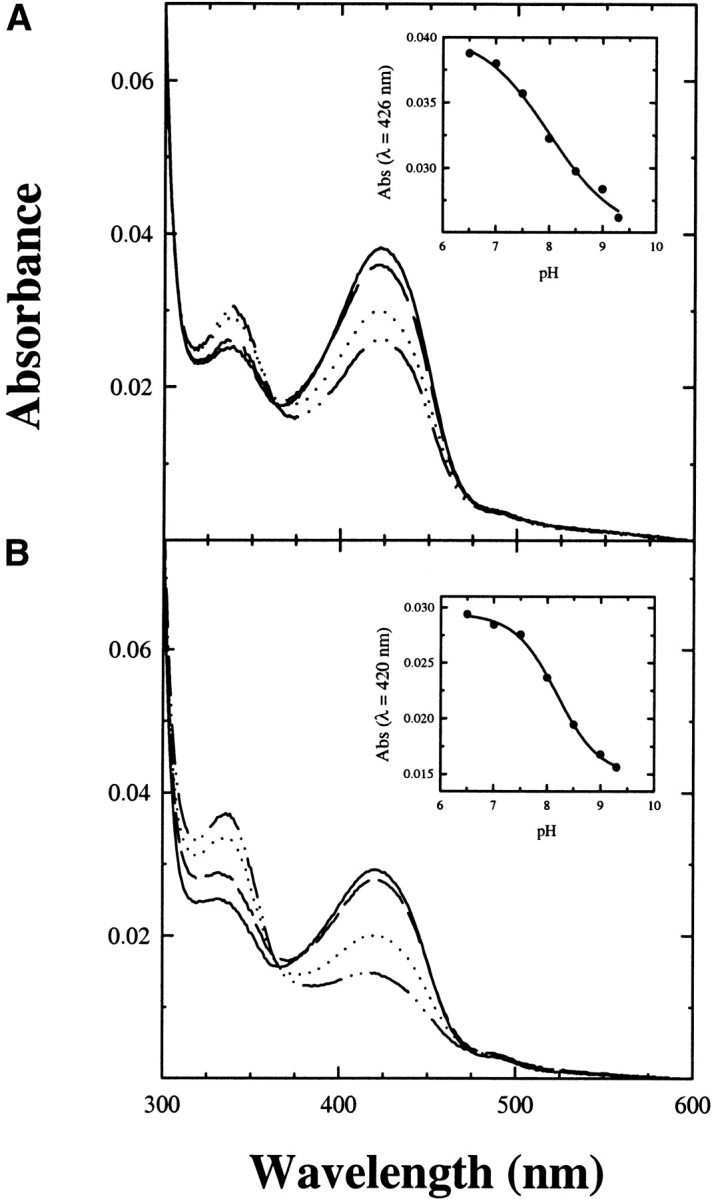
Absorption spectra of Trpase-doped silica gels and Trpase in solution as a function of pH. Enzyme-doped silica gels (A) and the soluble form (B) were exposed to solutions containing 50 mM potassium phosphate in the pH range 6.5–9.3. Absorption spectra at pH 6.5 (solid line), 7.5 (dashed line), 8.5 (dotted line), and 9.3 (dashed/dotted line) are reported. Insets: (A) Spectral changes at 426 nm as a function of pH were analyzed according to the equation for a single ionizable residue with pKa of 8.00 ± 0.17. (B) Spectral changes at 420 nm as a function of pH were analyzed according to the equation for a single ionizable residue with pKa of 8.18 ± 0.04. Silica gels cannot be exposed to higher pH values because siloxane bonds hydrolyze (Bettati et al. 2003).
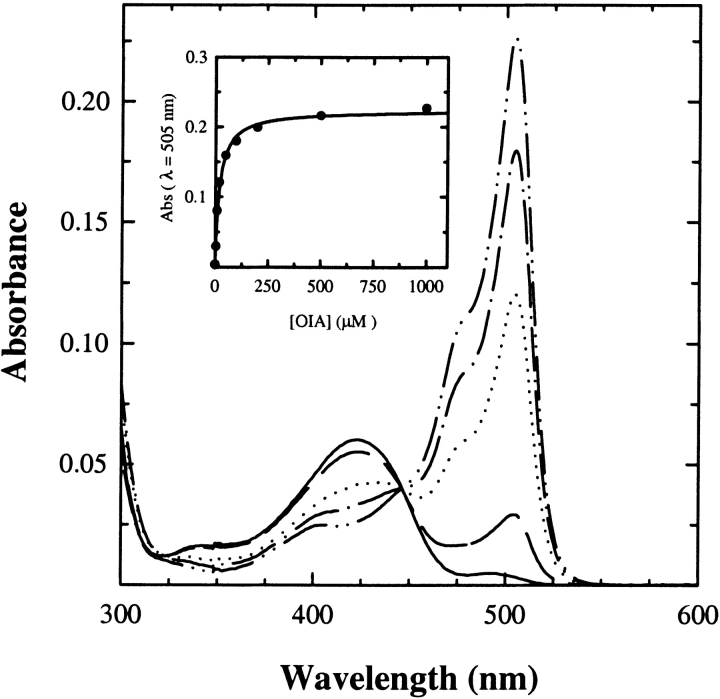
The reactivity of P. vulgaris Trpase-doped gels was characterized by investigating the reaction with inhibitors, substrate, and substrate analogs. The reaction of protein silica gels with oxindolyl-L-Ala (OIA), an enzyme inhibitor, results in the intense absorption peak of a quinonoid species at 505 nm (Fig. 6 ▶). The titration as a function of OIA concentration is well fitted by a binding isotherm with a dissociation constant of 20.0 ± 0.3 μM (Fig. 6 ▶, inset), very close to the value determined in solution (Table 1). For E. coli Trpase-doped gels, a dissociation constant of 73 μM was found (data not shown), about 10-fold higher than that measured in solution, 2.5–6 μM (Phillips et al. 1984; Kiick and Phillips 1988).
Figure 6.
Absorption spectra of Trpase-doped gels as a function of increasing concentrations of OIA. The titration was carried out in a solution containing 50 mM potassium phosphate (pH 7.5) at 25°C. (Inset) The absorbance change at λ = 505 nm as a function of OIA concentration was fitted to a binding isotherm (solid line).
The reaction of P. vulgaris Trpase-doped silica gels with the nonphysiological substrate L-serine was investigated in the absence (Fig. 7A ▶) and presence (Fig. 7B ▶) of benzimidazole, an uncompetitive inhibitor that was reported to stabilize the α-aminoacrylate intermediate of E. coli enzyme (Phillips 1991). An equilibrating mixture of the external aldimine (λmax = 420 nm) and the aminoacrylate intermediate (λmax = 350 nm) is slowly formed (Fig. 7A,B, ▶ inset). In solution this equilibrium is attained immediately upon mixing (Fig. 7C ▶). As observed for TPL, the slow attainment of equilibrium conditions might be due to the constraining effect of the gel matrix on protein dynamics as well as to slow ligand diffusion within the gel. The reaction leads also to the formation of pyruvate, as indicated by the linear increase of the absorbance at 340 nm (Fig. 7A,B, ▶ inset). Upon the subtraction of the contribution of pyruvate absorbance to the spectra of enzyme-doped gels (Fig. 7B ▶), it is evident that the amount of the aminoacrylate does not appreciably increase in the presence of benzimidazole, a behavior that parallels the reactivity of P. vulgaris Trpase in solution (Fig. 7C ▶), where benzimidazole only slightly increases the amount of aminoacrylate. In the gel, as in solution (Phillips 1991), benzimidazole affects the distribution of intermediates for the E. coli enzyme (data not shown). This finding indicates that there are subtle differences in the active-site geometry between E. coli and P. vulgaris Trpase.
Figure 7.
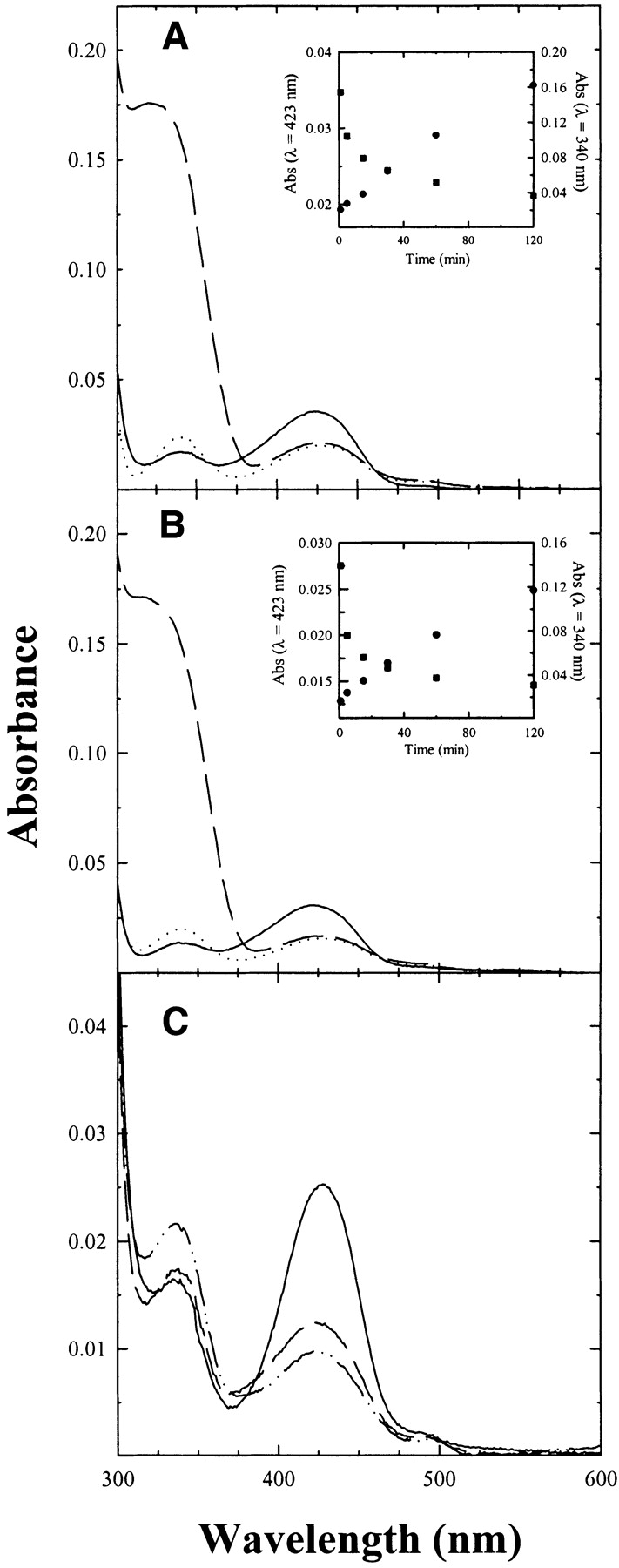
Reactivity of Trpase-doped silica gels and Trpase in solution with L-serine. Absorption spectra of Trpase-doped gels were recorded in a solution containing 50 mM potassium phosphate (pH 7.5) at 25°C, (A) in the absence (solid line) and presence of 0.5 M L-serine after 120 min of reaction (dashed line), and after subtraction of pyruvate absorbance (dotted line), (B) in the absence (solid line) and presence of 0.5 M L-serine and 5 mM benzimidazole, after 120 min of reaction (dashed line), and after subtraction of pyruvate absorbance (dotted line). Pyruvate concentration was evaluated by a coupled lactate dehydrogenase assay (see Materials and Methods). (Inset) Absorbance changes at 423 nm (▪) and 340 nm (•) as a function of time. (C) Absorption spectra of Trpase in a solution containing 50 mM potassium phosphate (pH 7.5) at 25°C in the absence (solid line) and presence of 0.5 M L-serine (dashed line) and 0.5 M L-serine and 5 mM benzimidazole (dashed/dotted line). Spectra were corrected for the contribution of pyruvate absorption.
Trpase-doped silica gels were also reacted with the natural substrate L-tryptophan. In the absence of benzimidazole, the amount of aminoacrylate in the gel (Fig. 8A ▶) is slightly higher that in solution. (Fig. 8C ▶). Similarly to the reaction with L-serine, results indicate that benzimidazole does not stabilize the aminoacrylate Schiff base (Fig. 8B ▶), as observed for the soluble enzyme (Fig. 8C ▶). This finding is, again, in contrast with the reactivity of the E. coli enzyme, in which binding of benzimidazole increases the amount of aminoacrylate in solution (Phillips 1991). The inability of benzimidazole to stabilize the aminoacrylate was also previously observed in the reaction of P. vulgaris Trpase crystals with L-tryptophan and, on the basis of the comparison with the reactivity of E. coli enzyme, erroneously attributed to lattice constraints (Phillips et al. 2002).
Figure 8.
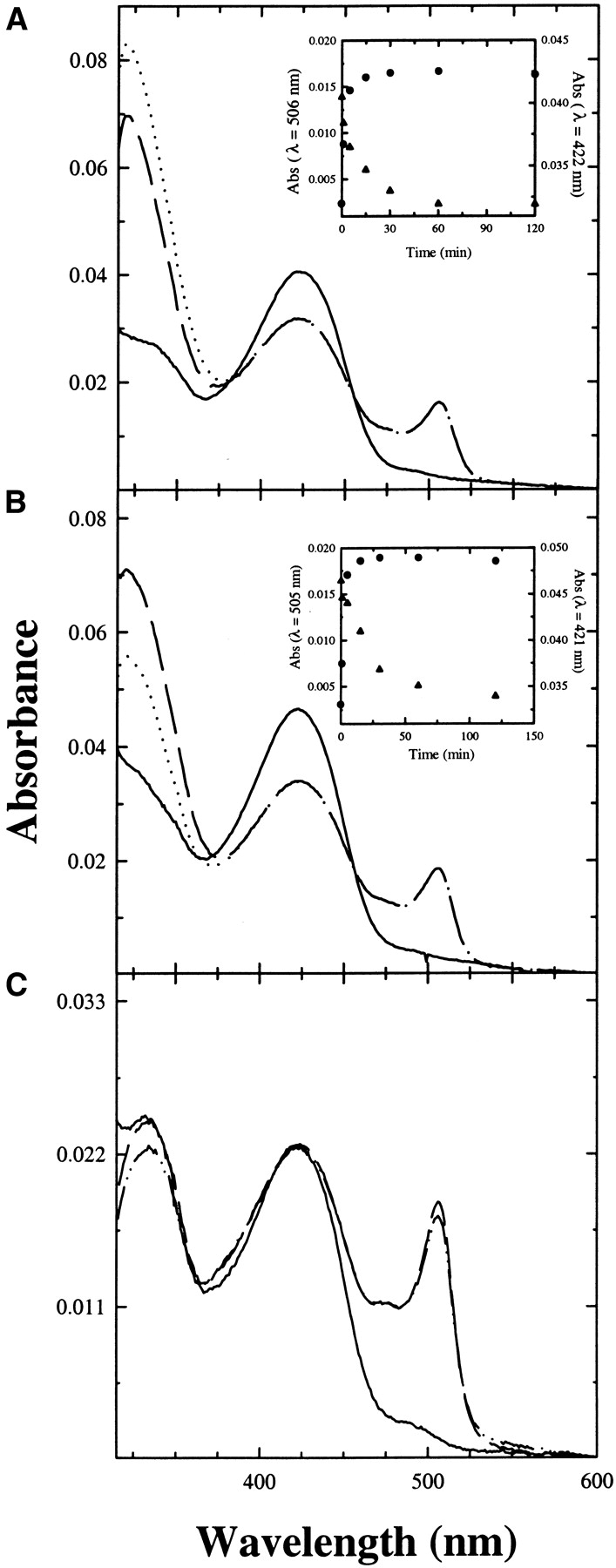
Reactivity of Trpase-doped gels and Trpase in solution with L-tryptophan. Absorption spectra of Trpase-doped gels were recorded in a solution containing 50 mM potassium phosphate (pH 7.5) at 25°C, (A) in the absence (solid line) and presence of 10 mM L-tryptophan after 120 min of reaction (dashed line), and after subtraction of pyruvate absorbance (dotted line), (B) in the absence (solid line) and presence of 10 mM L-tryptophan and 5 mM benzimidazole, after 120 min of reaction (dashed line), and after subtraction of pyruvate absorbance (dotted line). Pyruvate concentration was evaluated by a coupled lactate dehydrogenase assay (see Materials and Methods). Insets: (A) Absorbance changes at 506 nm (•) and 422 nm (▴) as a function of time; (B) absorption changes at 505 nm (•) and 421 nm (▴) as a function of time. (C) Absorption spectra of Trpase in a solution containing 50 mM potassium phosphate (pH 7.5) at 25°C in the absence (solid line), and presence of 10 mM L-tryptophan (dashed line) and 10 mM L-tryptophan and 5 mM benzimidazole (dashed/dotted line). Spectra were corrected for the contribution of pyruvate absorption.
TPL and Trpase activity assays in solution and in silica gel microsuspensions
The specific activity of encapsulated TPL and Trpase was determined on microsuspensions of enzyme-doped silica gels. Micrometer-size gels were obtained by a sonication procedure that does not affect enzyme activity. The size of the gel micro-fragments, about 2–4 μm, is such that the diffusion of substrates and products through the gel matrix is not rate-limiting the enzyme kinetics. In fact, the dc, that is, the fragment critical thickness over which rates are diffusion-controlled, was determined for each catalyzed reaction according to the equation (Ahmed et al. 1987)
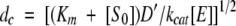 |
(1) |
where kcat and Km are the catalytic parameters obtained in solution for C. freundii TPL (Sundararaju et al. 1997) and P. vulgaris Trpase (Zakormirdina et al. 2002), E is the enzyme concentration expressed in mM, S0 is the substrate concentration expressed in mM, and D′ is the diffusion coefficient of the substrate inside the gel, calculated from the equation
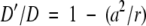 |
(2) |
where a is the average molecular radius (4 Å for molecules with molecular weights in the range 200–350 daltons), D (~6×10−6 cm2/sec) is the diffusion constant in water for substances with this range of molecular weights, and r is the average pore radius of the gel, 40–50 Å. The calculated values of dc, reported in Table 2, are significantly higher than the average gel thickness of 4 μm, indicating that diffusion of substrates within the gel microsuspensions cannot limit reaction rates. These values are obtained assuming that the negatively charged interior of the gel matrix does not affect the partitioning of the substrate between the solution phase and the gel matrix (Shen and Kostic 1997). At the pH of the assay, TPL and Trpase substrates exhibit only a moderately negative charge, and thus the effect, if any, is small.
Table 2.
Catalytic parameters of α-β elimination reactions catalyzed by Citrobacter freundii TPL and Proteus vulgaris Trpase, and critical thickness dc calculated according to Eq. 1
| TPLa | Trpaseb | |||
| Substrate | SOPC | S-Me-Cys | SOPC | S-Me-Cys |
| kcat (sec−1) | 9.7 | 0.9 | 33.4 | 4.1 |
| Km (mM) | 0.27 | 3.2 | 0.18 | 16 |
| dc (μM) | 27 | 548 | 13.8 | 480 |
The β-elimination activity for both enzymes was assayed using either the chromophoric substrate analog SOPC or S-Me-Cys. For the former substrate, the decrease of the concentration was directly monitored, whereas for the latter the formation of the final product pyruvate was monitored via the coupled lactate dehydrogenase assay. In both cases, the reaction was initiated by the addition of a small aliquot of the enzyme-doped gel microsuspensions to the assay mixture under continuous stirring. The specific activity of TPL gel microsuspensions was eightfold less than in solution for the reaction with SOPC, whereas no detectable activity was found for the slower reaction with S-Me-Cys (Table 3). The specific activity of Trpase gel microsuspensions was about eight- and sixfold lower than in solution for the reaction with SOPC and S-Me-Cys, respectively (Table 3).
Table 3.
Specific activities for α-β-elimination reaction catalyzed by TPL and Trpase encapsulated in silica gels and in solution
| Substrate | Soluble TPL (u/mg) | TPL-doped gel micro-suspension (u/mg) | Soluble Trpase (u/mg) | Trpase-doped gel micro-suspension (u/mg) |
| SOPC | 4.1 ± 0.1 | 0.49 ± 0.07 | 15.2 ± 0.5 | 1.8 ± 0.2 |
| S-Me-Cys | 0.21 ± 0.01 | close to 0 | 3.7 ± 0.5 | 0.62 ± 0.10 |
Discussion
Silica gel-encapsulated proteins generally maintain biological activity (Ellerby et al. 1992) and exhibit an increased stability (Eggers and Valentine 2001; Bettati et al. 2003). Therefore, the entrapment of enzymes appears to be a particularly promising method for the development of robust and efficient bioreactors and biosensors (Gill and Ballesteros 1998; Mozzarelli and Bettati 2001; Jin and Brennan 2002; Bettati et al. 2003). TPL and Trpase encapsulated in silica gels are able to catalyze the α-β-elimination reaction via the sequential formation of the external aldimine, the quinonoid, and the aminoacrylate catalytic intermediates. Furthermore, the accumulation of pyruvate in the reaction mixture indicates that aminoacrylate-coenzyme complexes undergo a water-assisted hydrolysis, as in solution. Therefore, TPL- and Trpase-doped gels are catalytically competent, although with a six- to eightfold decrease of the catalytic activity. Catalytic competence was also observed in a preliminary investigation of the reactivity of the PLP-dependent enzymes tryptophan synthase and O-acetylserine sulfhydrylase encapsulated in silica gels (Mozzarelli et al. 2000), with similar reductions of catalytic rates (B. Pioselli, S. Bettati, and A. Mozzarelli, unpubl.). Enzyme activities lower than in solution have frequently been reported for gel-encapsulated proteins (Jin and Brennan 2002; Besanger et al. 2003). However, in most cases this finding can be explained by catalytic rates limited by substrate diffusion within gels of uncontrolled thickness. In our case, the reduction of activity seems to be predominantly ascribed to the decrease of the rate of conformational changes that accompany the catalytic cycle, because the size of the gel particles was such that diffusion of substrates could not be rate-limiting the enzyme kinetics. Altered substrate partitioning within the gel matrix with respect to solution might affect the activity (Shen and Kostic 1997). In the experimental conditions of these enzyme assays, substrate partitioning is unlikely to play a major role, being substrates present at saturating concentration and only slightly negatively charged. An alternative explanation of the decreased activity, based on partial protein denaturation upon encapsulation, is also not supported by spectra of the native species. It is known that denaturation of PLP-enzymes leads to the release of the coenzyme, causing a significant decrease of the absorption intensity at 420 nm with a concomitant increase at 388 nm (Mozzarelli et al. 2000). We observed that the absorption ratios at 280 and (420 + 340) nm (we chose the sum of 420 and 340 nm absorption because of the alteration of tautomer equilibrium) for the native enzymes are very similar in solution and in silica gel, 6.16 and 6.11 for TPL and 5.6 and 5.37 for Trpase, respectively. Therefore, no significant protein denaturation has taken place upon encapsulation.
Previous studies on myoglobin and hemoglobin have clearly demonstrated that protein encapsulation influences the rates of conformational transitions (Bettati and Mozzarelli 1997; Das et al. 1998; Juszczak and Friedman 1999; Shibayama and Saigo 1999), allowing trapping of otherwise unstable conformational states (Juszczak and Friedman 1999; Khan et al. 2000; Bruno et al. 2001; Shibayama and Saigo 2001 Shibayama and Saigo 2003). The effect of encapsulation on local and global protein dynamics is strongly dependent on individual protein properties and protocols of encapsulation (Dave et al. 1995Dave et al. 1997; Jordan et al. 1995; Gottfried et al. 1999; Hartnett et al. 1999; Chirico et al. 2002; Gonnelli and Strambini 2003). Furthermore, the increased stability generally observed for encapsulated proteins can be explained either by limitations imposed by the gel network to a volume expansion (confinement effect; Zhou and Dill 2001) crossing the transition state towards denaturation and/or upon denaturation, or by stabilization of the native molecule by favorable interactions with the negatively charged pore surface of the matrix (Bettati et al. 2003). Recent computational studies demonstrated that caging helps protein folding (Klimov et al. 2002; Takagi et al. 2003; Thirumalai et al. 2003). The same mechanisms can operate for a catalytic process involving distinct conformational changes. Native PLP-dependent enzymes exist in two tautomeric forms, the enolimine and the ketoenamine, favored by an apolar and polar active site environment, respectively (Faeder and Hammes 1971). Furthermore, many PLP-dependent enzymes undergo an open to closed transition upon substrate binding that involves a reorientation of the two domains composing each subunit. These conformational events are crucial for the attainment of an efficient catalysis by PLP-enzymes (Schirch et al. 1991; McPhalen et al. 1992; Schneider et al. 1998; Burkhard et al. 1999). In the case of aspartate aminotransferase, it was demonstrated that crystallization-induced stabilization of the closed form of the enzyme increased by 5.8 kcal/mole the substrate affinity, because binding does not need to pay the energetic cost of the domain closure (Malashkevich et al. 1993). In the case of O-acetylserine sulfhydrylase, enzyme crystals with similar lattices exhibited striking differences in substrate reactivity, ranging from being completely inactive to fully active or undergoing crystal shattering due to subtle constraints imposed on the open-closed transition (Mozzarelli et al. 1998). We have found that encapsulation stabilizes in the opposite way the enolimine and the ketoenamine tautomers of TPL and Trpase with respect to solution, the enolimine being stabilized in TPL and the ketoenamine in Trpase. This finding is somewhat surprising, given the close similarity of the two enzymes, and emphasizes the relevance of subtle structural differences, for example, surface charge and amino acid distribution. Furthermore, we observed alterations of ligand binding affinities and equilibrium distribution of intermediates in silica gels with respect to solution only for TPL. These effects likely depend on changes of the relative rates of catalytic steps requiring conformational modifications, partially restrained by the silica matrix, as suggested by the decrease in the rate of quinonoid formation. The increased amount of the aminoacrylate species in the gel with respect to solution in the reaction of TPL with S-Me-Cys suggests a partial stabilization of the closed form of the enzyme. This conclusion is in agreement with the apolar enolimine tautomer of the internal aldimine of TPL being more favored in silica gels than in solution. Different results were observed for P. vulgaris Trpase-doped gels. In fact, (1) the ketoenamine was found to be more favored in the gel than in solution; (2) the dissociation constant for OIA was close to that determined in solution; (3) no significant differences in intermediates distribution was observed in the reaction with L-serine and L-tryptophan in the gel with respect to solution; and (4) the presence of benz-imidazole did not stabilize the aminoacrylate in the gel as in solution. It appears that Trpase encapsulation only slightly affects the tautomeric equilibrium of the internal aldimine, without any influence on steady-state distribution of intermediates, a result that might be due to the absence of any preferential stabilization by the silica matrix of the open with respect to the closed conformation and any restriction to the rate of the transition between open and closed conformations. However, the different reactivity of the P. vul-garis and E. coli enzymes in both solution and the gel is remarkable. This finding requires further investigation to be fully understood. The observed stabilization of the enolimine tautomer in the internal aldimine of E. coli Trpase gels and the absence of an absorption increase at 340 nm as a function of pH are in agreement with the attribution of the absorbing species accumulating at high pH in solution to a derivative different from the enolimine, likely the substituted aldamine (Ikushiro et al. 1998).
It would be of interest to modify the gelification protocol in order to pursue the attainment of full enzyme activity, by modulating (1) the pore size, because confinement length is expected to affect protein dynamics (Klimov et al. 2002), and (2) the gel matrix chemical properties. This goal was achieved in the case of lipase by using apolar silica precursors that make the environment of the gel matrix more hydrophobic, thus favoring the closure of the active site lid, a key requirement in this enzyme for an efficient catalysis. As a result, the encapsulated lipase exhibited a 1300-fold increase in activity with respect to the soluble form (Reetz and Jaeger 1998). For TPL and Trpase, a qualitatively similar result might be obtained by encapsulating the enzyme under conditions that favor the closed form, that is, in the presence of substrate or substrate analogs.
Materials and methods
Reagents
L-serine (Fluka), benzimidazole (Merck), L-tryptophan, S-methyl-L-cysteine (S-Me-Cys), pyridoxal 5′-phosphate (PLP; Sigma), NADH, lactate dehydrogenase (Boehringer), and 4-hydroxy-pyri-dine (4-OH-pyr; Aldrich) were used without further purification. S-(o-nitrophenyl)-L-cysteine (SOPC) was prepared as described (Phillips 1987). Oxindolyl-L-alanine (OIA) was prepared by oxidation of L-Trp as described (Phillips et al. 1984). 3-fluoro-L-tyrosine was prepared from 2-fluorophenol and ammonium pyru-vate using TPL (von Tersch et al. 1996).
Enzymes
Wild-type TPL from Citrobacter freundii and wild-type Trpase from Proteus vulgaris were expressed and purified as described (Chen et al. 1995b; Zakormirdina et al. 2002).
Protein encapsulation
TPL and TRPase were encapsulated in TMOS-derived wet silica gels. The sol-gel samples were prepared by mixing in a 0.5 : 0.5 : 1 ratio a sol derived from the acid-catalyzed hydrolysis of TMOS, 50 mM potassium phosphate buffer (pH 8.0), and a solution containing 20 mg/mL enzyme, 50 mM potassium phosphate buffer (pH 8.0), at 4°C. The mixture was quickly layered on a quartz slide, obtaining a thin gel film within a few minutes, at 4°C. The gels were stored in 50 mM potassium phosphate, 50 μM PLP (pH 7.0), at 4°C.
Spectrophotometric measurements on enzyme-doped gels
Enzyme-doped gels were placed in a cuvette containing 50 mM potassium phosphate buffer (pH 7.0). Absorption spectra were recorded with a CARY400 spectrophotometer. The absorption spectrum of a protein-free gel was subtracted to reduce the influence of light scattering originated from the nonperfect optical quality of the gel surface. This subtraction may introduce some spectral distortion, especially at low wavelengths (300–350 nm). Moreover, aging of PLP-dependent enzymes leads to an increase in the absorption intensity at 310–330 nm, introducing variability in the spectral properties of the internal aldimine species (Mozzarelli 1989). Titrations of TPL and Trpase gels were carried out by exposing silica gels to solutions containing increasing concentrations of inhibitors. Titration data were analyzed according to a binding isotherm using the software SigmaPlot 2000 (SPSS Science).
Activity assays
The enzyme activity of TPL and TRPase in solution and encapsulated in silica gels was assayed using either SOPC (Suelter et al. 1976) or S-Me-Cys (Watanabe and Snell 1977) as substrate analogs. The reaction of the chromophoric SOPC, carried out in a solution containing 0.6 mM SOPC, 50 mM potassium phosphate, 50 μM PLP (pH 8.0), at 25°C, was followed by recording the decrease in absorbance at 370 nm (Δɛ = −1.86*103 M−1 cm−1) as a function of time. The reaction of S-Me-Cys, carried out in a solution containing 50 mM potassium phosphate, 50 μM PLP, 0.2 mM NADH, 0.02 mg lactate dehydrogenase, and 30 mM or 100 mM S-Me-Cys for TPL and Trpase, respectively, (pH 8.0) at 25°C, was followed by the coupled assay with lactate dehydroge-nase and NADH, recording the absorbance decrease at 340 nm (Δɛ = −6.22*103 M−1 cm1). To avoid rate-limiting effects of substrate diffusion, enzyme-doped gels were sonicated at low power to obtain a micron-size gel particles suspension. Optical inspection indicated that the average dimension of gels was less than 2–4 μm. Sonication does not affect enzyme activity, as verified by applying the same protocol to the soluble enzymes.
Acknowledgments
The financial support of COFIN2001/2003 from the Italian Ministry of Education and University (to A.M.), FIRB Nanotechnology 2003 (to A.M.), NATO collaborative grant 977117 (to R.S.P., T.V.D., and A.M.), and Russian Foundation for Basic Investigations (02-04-48010 to T.V.D.) are gratefully acknowledged.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Abbreviations
TPL, Tyrosine phenol-lyase
Trpase, tryptophan indole-lyase
PLP, pyridoxal 5′-phosphate
SOPC, S-(o-Nitrophenyl)-L-cysteine
S-Me-Cys, S-methyl-L-cysteine
3-F-Tyr, 3-fluoro-L-tyrosine
4-OH-pyr, 4-hydroxy-pyridine
OIA, oxindolyl-L-alanine
TMOS, tetramethyl orthosilicate
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.03492904.
References
- Abbruzzetti, S., Viappiani, C., Bruno, S., and Mozzarelli, A. 2001. Enhanced geminate ligand rebinding upon photo-dissociation of silica gel-embedded myoglobin-CO. Chem. Phys. Lett. 346 430–436. [Google Scholar]
- Ahmed, S.A., Hyde, C.C., Thomas, G., and Miles, E.W. 1987. Microcrystals of tryptophan synthase α 2 β 2 complex from Salmonella typhimurium are catalytically active. Biochemistry 26 5492–5498. [DOI] [PubMed] [Google Scholar]
- Antson, A.A., Demidkina, T.V., Gollnick, P., Dauter, Z., von Tersch, R.L., Long, J., Berezhnoy, S.N., Phillips, R.S., Harutyunyan, E.H., and Wilson, K.S. 1993. Three-dimensional structure of tyrosine phenol-lyase. Biochemistry 32 4195–4206. [DOI] [PubMed] [Google Scholar]
- Bazhulina, N.P., Morozov, Y.V., Papisova, A.I., and Demidkina, T.V. 2000. Pyridoxal 5′-phoshate schiff base in Citrobacter freundii tyrosinephenol-lyase. Ionic and tautomeric equilibria. Eur. J. Biochem. 267 1830–1836. [DOI] [PubMed] [Google Scholar]
- Besanger, T.R., Chen, Y., Deisingh, A.K., Hodgson, R., Jin, W., Mayer, S., Brook, M.A., and Brennan, J.D. 2003. Screening of inhibitors using enzymes entrapped in sol-gel-derived materials. Anal. Chem. 75 2382–2391. [DOI] [PubMed] [Google Scholar]
- Bettati, S. and Mozzarelli, A. 1997. T state hemoglobin binds oxygen nonco-operatively with allosteric effects of protons, inositol hexaphosphate, and chloride. J. Biol. Chem. 272 32050–32055. [DOI] [PubMed] [Google Scholar]
- Bettati, S., Pioselli, B., Campanini, B., Viappiani, S., and Mozzarelli, A. 2004. Protein-doped nanoporous silica gels. In Encyclopedia of nanoscience and nanotechnology (ed. H.S. Nalwa), American Scientific Publishers, Steven-son Ranch, CA.
- Brinker, C.J. and Scherer, G.W. 1990. Sol-gel science: The physics and chemistry of sol-gel processing. Academic Press, Boston.
- Bruno, S., Bonaccio, M., Bettati, S., Rivetti, C., Viappiani, C., Abbruzzetti, S., and Mozzarelli, A. 2001. High and low oxygen affinity conformations of T state hemoglobin. Protein Sci. 10 2401–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhard, P., Tai, C.H., Ristroph, C.M., Cook, P.F., and Jansonius, J.N. 1999. Ligand binding induces a large conformational change in O-acetylserine sulfhydrylase from Salmonella typhimurium. J. Mol. Biol. 291 941–953. [DOI] [PubMed] [Google Scholar]
- Chen, H. and Phillips, R.S. 1993. Binding of phenol and analogues to alanine complexes of tyrosine phenol-lyase from Citrobacter freundii: Implications for the mechanism of α,β-elimination and alanine racemization. Biochemistry 32 11591–11599. [DOI] [PubMed] [Google Scholar]
- Chen, H., Gollnick, P., and Phillips, R.S. 1995a. Site-directed mutagenesis of His343→Ala in Citrobacter freundii tyrosine phenol-lyase. Effects on the kinetic mechanism and rate-determining step. Eur. J. Biochem. 229 540–549. [PubMed] [Google Scholar]
- Chen, H.Y., Demidkina, T.V., and Phillips, R.S. 1995b. Site-directed mutagen-esis of tyrosine-71 to phenylalanine in Citrobacter freundii tyrosine phenol-lyase: Evidence for dual roles of tyrosine-71 as a general acid catalyst in the reaction mechanism and in cofactor binding. Biochemistry 34 12276–12283. [DOI] [PubMed] [Google Scholar]
- Chirico, G., Cannone, F., Beretta, S., Diaspro, A., Campanini, B., Bettati, S., Ruotolo, R., and Mozzarelli, A. 2002. Dynamics of green fluorescent protein mutant2 in solution, on spin-coated glasses, and encapsulated in wet silica gels. Protein Sci. 11 1152–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, T.K., Khan, I., Rousseau, D.L., and Friedman, J.M. 1998. Preservation of the native structure in myoglobin at low pH by sol-gel encapsulation. J. Am. Chem. Soc. 120 10268–10269. [Google Scholar]
- Dave, B.C., Soyez, H., Miller, J.M., Dunn, B., Valentine, J.S., and Zink, J.I. 1995. Synthesis of protein-doped sol-gel SiO2 thin-films—evidence for rotational mobility of encapsulated cytochrome c. Chem. Mater. 7 1431–1434. [Google Scholar]
- Demidkina, T.V., Barbolina, M.V., Faleev, N.G., Sundararaju, B., Gollnick, P.D., and Phillips, R.S. 2002. Threonine-124 and phenylalanine-448 in Ci-trobacter freundii tyrosine phenol-lyase are necessary for activity with L-tyrosine. Biochem. J. 363 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers, D.K. and Valentine, J.S. 2001. Molecular confinement influences protein structure and enhances thermal protein stability. Protein Sci. 10 250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliot, A.C. and Kirsch, J.F. 2002. Modulation of the internal aldimine pK(a)’s of 1-aminocyclopropane-1-carboxylate synthase and aspartate aminotrans-ferase by specific active site residues. Biochemistry 41 3836–3842. [DOI] [PubMed] [Google Scholar]
- Ellerby, L.M., Nishida, C.R., Nishida, F., Yamanaka, S.A., Dunn, B., Valentine, J.S., and Zink, J.I. 1992. Encapsulation of proteins in transparent porous silicate-glasses prepared by the sol-gel method. Science 255 1113–1115. [DOI] [PubMed] [Google Scholar]
- Faeder, E.V. and Hammes, G.G. 1971. Kinetics studies of tryptophan synthetase. Interaction of substrates with the B subunit. Biochemistry 10 1041–1045. [DOI] [PubMed] [Google Scholar]
- Gill, I. 2001. Bio-doped nanocomposite polymers: Sol-gel bioencapsulates. Chem. Mater. 13 3404–3421. [Google Scholar]
- Gill, I. and Ballesteros, A. 1998. Encapsulation of biologicals within silicate, siloxane, and hybrid sol-gel polymers: An efficient and generic approach. J. Am. Chem. Soc. 120 8587–8598. [Google Scholar]
- ———. 2000. Bioencapsulation within synthetic polymers (Part 1): Sol-gel encapsulated biologicals. Trends Biotech. 18 282–296. [DOI] [PubMed] [Google Scholar]
- Gonnelli, M. and Strambini, G.B. 2003. Structure and dynamics of proteins encapsulated in silica hydrogels by Trp phosphorescence. Biophys. Chem. 104 155–169. [DOI] [PubMed] [Google Scholar]
- Gottfried, D.S., Kagan, A., Hoffman, B.M., and Friedman, J.M. 1999. Impeded rotation of a protein in a sol-gel matrix. J. Phys. Chem. B 103 2803–2807. [Google Scholar]
- Hartnett, A.M., Ingersoll, C.M., Baker, G.A., and Bright, F.V. 1999. Kinetics and thermodynamics of free flavins and the flavin-based redox active site within glucose oxidase dissolved in solution or sequestered within a sol-gel-derived glass. Anal. Chem. 71 1215–1224. [DOI] [PubMed] [Google Scholar]
- Ikushiro, H., Hayashi, H., Kawata, Y., and Kagamiyama, H. 1998. Analysis of the pH- and ligand-induced spectral transitions of tryptophanase: Activation of the coenzyme at the early steps of the catalytic cycle. Biochemistry 37 3043–3052. [DOI] [PubMed] [Google Scholar]
- Isupov, M.N., Antson, A.A., Dodson, E.J., Dodson, G.G., Dementieva, I.S., Zakomirdina, L.N., Wilson, K.S., Dauter, Z., Lebedev, A.A. and Harutyun-yan, E.H. 1998. Crystal structure of tryptophanase. J. Mol. Biol. 276 603–623. [DOI] [PubMed] [Google Scholar]
- Jin, W. and Brennan, J.D. 2002. Properties and applications of proteins encapsulated within sol-gel derived materials. Anal. Chim. Acta 461 1–36. [Google Scholar]
- Johnson, P. and Whateley, T.L. 1971. Use of polymerizing silica gel systems for the immobilization of trypsin. J. Colloid Interface Sci. 37 557–563. [Google Scholar]
- Jordan, J.D., Dunbar, R.A., and Bright, F.V. 1995. Dynamics of acrylodan-labeled bovine and human serum-albumin entrapped in a sol-gel-derived biogel. Anal. Chem. 67 2436–2443. [DOI] [PubMed] [Google Scholar]
- Juszczak, L.J. and Friedman, J.M. 1999. UV resonance Raman spectra of ligand binding intermediates of sol-gel encapsulated hemoglobin. J. Biol. Chem. 274 30357–30360. [DOI] [PubMed] [Google Scholar]
- Kallen, R.G., Korpela, T., Martell, A.E., Matsushina, Y., Metzler, C.M., Metz-ler, D.E., Morozov, Y.V., Ralston, I.M., Savin, F.A., Torchinsky, Y.M., et al. 1985. Chemical and spectroscopic properties of pyridoxal and pyridox-amine phosphates. In Transaminases (eds. P. Christen and D.E. Metzler), pp. 38. Wiley, New York.
- Khan, I., Shannon, C.F., Dantsker, D., Friedman, A.J., Perez-Gonzalez-de-Apo-daca, J., and Friedman, J.M. 2000. Sol-gel trapping of functional intermediates of hemoglobin: Geminate and bimolecular recombination studies. Biochemistry 39 16099–16109. [DOI] [PubMed] [Google Scholar]
- Kiick, D.M. and Phillips, R.S. 1988. Mechanistic deductions from kinetic isotope effects and pH studies of pyridoxal phosphate dependent carbon–carbon lyases: Erwinia herbicola and Citrobacter freundii tyrosine phenollyase. Biochemistry 27 7333–7338. [DOI] [PubMed] [Google Scholar]
- Klimov, D.K., Newfield, D., and Thirumalai, D. 2002. Simulations of β-hairpin folding confined to spherical pores using distributed computing. Proc. Natl. Acad. Sci. 99 8019–8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai, H., Yamada, H., Matsui, H., Ohkishi, H., and Ogata, K. 1970. Ty-rosine phenol lyase I. Purification, crystallization, and properties. J. Biol. Chem. 245 1767–1772. [PubMed] [Google Scholar]
- Malashkevich, V.N., Toney, M.D., and Jansonius, J.N. 1993. Crystal structures of true enzymatic reaction intermediates: Aspartate and glutamate ketimines in aspartate aminotransferase. Biochemistry 32 13451–13462. [DOI] [PubMed] [Google Scholar]
- McIninch, J.K. and Kantrowitz, E.R. 2001. Use of silicate sol-gels to trap the R and T quaternary conformational states of pig kidney fructose-1,6-bisphos-phatase. Biochim. Biophys. Acta 1547 320–328. [DOI] [PubMed] [Google Scholar]
- McPhalen, C.A., Vincent, M.G., Picot, D., Jansonius, J.N., Lesk, A.M., and Chothia, C. 1992. Domain closure in mitochondrial aspartate aminotrans-ferase. J. Mol. Biol. 227 197–213. [DOI] [PubMed] [Google Scholar]
- Metzler, C.M., Viswanath, R., and Metzler, D.E. 1991. Equilibria and absorption spectra of tryptophanase. J. Biol. Chem. 266 9374–9381. [PubMed] [Google Scholar]
- Mozzarelli, A. and Bettati, S. 2001. Functional properties of immobilized proteins. In Physical properties and applications (ed. H.S. Nalwa), pp. 55–97. Overseas Publishers Association, Singapore.
- Mozzarelli, A., Peracchi, A., Rossi, G.L., Ahmed, S.A., and Miles, E.W. 1989. Microspectrophotometric studies on single crystals of the tryptophan synthase α 2 β 2 complex demonstrate formation of enzyme-substrate intermediates. J. Biol. Chem. 264 15774–15780. [PubMed] [Google Scholar]
- Mozzarelli, A., Bettati, S., Pucci, A.M., Burkhard, P., and Cook, P.F. 1998. Catalytic competence of O-acetylserine sulfhydrylase in the crystal probed by polarized absorption microspectrophotometry. J. Mol. Biol. 283 135–146. [DOI] [PubMed] [Google Scholar]
- Mozzarelli, A., Campanini, B., Bettati, S., and Peracchi, A. 2000. Functional properties of immobilized pyroxidal 5′phosphate dependent enzymes probed by absorption microspectrophotometry. In Biochemistry and molecular biology of vitamin B6 and PQQ-dependent proteins (eds. A. Iriarte et al.), pp. 230–232. Birkhauser Verlag, Basel, Switzerland.
- Phillips, R.S. 1987. Reactions of O-acyl-L-serines with tryptophanase, tyrosine phenol-lyase, and tryptophan synthase. Arch. Biochem. Biophys. 256 302–310. [DOI] [PubMed] [Google Scholar]
- ———. 1991. Reaction of indole and analogues with amino acid complexes of Escherichia coli tryptophan indole-lyase: Detection of a new reaction intermediate by rapid-scanning stopped-flow spectrophotometry. Biochemistry 30 5927–5934. [DOI] [PubMed] [Google Scholar]
- Phillips, R.S., Miles, E.W., and Cohen, L.A. 1984. Interactions of tryptophan synthase, tryptophanase, and pyridoxal phosphate with oxindolyl-L-alanine and 2,3-dihydro-L-tryptophan: Support for an indolenine intermediate in tryptophan metabolism. Biochemistry 23 6228–6234. [DOI] [PubMed] [Google Scholar]
- Phillips, R.S., Demidkina, T.V., Zakormirdina, L.N., Bruno, S., Ronda, L., and Mozzarelli, A. 2002. Crystals of tryptophan indole-lyase and tyrosine phenol-lyase form stable quinonoid complexes. J. Biol. Chem. 277 21592–21597. [DOI] [PubMed] [Google Scholar]
- Phillips, R.S., Demidkina, T.V., and Faleev, N.G. 2003. Structure and mechanism of tryptophan indole-lyase and tyrosine phenol-lyase. Biochim. Bio-phys. Acta 1647 167–172. [DOI] [PubMed] [Google Scholar]
- Reetz, M.T. and Jaeger, K.E. 1998. Overexpression, immobilization and bio-technological application of Pseudomonas lipases. Chem. Phys. Lipids 93 3–14. [DOI] [PubMed] [Google Scholar]
- Schirch, V., Shostak, K., Zamora, M., and Guatam-Basak, M. 1991. The origin of reaction specificity in serine hydroxymethyltransferase. J. Biol. Chem. 266 759–764. [PubMed] [Google Scholar]
- Schneider, T.R., Gerhardt, E., Lee, M., Liang, P.H., Anderson, K.S., and Schlichting, I. 1998. Loop closure and intersubunit communication in tryp-tophan synthase. Biochemistry 37 5394–5406. [DOI] [PubMed] [Google Scholar]
- Shen, C.Y. and Kostic, N.M. 1997. Kinetics of photoinduced electron-transfer reactions within sol-gel silica glass doped with zinc cytochrome c. Study of electrostatic effects in confined liquids. J. Am. Chem. Soc. 119 1304–1312. [Google Scholar]
- Shibayama, N. and Saigo, S. 1995. Fixation of the quaternary structures of human adult haemoglobin by encapsulation in transparent porous silica gels. J. Mol. Biol. 251 203–209. [DOI] [PubMed] [Google Scholar]
- ———. 1999. Kinetics of the allosteric transition in hemoglobin within silicate sol-gels. J. Am. Chem. Soc. 121 444–445. [Google Scholar]
- ———. 2001. Direct observation of two distinct affinity conformations in the T state human deoxyhemoglobin. FEBS Lett. 492 50–53. [DOI] [PubMed] [Google Scholar]
- ———. 2003. Oxygen equilibrium properties of myoglobin locked in the li-ganded and unliganded conformations. J. Am. Chem. Soc. 125 3780–3783. [DOI] [PubMed] [Google Scholar]
- Suelter, C.H., Wang, J., and Snell, E.E. 1976. Direct spectrophotometric assay of tryptophanase. FEBS Lett. 66 230–. [DOI] [PubMed] [Google Scholar]
- Sundararaju, B., Antson, A.A., Phillips, R.S., Demidkina, T.V., Barbolina, M.V., Gollnick, P., Dodson, G.G., and Wilson, K.S. 1997. The crystal structure of Citrobacter freundii tyrosine phenol-lyase complexed with 3–(4′-hydroxyphenyl)propionic acid, together with site-directed mutagen-esis and kinetic analysis, demonstrates that arginine 381 is required for substrate specificity. Biochemistry 36 6502–6510. [DOI] [PubMed] [Google Scholar]
- Takagi, F., Koga, N., and Takada, S. 2003. How protein thermodynamics and folding mechanisms are altered by the chaperonin cage: Molecular simulations. Proc. Natl. Acad. Sci. 100 11367–11372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirumalai, D., Klimov, D.K., and Lorimer, G.H. 2003. Caging helps protein fold. Proc. Natl. Acad. Sci. 100 11195–11197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Tersch, R.L., Secundo, F., Phillips, R.S., and Newton, M.G. 1996. Preparation of fluorinated aminoacid with tyrosine phenol lyase: Effects of fluorination on the reaction kinetics and mechanism of tyrosine phenol lyase and tyrosine protein kinase. In Biomedical application of fluorine chemistry (eds. I. Ojima et al.), pp. 95–104. American Chemical Society, Washington, DC.
- Watanabe, T. and Snell, E.E. 1977. The interaction of Escherichia coli trypto-phanase with various amino and their analogs. Active site mapping. J. Biochem. (Tokyo) 82 733. [DOI] [PubMed] [Google Scholar]
- West, J.M. and Kantrowitz, E.R. 2003. Trapping specific quaternary states of the allosteric enzyme aspartate transcarbamoylase in silica matrix sol-gels. J. Am. Chem. Soc. 125 9924–9925. [DOI] [PubMed] [Google Scholar]
- Zakormirdina, L.N., Kulikova, V.V., Gogoleva, O.I., Dementieva, I.S., Faleev, N.G., and Demidkina, T.V. 2002. Tryptophan indole-lyase from Proteus vulgaris: Kinetic and spectral properties. Biochemistry (Moscow) 67 1437–1445. [DOI] [PubMed] [Google Scholar]
- Zhou, H.X. and Dill, K.A. 2001. Stabilization of proteins in confined spaces. Biochemistry 40 11289–11293. [DOI] [PubMed] [Google Scholar]