Abstract
Fatty acid binding proteins (FABPs) exhibit a β-barrel topology, comprising 10 antiparallel β-sheets capped by two short α-helical segments. Previous studies suggested that fatty acid transfer from several FABPs occurs during interaction between the protein and the acceptor membrane, and that the helical domain of the FABPs plays an important role in this process. In this study, we employed a helix-less variant of intestinal FABP (IFABP-HL) and examined the rate and mechanism of transfer of fluorescent anthroyloxy fatty acids (AOFA) from this protein to model membranes in comparison to the wild type (wIFABP). In marked contrast to wIFABP, IFABP-HL does not show significant modification of the AOFA transfer rate as a function of either the concentration or the composition of the acceptor membranes. These results suggest that the transfer of fatty acids from IFABP-HL occurs by an aqueous diffusion-mediated process, i.e., in the absence of the helical domain, effective collisional transfer of fatty acids to membranes does not occur. Binding of wIFABP and IFABP-HL to membranes was directly analyzed by using a cytochrome c competition assay, and it was shown that IFABP-HL was 80% less efficient in preventing cytochrome c from binding to membranes than the native IFABP. Collectively, these results indicate that the α-helical region of IFABP is involved in membrane interactions and thus plays a critical role in the collisional mechanism of fatty acid transfer from IFABP to phospholipid membranes.
The intestinal fatty acid binding protein (IFABP) belongs to a family of 14- to 15-kDa intracellular lipid-binding proteins (1, 2). It binds a single molecule of long-chain fatty acid in an interior cavity surrounded by two five-stranded antiparallel β-sheets (3). The structure also contains a small helix–turn–helix motif that is interspersed between the first and second β-strands. The α-helical domain covers one end of the binding cavity, and the second helix participates in a flexible portal that may regulate the association and dissociation of fatty acids (4–6).
It has been often proposed that IFABP, which is abundantly produced in the enterocyte, is important for intracellular trafficking and processing of the large quantities of dietary fatty acid absorbed by the small intestine (7). To address the putative transport function of the FABPs, we have previously used an in vitro fluorescence resonance energy-transfer assay to examine the rate and mechanism of transfer of fluorescently tagged fatty acids from FABPs to phospholipid membranes. The results have suggested that different members of the FABP family transfer fatty acids to phospholipid bilayers via distinct kinetic mechanisms (8–12). Liver FABP transfer of fatty acids to membranes is thought to involve an initial and obligatory release of ligand to the aqueous milieu prior to membrane association. In contrast, transfer of fatty acids from IFABP appears to occur during direct collisional interactions between the protein and the acceptor membrane (12).
The structural elements underlying collisional transfer of the fatty acid from IFABP to membranes, i.e., the specific binding protein–membrane interactions, could have important physiological consequences, as they may dictate the directed intracellular trafficking of fatty acids. Therefore, it would be of great interest to determine the region or regions of the protein involved in the putative interaction. Despite their relatively short lengths, the α-helical segments of IFABP may be expected to be membrane-interactive, particularly the α-I helix, which is amphipathic (13, 14). Recently, we employed site-directed mutagenesis and showed that specific lysine residues of the α-helical domain of the homologous heart FABP are involved in the collisional transfer of fatty acids to phospholipid membranes (15).
To test the hypothesis that the IFABP helical domain plays an important role in the collision-based transfer of fatty acids to membranes, we have used a variant of IFABP engineered by deleting 17 residues corresponding to the α-helical domain and inserting a 2-residue linker after residue 14 (16). The three-dimensional structure of this variant has been determined by triple-resonance three-dimensional NMR. It exhibits a helix-less conformation that is nearly superimposable with the β-sheet domain of wild-type IFABP (wIFABP) (17). The selective deletion of the α-helical domain results in a large opening that connects the interior fatty acid binding cavity with exterior solvent. Unlike wIFABP, the association and dissociation of fatty acids is structurally and kinetically unimpeded with helix-less IFAPB (IFABP-HL) (16, 18).
Here we have examined the rate and mechanism of transfer of anthroyloxy-labeled fatty acids (AOFA) from IFABP-HL to phospholipid membranes, compared with wIFABP. We have also assessed the interaction between each of these proteins and membranes. The results indicate that the helical region of IFABP is a critical domain for membrane–protein interactions and participates in the mechanism of collisional transfer of fatty acids from IFABP to membranes.
MATERIALS AND METHODS
Materials.
The fluorescently labeled AOFA, 12-(9-anthroyloxy)oleic acid (12AO), was purchased from Molecular Probes. Egg phosphatidylcholine (EPC), egg phosphatidylethanolamine (EPE), dansyl-phosphatidylethanolamine (DPE), 1-palmitoyl-2-[12-[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]dodecanoyl]-sn-glycero-3-phosphocholine (NBD-PC), brain phosphatidylserine (PS), and bovine heart cardiolipin (CL) were obtained from Avanti Polar Lipids. Lipids were stored in chloroform under nitrogen at −20°C. Lipidex-1000 and nalidixic acid were purchased from Sigma. Isopropyl-β-d-thiogalactoside (IPTG) was obtained from Fisher. All other chemicals were reagent grade or better.
FABP Purification.
Recombinant rat IFABP plasmid was generously provided by Alan Kleinfeld and Ron Ogata (Medical Biology Institute, La Jolla, CA). The helix-less mutant was constructed by using site-directed mutagenesis, and the mutant protein was overexpressed in Escherichia coli harboring pMON-IFABP, as detailed elsewhere (16). Both proteins were purified from E. coli as described previously for the wild-type protein (12), except for the induction, which was done with 0.4 mM IPTG for the wild type and 0.1 mM nalidixic acid for the helix-less variant.
Vesicle Preparation.
Small unilamellar vesicles (SUVs) were prepared by sonication and ultracentrifugation as described previously (19, 20). The standard vesicles were prepared to contain 90 mol % of EPC and 10 mol % of NBD-PC, which served as the fluorescent quencher. For some experiments, as indicated in the text, 25 mol % of other lipids were substituted for EPC in the vesicles. Vesicles were prepared in TBS buffer (40 mM Tris/100 mM NaCl, pH 7.4) except for SUVs containing CL, which were prepared in TBS with 1 mM EDTA.
Transfer of AOFA from FABPs to SUVs.
A fluorescence resonance transfer assay was used to monitor the transfer of AOFA from wIFABP or IFABP-HL to acceptor model membranes as described in detail (9–12). Briefly, FABP with bound AOFA was mixed with SUV by using a Stopped-Flow Spectrofluorometer SX-18MV (Applied Photophysics, Surrey, U.K.). The NBD moiety is an energy-transfer acceptor of the anthroyloxy group donor; therefore, the fluorescence of the AOFA is quenched when the ligand is bound to SUVs that contain NBD-PC. On mixing, transfer of AOFA from protein to membrane is directly monitored by the time-dependent decrease in anthroyloxy group fluorescence.
AOFA binding constants were determined by using a fluorescent titration assay (21). The apparent Kd values for 12AO were approximately 10-fold higher for binding to the IFABP-HL (1.7 μM) than the wild-type (0.16 μM), consistent with previous results using native fatty acids (18). To maintain a low level (<4%) of unbound fatty acid concentration in the transfer experiments, the protein/probe ratio was higher for the IFABP-HL. Final transfer assay conditions, unless otherwise noted, were 15 μM wIFABP with 1.5 μM 12AO and 150 μM SUV, or 45 μM IFABP-HL with 0.75 μM AOFA and 450 μM SUV. Transfer was monitored at 25°C. Controls to ensure that photobleaching was eliminated were performed before each experiment, as previously described (12). Data were analyzed by using software provided with the instrument, and all curves were well described by a single exponential function. For each experimental condition, at least five replicates were done. Average values ± SD for three or more separate experiments are reported.
FABP Interaction with Membranes.
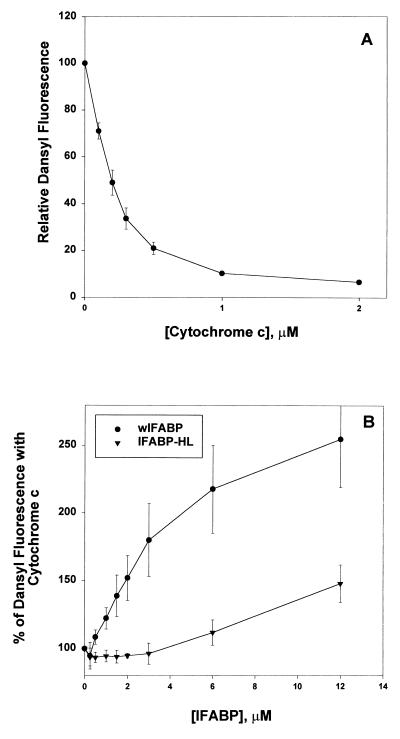
To assess more directly the putative association of IFABP with vesicles, an assay that exploits the well known membrane-interactive properties of cytochrome c was employed. The binding of cytochrome c to acidic membranes can be monitored by using a resonance-energy transfer assay (22) in which the dansyl fluorescence of DPE-labeled SUV is quenched on binding of cytochrome c, which contains the heme moiety quencher. Competition of FABP with cytochrome c for binding to SUVs was determined by the relief of cytochrome c-related quenching of the dansyl fluorescence. In a final volume of 2 ml, 0–12 μM FABP was added to 12.5 μM SUV (EPC/EPE/CL/DPE, 64:10:25:1) in 20 mM Tris⋅HCl/0.1 mM EDTA, pH 7.4. After a 5-min equilibration, cytochrome c was added (0.75 μM final concentration), and the mixture equilibrated an additional 2 min before monitoring fluorescence emission at 520 nm (λex = 335 nm). In the absence of bound FABP, the dose-dependent quenching of dansyl fluorescence is observed (see Fig. 3A). An inhibition of cytochrome c-dependent quenching is interpreted as evidence for FABP interaction with the SUV, i.e., FABP prevention of subsequent cytochrome c interaction with the bilayer.
Figure 3.
Inhibition of cytochrome c binding to anionic membranes by FABP. (A) Increasing concentrations of cytochrome c were incubated with 12.5 μMSUV (EPC/EPE/CL/DPE, 64:10:25:1). The quenching of dansyl fluorescence is expressed relative to the fluorescence intensity of vesicles without cytochrome c. (B) 12.5 μM SUV containing 1% DPE were incubated with increasing concentrations of IFABP (•) or FABP-HL (▾) for 5 min, and 0.75 μM cytochrome c was then added as described in Materials and Methods. Results are expressed as the percent relative fluorescence intensity, where 100% represents the relative fluorescence intensity of SUV incubated in the presence of cytochrome c, but without FABP. Results are the average of three experiments ± SD.
RESULTS
Effect of Vesicle Concentration and Charge on Fatty Acid Transfer from FABPs to Membranes.
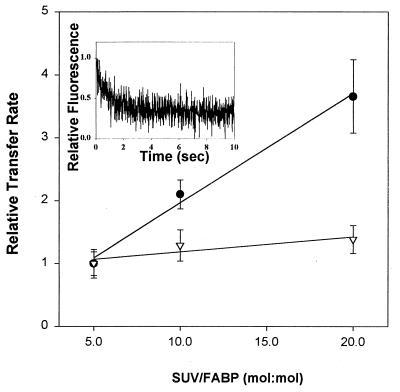
Transfer of a hydrophobic ligand from a protein to a membrane can occur by different mechanisms. One possibility is aqueous diffusion, in which the rate-limiting step is the release of the fatty acid from the protein. Another is a collisional mechanism, in which effective interaction between protein and membrane is rate-limiting for ligand transfer (10–12). To distinguish between these transfer mechanisms, AOFA transfer from the helix-less IFABP to model membranes, in comparison to the wild-type FABP, was examined as a function of increasing membrane concentration and as a function of the surface characteristics of the acceptor vesicles. In studies involving increasing acceptor-membrane level, no change in transfer rate is expected for a diffusional mechanism because the rate of ligand dissociation from the protein is independent of the acceptor. For collisional transfer, the rate of ligand movement will increase as the number of protein–membrane collisions increases and, hence, as the acceptor-membrane concentration increases. Fig. 1 shows the results obtained when a constant concentration of wIFABP or IFABP-HL was mixed with increasing concentrations of EPC SUVs. Increasing the concentration of acceptor lipid did not affect substantially the rate of transfer of 12AO from IFABP-HL, whereas the wIFABP shows a proportional increase in transfer rate with SUV concentration, in agreement with previous observations (12). These results support the hypothesis that AOFA transfer from IFABP is occurring during effective collisional interactions with acceptor membranes. In contrast, the IFABP-HL behaves quite differently, showing very minimal increases in AOFA transfer rate as a function of SUV concentration. In addition, at a 5:1 (mol/mol) ratio of SUV/FABP, the absolute rate of 12AO transfer was approximately 4-fold higher from IFABP-HL than from wIFABP (Fig. 1). This more rapid dissociation of ligand from IFABP-HL that occurs in the absence of the helical domain appears to reflect the higher dissociation constants found for IFABP-HL using both native (18) and fluorescent fatty acids.
Figure 1.
Effect of acceptor membrane concentration on AOFA transfer from FABP. Transfer of 1.5 μM 12AO from 15 μM wIFABP to EPC/NBD-PC SUV (•) and of 0.75 μM 12AO from 45 μM IFABP-HL to EPC/NBD-PC SUV (▿). Results are expressed relative to the 12AO transfer rates for 5:1 FABP/SUV, which were 0.38 ± 0.07 per sec for wIFABP and 1.25 ± 0.28 per sec for IFABP-HL. Averages of three different experiments ± SD are shown. A representative scan of experimental data is shown in the Inset (0.75 μM 12AO, 45 μM IFABP-HL, 225 μM EPC/NBD-PC SUV), with the rate of 1.35 per sec obtained by exponential fitting, as described in Materials and Methods.
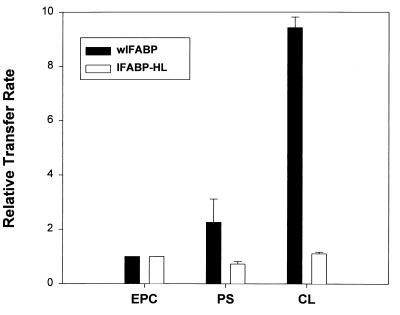
Changes in the surface charge density of the acceptor vesicles can also influence ligand transfer rates if electrostatic interactions between donor protein and acceptor membranes are involved, whereas in the case of aqueous diffusion, characteristics of the acceptor membrane would not be expected to regulate the transfer rate. Fig. 2 shows that the 12AO transfer rate from wIFABP is substantially increased by incorporation of 25 mol % PS or CL into EPC/NBD-PC acceptor membranes. 12AO transfer from IFABP-HL, on the other hand, was essentially unaffected by the presence of negatively charged phospholipid. These results confirm our previous observations for AOFA transfer from wild-type IFABP (12) and imply that the mechanism of fatty acid transfer from the IFABP-HL is likely via aqueous diffusion, qualitatively different than the collisional mechanism suggested for IFABP.
Figure 2.
Effect of vesicle charge on AOFA transfer from FABP. Transfer of 1.5 μM 12AO from 15 μM wIFABP to 150 μM EPC/NBD-PC SUV containing 25 mol % of PS or CL (solid bars) or 0.75 μM 12AO from 45 μM IFABP-HL (open bars) to 450 μM EPC/NBD-PC SUV containing 25 mol % of PS or CL. Results are expressed relative to the transfer rate of 12AO to EPC/NBD-PC membranes. Averages from three different experiments ± SD are shown.
IFABP Interaction with Membranes.
As inferred from the AOFA transfer results, the putative interaction of IFABP with membranes is sensitive to surface charge density. Because cytochrome c is known to interact as a peripheral membrane protein with acidic membranes (23–25), we determined whether IFABP could compete with cytochrome c for binding to membranes containing CL. In agreement with previous results (26), cytochrome c caused a concentration-dependent quenching of dansyl fluorescence (Fig. 3A). The results in Fig. 3B show that preincubation of CL-containing vesicles with wIFABP was effective in preventing subsequent cytochrome c binding in a concentration-dependent manner. For example, preincubation with 12 μM wIFABP resulted in a 150% increase in dansyl fluorescence over that seen in the absence of FABP. In other words, wIFABP prevented the binding of cytochrome c to the membrane and its consequent quenching of the dansyl fluorescence. IFABP-HL, in distinct contrast, was 20% as effective as wIFABP in preventing cytochrome c from binding to membranes.
DISCUSSION
Although the functions of members of the FABP family in vivo remain uncertain, it is generally thought that they include the intracellular transport of fatty acids (2). We have elucidated distinct mechanisms by which different members of this protein family transfer fatty acids to phospholipid membranes in vitro, and it is hypothesized that these mechanisms play relevant physiological roles in the intracellular transport and utilization of fatty acids (27). IFABP was shown to use direct collisions with phospholipid membranes to transfer fatty acids, and the presence of anionic phospholipids in the acceptor membranes was found to dramatically increase the rate of fatty acid transfer (12). Similar results were found for the homologous heart FABP, and subsequent point-mutagenesis studies demonstrated that basic lysine residues in the helical domain of heart FABP were critical for the enhancement of the fatty acid transfer rate to negatively charged membranes (11, 15). The deletion of the helical domain of IFABP, resulting in an all-β-sheet variant of IFABP that is remarkably stable and retains the ability to bind fatty acids (16–18) provides a unique model to study the role of this domain in the mechanism of ligand transfer. Results of the AOFA transfer and protein–membrane interaction experiments for IFABP-HL were contrasted with those for wIFABP, and this provided insight into the nature of the mechanism of transfer of fatty acids to membranes.
The absence of the α-helical domain dramatically altered the regulation of AOFA transfer from IFABP. Whereas transfer from wIFABP was directly proportional to the product of [donor FABP] × [acceptor phospholipid], a central characteristic of collision-mediated transfer processes (11), the response of IFABP-HL to SUV concentration was dampened by approximately 90%. As well, the absence of the α-helical region resulted in the total loss of sensitivity to acceptor-membrane surface charge, such that the rate of AOFA transfer from IFABP-HL was completely unmodified by the charge composition of the acceptor membranes. In contrast, and as expected from previous results, the wIFABP demonstrated a marked enhancement of fatty acid transfer rate to acidic vesicles (12). The significance of amphipathic helices in the targeting of proteins to membranes is well appreciated, and the charge characteristics of the helix appear to modulate interactions with membranes (13). In wIFABP, the α-I helix is amphipathic, with the polar face containing two basic lysine residues at positions 16 and 20 and two negatively charged glutamate residues at positions 15 and 19 (14). It is hypothesized that the charged face of the α-I helix participates in the membrane interactions that lead to the dramatic increase in AOFA transfer to anionic membranes.
The movement of fatty acids to phospholipid bilayers from wIFABP, as well as from heart and adipocyte FABPs, is characterized by a proportional increase in transfer rate with membrane concentration that is observed in the absence or presence of a net charge on the membranes, i.e., to zwitterionic as well as charged phospholipids (11, 12, 15). These results suggest that, in addition to electrostatic interactions between FABPs and membranes, some form of hydrophobic interaction may also occur. Indeed, we previously demonstrated that the acyl chain, sterol, and zwitterionic phospholipid composition of acceptor membranes modulated AOFA transfer rates from IFABP, adipocyte FABP and heart FABP (11, 12), although to a lesser magnitude than changes induced by membrane charge. Alternatively, charge separation between the phosphate and trimethylammonium groups on phosphatidylcholine may lend sufficient ionic character to promote charge–charge interactions between FABPs and membranes. In the present study, the absence of the helical domain and, in particular, the amphipathic α-I helix did not entirely eliminate the increase in AOFA transfer rate with zwitterionic phospholipid membrane concentration, indicating that additional, albeit far weaker, associations with membranes may remain for IFABP-HL. It is currently not known whether these relatively minor putative interactions are ionic and/or hydrophobic in nature.
To directly investigate the interaction between the IFABP-HL and membranes, we analyzed the inhibition of cytochrome c binding to anionic membranes caused by preincubation with IFABP-HL compared with the inhibition produced by the wIFABP. It was found that native IFABP was able to prevent the subsequent binding of cytochrome c. These results directly demonstrate that IFABP may be a membrane-interactive protein. The IFABP-HL protein, in contrast, was markedly less efficient in preventing cytochrome c binding to anionic phospholipid membranes, indicating that the α-helical domain is likely to be involved in the interaction with membranes. The absence of the helical region either reduces the degree of interaction, or the interaction is weak such that the helix-less protein is easily displaced in the presence of cytochrome c. This finding corroborates results of the kinetic experiments that indicated that the α-helical domain is a critical structural element in the process of fatty acid transfer to phospholipid membranes.
It is not known whether the α-I, α-II, or both helices are necessary for effective collisional fatty acid transfer to take place. As discussed above, the amphipathic α-I is thought to be critical for forming ion-pair interactions with membranes. The α-II helix, although not amphipathic in nature, is nevertheless a key structural element of the putative fatty acid portal, and forms long-range interactions with the β-turn between strands C and D. It is thus likely to play a role in the fatty acid transfer process as well. The NMR structures of the apo- and holo- forms of IFABP in solution have indicated considerably greater differences than those initially suggested by crystallographic analysis (5). Interestingly, the regions of the protein that exhibit the largest differences include the distal half of the α-II helix and the turn between β-strands C and D. These structural elements are considerably more disordered and flexible in the absence of bound fatty acids and exhibit diminished long-range interactions (5, 6). The fatty acid presumably enters the interior binding cavity through this locally disordered, dynamic portal. In addition, mutations in the α-II helix and C–D turn of heart FABP were shown to alter the rate of AOFA transfer to membranes, further indicating the participation of α-II in collisional transfer of fatty acid (15).
We previously suggested that the transfer of fatty acids from the FABP binding site to model membranes occurs in a multistage process (15, 5). These stages may be depicted as (i) an initial interaction between the protein surface and the target membrane, (ii) a conformational transition of the dynamic portal from the ordered closed state to a manifold of disordered open states, (iii) dissociation of the fatty acid from the protein binding site, and (iv) the association the of fatty acids with the membrane. For wIFABP, we propose that these four steps are temporally and structurally linked in that the effective collision between holo-IFABP and the membrane destabilizes the dynamic portal and catalyzes the release of the fatty acid. In this case step (i) is rate-limiting, and the transfer process exhibits collisional kinetics. For IFABP-HL, steps (i) and (ii) are eliminated, because both the surface recognition sites and the dynamic portal have been deleted. In this case, step (iii) is rate-limiting and the transfer process exhibits diffusional kinetics. It may be possible to find other IFABP variants in which the dynamic portal is destabilized without disrupting the helical domain, or vice versa. These variants should allow us to selectively eliminate individual steps in the multistage transfer of fatty acids from the protein-binding site to the acceptor membrane.
The present results with IFABP-HL provide support for this multistage transfer process, and further demonstrate that wIFABP possesses a mechanism for the collisional, targeted release of fatty acids. In the intestinal enterocyte, IFABP may use such a collisional mechanism to specifically target fatty acids to or from particular organelles or metabolic pathways. It is also possible that IFABP may interact not only with acidic phospholipids localized to the cytoplasmic leaflet of organellar membranes but also with acidic peptide domains of membrane proteins. In contrast, liver FABP exhibits diffusional transfer kinetics in vitro (12). Liver FABP may therefore be unable to target fatty acids within the enterocyte and may function as a buffer to modulate the intracellular levels of unbound fatty acids.
Acknowledgments
This work was supported by National Institutes of Health Grants DK38389 (J.S.), DK48046 (D.P.C.), and DK13332 (C.F.), and by State funds (J.S.).
ABBREVIATIONS
- FABP
fatty acid binding protein
- IFABP
intestinal FABP
- wIFABP
wild-type IFAPB
- IFABP-HL
helix-less IFABP
- SUV
small unilamellar vesicles
- AOFA
anthroyloxy-labeled fatty acid
- 12AO
12-(9-anthroyloxy)oleic acid
- EPC
egg phosphatidylcholine
- NBD-PC
N-(7-nitro-2,1,3-benzoxadiazol-4-yl) egg phosphatidylcholine
- PS
brain phosphatidylserine
- CL
bovine heart cardiolipin
- EPE
egg phosphatidylethanolamine
- DPE
dansyl-phosphatidylethanolamine
References
- 1. Banaszak L, Winter N, Xu Z, Bernlohr D A, Cowan S, Jones T A. Adv Protein Chem. 1994;45:89–151. doi: 10.1016/s0065-3233(08)60639-7. [DOI] [PubMed] [Google Scholar]
- 2.Veerkamp J H, Maatman R G. Prog Lipid Res. 1995;34:17–52. doi: 10.1016/0163-7827(94)00005-7. [DOI] [PubMed] [Google Scholar]
- 3.Sacchetini J C, Gordon J I. J Biol Chem. 1993;268:18399–18402. [PubMed] [Google Scholar]
- 4.Sacchettini J C, Gordon J I, Banaszak L. J Mol Biol. 1989;208:327–339. doi: 10.1016/0022-2836(89)90392-6. [DOI] [PubMed] [Google Scholar]
- 5.Hodsdon M E, Cistola D P. Biochemistry. 1997;36:2278–2290. doi: 10.1021/bi962018l. [DOI] [PubMed] [Google Scholar]
- 6.Hodsdon M E, Cistola D P. Biochemistry. 1997;36:1450–1460. doi: 10.1021/bi961890r. [DOI] [PubMed] [Google Scholar]
- 7.Bass N M. Chem Phys Lipids. 1985;38:95–114. doi: 10.1016/0009-3084(85)90060-x. [DOI] [PubMed] [Google Scholar]
- 8.Wootan M G, Bernlohr D A, Storch J. Biochemistry. 1993;32:8622–8627. doi: 10.1021/bi00084a033. [DOI] [PubMed] [Google Scholar]
- 9.Kim H K, Storch J. J Biol Chem. 1992;267:20051–20056. [PubMed] [Google Scholar]
- 10.Kim H K, Storch J. J Biol Chem. 1992;267:77–82. [PubMed] [Google Scholar]
- 11.Wootan M G, Storch J. J Biol Chem. 1994;269:10517–10523. [PubMed] [Google Scholar]
- 12.Hsu K T, Storch J. J Biol Chem. 1996;271:13317–13323. doi: 10.1074/jbc.271.23.13317. [DOI] [PubMed] [Google Scholar]
- 13.Anantharamaiah G M, Jones M K, Segrest J P. The Amphipathic Helix. Boca Raton, FL: CRC; 1993. pp. 110–143. [Google Scholar]
- 14.Scapin G, Gordon J I, Sacchettini J C. J Biol Chem. 1992;267:4253–4269. doi: 10.2210/pdb1ifc/pdb. [DOI] [PubMed] [Google Scholar]
- 15.Herr F M, Aronson J, Storch J. Biochemistry. 1996;35:1296–1303. doi: 10.1021/bi952204b. [DOI] [PubMed] [Google Scholar]
- 16.Kim K, Cistola D P, Frieden C. Biochemistry. 1996;35:7553–7558. doi: 10.1021/bi9529115. [DOI] [PubMed] [Google Scholar]
- 17.Steele R A, Emmert D A, Kao J, Hodson M E, Frieden C, Cistola D P. Protein Sci. 1998;7:1332–1339. doi: 10.1002/pro.5560070609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cistola D P, Kim K, Rogl H, Frieden C. Biochemistry. 1996;35:7559–7565. doi: 10.1021/bi952912x. [DOI] [PubMed] [Google Scholar]
- 19.Huang C, Thompson T E. Methods Enzymol. 1974;32:485–489. doi: 10.1016/0076-6879(74)32048-4. [DOI] [PubMed] [Google Scholar]
- 20.Storch J, Kleinfeld A M. Biochemistry. 1986;25:1717–1726. doi: 10.1021/bi00355a041. [DOI] [PubMed] [Google Scholar]
- 21.Xu Z, Buelt M K, Banaszak L J, Bernlohr D A. J Biol Chem. 1991;266:14367–14370. [PubMed] [Google Scholar]
- 22.Mustonen P, Virtanen J A, Somerharju P J, Kinnunen P K. Biochemistry. 1987;26:2991–2997. doi: 10.1021/bi00385a006. [DOI] [PubMed] [Google Scholar]
- 23.Vik S B, Georgvich G, Capaldi R A. Proc Natl Acad Sci USA. 1981;78:1456–1460. doi: 10.1073/pnas.78.3.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rytömaa M, Kinnunen P K. J Biol Chem. 1994;269:1770–1774. [PubMed] [Google Scholar]
- 25.Speck S H, Neu C A, Swanson M S, Margoliash E. FEBS Lett. 1982;164:379–382. doi: 10.1016/0014-5793(83)80321-4. [DOI] [PubMed] [Google Scholar]
- 26.Faucon J F, Dufourc E J, Lussan C, Bernon R. Biochem Biophys Acta. 1976;435:283–294. doi: 10.1016/0005-2736(76)90193-0. [DOI] [PubMed] [Google Scholar]
- 27.Storch J, Herr F, Hsu K, Kim H, Liou H, Smith E. Comp Biochem Physiol B. 1996;115:333–339. [Google Scholar]