Abstract
Sequence analysis of a heat-stable protein necessary for the activation of ADP ribosylation factor-dependent phospholipase D (PLD) reveals that this protein has a structure highly homologous to the previously known GM2 ganglioside activator whose deficiency results in the AB-variant of GM2 gangliosidosis. The heat-stable activator protein indeed has the capacity to enhance enzymatic conversion of GM2 to GM3 ganglioside that is catalyzed by β-hexosaminidase A. Inversely, GM2 ganglioside activator purified separately from tissues as described earlier [Conzelmann, E. & Sandhoff, K. (1987) Methods Enzymol. 138, 792–815] stimulates ADP ribosylation factor-dependent PLD in a dose-dependent manner. At higher concentrations of ammonium sulfate, the PLD activator protein apparently substitutes for protein kinase C and phosphatidylinositol 4,5-bisphosphate, both of which are known as effective stimulators of the PLD reaction. The mechanism of action of the heat-stable PLD activator protein remains unknown.
Keywords: ADP ribosylation factor phosphatidylcholine
Phospholipase D (PLD) in mammalian tissues is activated by a wide variety of external signals (for review, see ref. 1). The enzyme catalyzes the hydrolysis of phosphatidylcholine (PtdCho) to generate lipid mediators for the control of a broad range of physiological and pathological processes including intracellular protein trafficking, vesicular transport, membrane ruffling, cell motility, mitogenesis, oncogenesis, and inflammation (for review, see ref. 2). Biochemical studies have shown that there are at least two types of PLD in mammalian tissues. One is activated by small G protein such as ADP ribosylation factor (ARF) (3, 4), and the other is insensitive to ARF (5). One form of the latter type purified from pig lung is stimulated by sodium oleate (6), although the significance of this stimulation is unclear. Thus far, several PLDs have been cloned and their primary structures are defined (7–9). PLD1a is an ARF-dependent enzyme with a molecular mass of 124 kDa (7). A shorter splice variant PLD1b, which lacks 38 amino acids, shows similar enzymatic properties (9). PLD1 is activated by phosphatidylinositol 4,5-bisphosphate (PtdIns-4, 5-P2) (3) and to a lessor extent by phosphatidylinositol 3,4,5-trisphosphate (9). PLD1 also is activated by protein kinase C (PKC) (10). This activation is enhanced by phorbol ester in the absence of ATP. On the other hand, PLD2 with a molecular mass of 106 kDa shows ≈50% homology to PLD1 and does not require ARF but is activated by PtdIns-4,5-P2 but not by PKC (8).
Several protein factors are shown to be involved in the activation of ARF-dependent PLD. An enzyme obtained from hematopoietic cell lines, which resembles PLD1, is activated by coexistence of a 50-kDa soluble protein and ARF (11, 12).
In earlier reports from this laboratory, cytosol from the rat kidney was essential for the activation of ARF-dependent PLD purified partially from the same tissue (13). The kidney PLD is an ARF-dependent enzyme resembling PLD1. The active components in the cytosol consist of at least three protein factors; ARF, RhoA, and a heat-stable protein (13). The heat-stable protein shows a molecular size of 23 kDa upon SDS/PAGE (14). Sequence analysis now reveals that this protein is highly homologous to previously known GM2 ganglioside activator. Deficiency of this activator results in the AB-variant of GM2 gangliosidosis (for review, see ref. 15).
MATERIALS AND METHODS
Materials.
1,2-Di[1-14C]palmitoyl-sn-glycero-3-phosphocholine ([14C]PtdCho, 115 mCi/mmol) and 1-[1-14C]palmitoyl-2-lyso-sn-glycero-3-phosphocholine ([14C]lysoPtdCho, 57.0 mCi/mmol) were purchased from DuPont/NEN. Phosphatidylethanol, a standard for TLC, was from Avanti Polar-Lipids. Plasmalogen-rich phosphatidylethanolamine (PtdEtn) (60% plasmalogen) was from Serdary Research Laboratories (Englewood Cliffs, NJ). Guanosine 5′-O-(3-thiotriphosphate) (GTP-γ-S) was from Boehringer Mannheim. Glass-backed silica gel 60 was purchased from Merck. Phorbol 12-myristate 13-acetate (PMA) was a product of LC services (Woburn, MA). Other chemicals were of analytical grade.
Preparation of PLD.
PLD was purified ≈200-fold as described (13) except that the rat kidney was used instead of the bovine kidney as enzyme source and that hydroxyapatite column chromatography was omitted from the purification procedure.
Preparation of ARF.
ARF was purified from the bovine brain as described (16). The final preparation of ARF after HiLoad 16/60 Superdex 75 (Pharmacia; 1.6 × 60 cm) was ≈87% pure by silver stain. This preparation was free of heat-stable PLD activator.
Preparation of β-Hexosaminidase A.
β-Hexosaminidase A was prepared as described (17) except that the rat liver (100 g) was used instead of the human liver as enzyme source. β-Hexosaminidase A was separated from β-hexosaminidase B by DEAE-Sephadex A-50 column (1.6 × 5 cm; 10 ml) (17).
Preparation of GM2 Ganglioside Activator.
GM2 ganglioside activator was purified from the rat kidney by the method originally described for the human kidney (18). Purification procedure included heat treatment of the kidney cytosol, adjustment to pH 3.0 with trichloroacetic acid, and column chromatography on DEAE-cellulose, Octyl-Sepharose, and Superdex 200 columns (Pharmacia).
Gel Filtration of PLD Activator.
The purified heat-stable PLD activator (100 μg) was applied to a HiLoad 16/60 Superdex 200 column (Pharmacia; 1.6 × 60 cm), which had been equilibrated with a buffer (50 mM Hepes-NaOH at pH 7.4, 1 mM MgCl2, 1 mM EGTA, and 1 μg/ml leupeptin), and eluted from the column with the same buffer at a flow rate of 1 ml/min by using an FPLC system (Pharmacia). Fractions (5 ml each) were collected and assayed for the ability to activate PLD and also to enhance the enzymatic conversion of GM2 to GM3 ganglioside catalyzed by β-hexosaminidase A.
PLD Assay.
PLD activity was routinely assayed by measuring the formation of [14C]phosphatidylethanol from [14C]PtdCho in the presence of ethanol. The reaction mixture (100 μl) contained 100 ng of PLD partially purified from the rat kidney, 200 nM ARF, 100 μM GTP-γ-S, 1.6 M ammonium sulfate, 60 μM [14C]PtdCho (4, 583 dpm/nmol), 70 μM PtdEtn, 2% ethanol, 1 mM MgCl2, and 20 mM Hepes-NaOH at pH 7.4 and each activator fraction to be assayed. [14C]PtdCho and PtdEtn were separately dried, dispersed in distilled water by sonication, and added to the reaction mixture. The detailed conditions were described elsewhere (19).
Where indicated, PLD activity was alternatively determined with PtdIns-4,5-P2-containing mixed lipid vesicle as substrate under the condition described by Brown et al. (3). The reaction mixture (100 μl) contained 100 ng of PLD purified partially from the rat kidney, 200 nM ARF, 100 μM GTP-γ-S, 5 μM [14C]PtdCho (55,000 dpm/nmol), 80 μM PtdEtn, 7 μM PtdIns-4,5-P2, 2% ethanol, 1 mM MgCl2, 20 mM Hepes-NaOH at pH 7.4, and GM2 ganglioside activator or PKC to be assayed. Lipids were mixed first in chloroform, dried, dispersed in distilled water by sonication as described in ref. 3, and added to the reaction mixture. Where indicated, PtdIns-4,5-P2 was omitted from the lipid vesicles, and 1.6 M ammonium sulfate was added to the reaction mixture.
Assay of Hydrolysis of GM2 Ganglioside.
Hydrolysis of GM2 ganglioside by β-hexosaminidase A was assayed in the presence of heat-stable PLD activator or GM2 ganglioside activator under the condition specified (20). The reaction mixture (100 μl) contained 100 μM GM2 ganglioside, 0.5 unit of β-hexosaminidase A, 25 μg of ovalbumin, 10 mM acetate buffer at pH 4.6, and either the heat-stable PLD activator or GM2 ganglioside activator as specified. After incubation, GM2 and GM3 gangliosides were extracted and separated by TLC (20). The TLC plate was sprayed with resorcinol, and intensity of the bands was quantitated by a digital image processing program, image (National Institutes of Health) by using a Macintosh Computer.
Other Procedure.
Conventional PKC (mixture of PKCα, PKCβI, PKCβII, and PKCγ) was purified from rat brain as described (21). Protein was determined by the method of Bradford (22).
RESULTS
Amino Acid Sequence of PLD Activator.
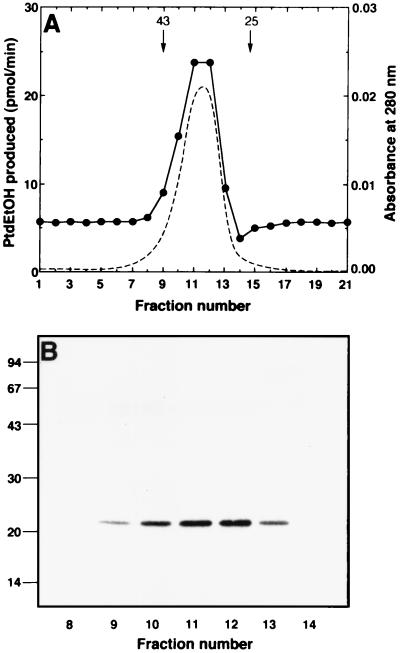
The heat-stable PLD activator was subjected to gel filtration analysis on a Superdex 200 column. The protein appeared as a symmetric peak with a molecular mass of ≈30 kDa, and its elution profile coincided with that of the PLD-stimulating activity (Fig. 1A). This protein showed a single band with 23 kDa upon SDS/PAGE (Fig. 1B).
Figure 1.
Gel filtration analysis of PLD activator. (A) Purified PLD activator was loaded on a Superdex 200 column. Each fraction was assayed for PLD activation in the presence of 200 nM ARF. •, PLD activity; dotted line, A at 280 nm. Arrows indicate the elution position of ovalbumin (43 kDa) and chymotrypsinogen A (25 kDa), which were run in parallel experiments. (B) Aliquots of the samples from Superdex 200 column were subjected to SDS/PAGE on a 12.5% gel followed by silver stain. The positions of molecular mass markers are indicated in kilodaltons.
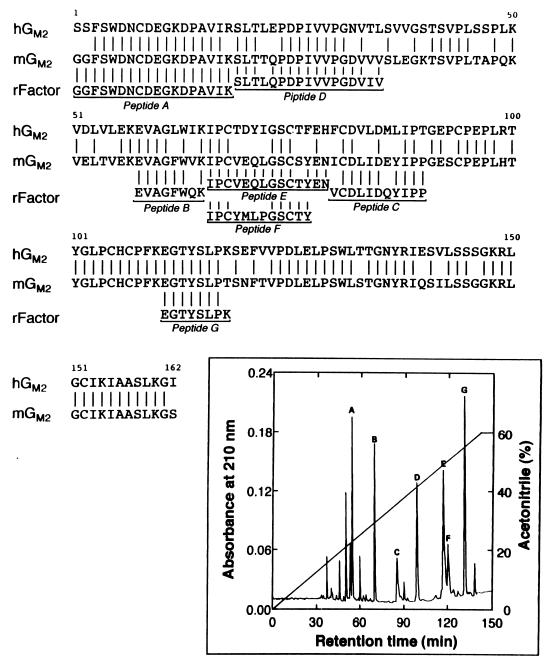
The activator protein (3 μg) was reduced with DTT in the presence of guanidine-HCl and S-pyridylethylated with 4-vinylpyridine as described (23). The protein was collected by 10% trichloroacetic acid precipitation and then subjected to SDS/PAGE. The band corresponding to the 23-kDa protein was treated overnight with 1 μg of lysylendopeptidase (24). The resulting peptide fragments were applied to a C18 column equipped to a Capillary HPLC Microblotter System (Perkin–Elmer Applied Biosystem, model 173A), which had been equilibrated with 0.1% trifluoroacetic acid. The column was washed with the same buffer at a flow rate of 5 μl/min. Peptides were separated with a 700-μl linear concentration gradient (0–60%) of acetonitrile in 0.1% trifluoroacetic acid. Several peptides were obtained (Fig. 2 Inset). The seven peptides indicated were each subjected to a Protein Sequencer Procise (Perkin–Elmer Applied Biosystems, model 492). Total 76 amino acids were sequenced from these seven peptides by automated Edman degradation analysis. The sequence shows 90% homology with the primary structure of the mouse GM2 ganglioside activator (ref. 25; Fig. 2). Peptide F, whose sequence was related to that of peptide E, also was recovered with the amount of 1:5 ratio, suggesting that the sample of the original activator isolated is a mixture of GM2 ganglioside activator isoforms. By comparison, the sequence homology between human and mouse GM2 ganglioside activators was 74%. After Superdex 200 column (Fig. 1), an aliquot of the heat-stable PLD activator was subjected directly to the sequence analysis. The only amino acid sequence detected was GGFSWDNCDEGKDPAVIKSL, which corresponded to the amino-terminal sequence of GM2 ganglioside activator, verifying the purity of the preparation.
Figure 2.
Alignment of amino acid sequences of heat-stable PLD activator and GM2 ganglioside activator. Partial peptide sequence of the rat heat-stable activator (rFactor) was compared with those of human (hGM2) (30, 31) and mouse (mGM2) (25) GM2 ganglioside activators. Vertical bars, identical amino acids. (Inset) Elution profile of peptides derived from lysylendopeptidase-treated PLD activator on a C18 column.
PLD Activator as GM2 Ganglioside Activator.
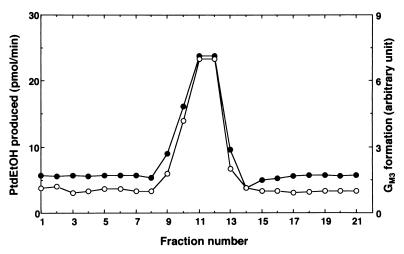
The purified PLD activator, after Superdex 200 column, was assayed for its ability to enhance enzymatic conversion of GM2 to GM3 ganglioside that is catalyzed by β-hexosaminidase A. The elution profile to stimulate PLD and that to enhance enzymatic conversion of GM2 to GM3 ganglioside support the notion that a single protein possesses the two separate functions (Fig. 3). The properties of the PLD activator, including thermal stability and abundance in the kidney, were all consistent with those of GM2 ganglioside activator described (for review, see ref. 15).
Figure 3.
Enhancement by PLD activator of enzymatic conversion of GM2 to GM3 ganglioside catalyzed by β-hexosaminidase A. Purified PLD activator was loaded on a Superdex 200 column (Fig. 1). Each fraction was assayed for the ability to stimulate enzymatic conversion of GM2 to GM3 ganglioside catalyzed by β-hexosaminidase A. PLD activation also is plotted in the same figure. •, PLD activity; E, GM3 formation.
GM2 Ganglioside Activator as PLD Activator.
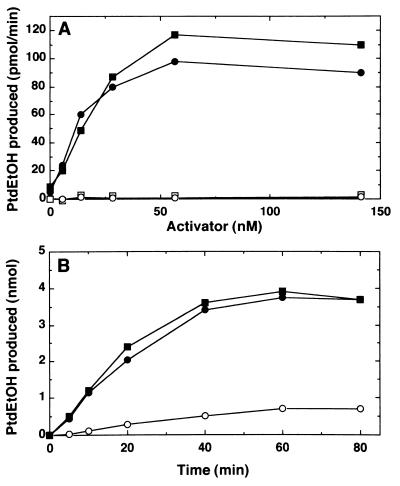
In the next experiment, GM2 ganglioside activator was separately purified from the rat kidney cytosol by the method described (18). The final preparation of the GM2 ganglioside activator was >95% pure as judged by silver stain using SDS/PAGE. In the both presence and absence of ARF, PLD alone was practically inactive, but exhibited its enzymatic activity almost linearly with increasing amounts of GM2 ganglioside activator added, and reached a plateau (Fig. 4A). GM2 ganglioside activator alone showed no effect unless ARF was added. The specific activity of GM2 ganglioside activator was comparable with the heat-stable activator. The reaction of PLD in the presence of both ARF and GM2 ganglioside activator proceeded linearly with time for 40 min (Fig. 4B). Under the given conditions, ≈40% of total PtdCho was converted to phosphatidylethanol after a 60-min incubation.
Figure 4.
Stimulation of PLD by GM2 ganglioside activator or heat-stable PLD activator. (A) Stimulation of PLD by various amounts of purified GM2 ganglioside activator or by heat-stable PLD activator. • and ○, with GM2 ganglioside activator; ■ and □, with heat-stable PLD activator; • and ■, with 200 nM ARF; E and G, without ARF. (B) Time course of PLD reaction. •, with 56 nM GM2 ganglioside activator and 200 nM ARF; ■, with 56 nM heat-stable PLD activator and 200 nM ARF; E, with 200 nM ARF alone.
PtdIns-4,5-P2 and PKC for PLD Activation.
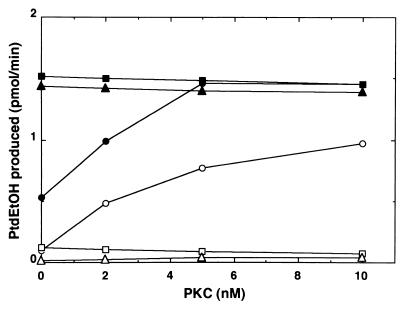
PLD1 has been shown to require PtdIns-4,5-P2 for enzymatic activity (3). The enzyme also is activated by PKC, and this activation depends on phorbol ester but occurs in the absence of ATP (10). Under the condition comparable with that made for these observations (9), PLD from the kidney exhibited considerable activity in the simultaneous addition of PKC and PtdIns-4,5-P2 (Fig. 5). It is worth noting, however, that the enzymatic activity thus obtained was enhanced further by the addition of GM2 ganglioside activator and that the activity disappeared in the presence of higher concentrations of ammonium sulfate. Further, in the presence of ammonium sulfate, the enzyme was fully active when GM2 ganglioside activator was added irrespective of the presence or absence of PtdIns-4,5-P2 and PKC. ARF alone was very weak to activate the enzyme (Figs. 4 and 5). In this experiment, PKC was added together with PMA, but ATP was omitted from the reaction mixture.
Figure 5.
Stimulation of PLD by GM2 ganglioside activator and PKC under PtdIns-4,5-P2-containing mixed lipid vesicle assay. PLD activity was measured with various concentrations of purified PKC plus 100 nM PMA in the presence of various combinations of activators as indicated. •, with 7 μM PtdIns-4,5-P2, 200 nM ARF, and 56 nM GM2 ganglioside activator; ○, with 7 μM PtdIns-4,5-P2, and 200 nM ARF; ■, with 7 μM PtdIns-4,5-P2, 1.6 M ammonium sulfate, 200 nM ARF, and 56 nM GM2 ganglioside activator; □, with 7 μM PtdIns-4,5-P2, 1.6 M ammonium sulfate, and 200 nM ARF; ▴, with 1.6 M ammonium sulfate, 200 nM ARF, and 56 nM GM2 ganglioside activator; ▵, with 200 nM ARF.
DISCUSSION
Several protein factors are known as activators of mammalian PLD1 enzyme, that include small G protein such as ARF (3, 4) and RhoA (26), PKC (9, 10), unidentified cytosolic proteins with 50 kDa (11, 12) and 23 kDa (14). PtdIns-4,5-P2 (3) and PtdEtn (27) also are involved in the reaction, although these lipids do not serve as the substrates. The present study has shown that the cytosolic 23-kDa protein is indistinguishable from the previously known GM2 ganglioside activator. Deficiency of this protein causes the accumulation of a lethal quantity of GM2 ganglioside in the lysosomes, that is Tay-Sachs disease (for review, see ref. 15). The kidney PLD enzyme used in this study is ARF-dependent and shows properties similar to PLD1 (13). The enzymatic activity obtained in the presence of both PKC and PtdIns-4,5-P2 disappears by the addition of higher concentrations of ammonium sulfate (Fig. 5), which presumably counteracts the proper association of PLD with PKC and PtdIns-4,5-P2. Yet, in the presence of this salt, the enzyme is fully activated by the addition of both ARF and the heat-stable activator protein irrespective of the presence or absence of PKC and PtdIns-4,5-P2. Apparently, this activator protein overcomes the inhibitory action of this salt, and it substitutes for PKC and PtdIns-4,5-P2. ARF is always essential for PLD activity, and PtdEtn is necessary as cosubstrate as described earlier (27). In the reaction of β-hexosaminidase A, GM2 ganglioside activator is proposed to be associated with this lipid substrate rather than acts on the enzyme (for review, see ref. 15). However, no evidence has been available thus far, suggesting that this activator protein is bound to PtdCho, which is the substrate of PLD. It is proposed that PKC activates PLD1 by protein–protein interaction through its regulatory region rather than by protein phosphorylation through its catalytic region (9, 10). Contrarily, several reports have suggested direct involvement of protein phosphorylation in this PKC action (28, 29). Obviously, such a higher concentration of ammonium sulfate is unphysiological, and thus the biological significance of this heat-stable activator protein in the PLD reaction remains to be explored. Equally, the mechanism of PtdIns-4,5-P2 action in the PLD reaction needs to be clarified further.
Acknowledgments
We thank Dr. Y. Hirabayashi (Institute of Physical and Chemical Research (RIKEN), Japan) for valuable comments on the enzymatic conversion of GM2 to GM3 ganglioside. We also thank Miss M. Honma for her skillful secretarial assistance. This work was supported in part by research grants from the Special Research Fund of Ministry of Education, Science, and Culture, Japan, the Yamanouchi Foundation for Research on Metabolic Disorders, Sankyo Neuroscience Research Institute, and Merck Sharp & Dohme Research Laboratories.
ABBREVIATIONS
- PLD
Phospholipase D
- PtdCho
phosphatidylcholine
- ARF
ADP ribosylation factor
- PtdIns-4
5-P2, phosphatidylinositol 4,5-bisphosphate
- PKC
protein kinase C
- [14C]PtdCho
1,2-di[1-14C]palmitoyl-sn-glycero-3-phosphocholine
- [14C]lysoPtdCho
1-[1-14C]palmitoyl-2-lyso-sn-glycero-3-phosphocholine
- PtdEtn
phosphatidylethanolamine
- GTP-γ-S
guanosine 5′-O-(3-thiotriphosphate)
- PMA
phorbol 12-myristate 13-acetate
References
- 1. Billah M M, Anthes J C. Biochem J. 1990;269:281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Exton J H. J Biol Chem. 1997;272:15579–15582. doi: 10.1074/jbc.272.25.15579. [DOI] [PubMed] [Google Scholar]
- 3.Brown H A, Gutowski S, Moomaw C R, Slaughter C, Sternweis P C. Cell. 1993;75:1137–1144. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- 4.Cockcroft S, Thomas G M H, Fensome A, Geny B, Cunningham E, Gout I, Hiles I, Totty N F, Truong O, Hsuan J J. Science. 1994;263:523–526. doi: 10.1126/science.8290961. [DOI] [PubMed] [Google Scholar]
- 5.Chalifa V, Möhn H, Liscovitch M. J Biol Chem. 1990;265:17512–17519. [PubMed] [Google Scholar]
- 6.Okamura S, Yamashita S. J Biol Chem. 1994;269:31207–31213. [PubMed] [Google Scholar]
- 7.Hammond S H, Altshuller Y M, Sung T-C, Rudge S A, Rose K, Engebrecht J, Morris A J, Frohman M A. J Biol Chem. 1995;270:29640–29643. doi: 10.1074/jbc.270.50.29640. [DOI] [PubMed] [Google Scholar]
- 8.Kodaki T, Yamashita S. J Biol Chem. 1997;272:11408–11413. doi: 10.1074/jbc.272.17.11408. [DOI] [PubMed] [Google Scholar]
- 9.Hammond S M, Jenco J M, Nakashima S, Cadwallader K, Gu Q-m, Cook S, Nozawa Y, Prestwich G D, Frohman M A, Morris A J. J Biol Chem. 1997;272:3860–3868. doi: 10.1074/jbc.272.6.3860. [DOI] [PubMed] [Google Scholar]
- 10.Conricode K M, Brewer K A, Exton J H. J Biol Chem. 1992;267:7199–7202. [PubMed] [Google Scholar]
- 11.Lambeth J D, Kwak J-Y, Bowman E P, Perry D, Uhlinger D J, Lopez I. J Biol Chem. 1995;270:2431–2434. doi: 10.1074/jbc.270.6.2431. [DOI] [PubMed] [Google Scholar]
- 12.Bourgoin S, Harbor D, Desmarais Y, Takai Y, Beaulieu A. J Biol Chem. 1995;270:3172–3178. doi: 10.1074/jbc.270.7.3172. [DOI] [PubMed] [Google Scholar]
- 13.Shimooku K, Akisue T, Jinnai H, Hitomi T, Ogino C, Yoshida K, Nakamura S, Nishizuka Y. FEBS Lett. 1996;387:141–144. doi: 10.1016/0014-5793(96)00483-8. [DOI] [PubMed] [Google Scholar]
- 14.Akisue T, Jinnai H, Hitomi T, Miwa N, Yoshida K, Nakamura S. FEBS Lett. 1998;422:108–112. doi: 10.1016/s0014-5793(97)01611-6. [DOI] [PubMed] [Google Scholar]
- 15.Fürst W, Sandhoff K. Biochim Biophys Acta. 1992;1126:1–16. doi: 10.1016/0005-2760(92)90210-m. [DOI] [PubMed] [Google Scholar]
- 16.Taylor T C, Kahn R A, Melançon P. Cell. 1992;70:69–79. doi: 10.1016/0092-8674(92)90534-j. [DOI] [PubMed] [Google Scholar]
- 17.Li S-C, Hirabayashi Y, Li Y-T. J Biol Chem. 1981;256:6234–6240. [PubMed] [Google Scholar]
- 18.Conzelmann E, Sandhoff K. Methods Enzymol. 1987;138:792–815. doi: 10.1016/0076-6879(87)38068-1. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura S, Shimooku K, Akisue T, Jinnai H, Hitomi T, Kiyohara Y, Ogino C, Yoshida K, Nishizuka Y. Proc Natl Acad Sci USA. 1995;92:12319–12322. doi: 10.1073/pnas.92.26.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S-C, Li Y-T. J Biol Chem. 1976;251:1159–1163. [PubMed] [Google Scholar]
- 21.Kikkawa U, Go M, Koumoto J, Nishizuka Y. Biochem Biophys Res Commun. 1986;135:636–643. doi: 10.1016/0006-291x(86)90040-9. [DOI] [PubMed] [Google Scholar]
- 22.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 23.Hermodson M A, Ericsson L H, Neurath H, Walsh K A. Biochemistry. 1973;12:3146–3153. doi: 10.1021/bi00741a002. [DOI] [PubMed] [Google Scholar]
- 24.Jekel P A, Weijer W J, Beintema J J. Anal Biochem. 1983;134:347–354. doi: 10.1016/0003-2697(83)90308-1. [DOI] [PubMed] [Google Scholar]
- 25.Yamanaka S, Johnson O N, Lyu M S, Kozak C A, Proia R L. Genomics. 1994;24:601–604. doi: 10.1006/geno.1994.1674. [DOI] [PubMed] [Google Scholar]
- 26.Bowman E P, Uhlinger D J, Lambeth J D. J Biol Chem. 1993;268:21509–21512. [PubMed] [Google Scholar]
- 27.Nakamura S, Kiyohara Y, Jinnai H, Hitomi T, Ogino C, Yoshida K, Nishizuka Y. Proc Natl Acad Sci USA. 1996;93:4300–4304. doi: 10.1073/pnas.93.9.4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue H, Shimooku K, Akisue T, Nakamura S. Biochem Biophys Res Commun. 1995;210:542–548. doi: 10.1006/bbrc.1995.1694. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt M, Voss M, Thiel M, Bauer B, Grannass A, Tapp E, Cool R H, de Gunzburg J, von Eichel-Streiber C, Jakobs K H. J Biol Chem. 1998;273:7413–7422. doi: 10.1074/jbc.273.13.7413. [DOI] [PubMed] [Google Scholar]
- 30.Xie B, McInnes B, Neote K, Lamhonwah A-M, Mahuran D. Biochem Biophys Res Commun. 1991;177:1217–1223. doi: 10.1016/0006-291x(91)90671-s. [DOI] [PubMed] [Google Scholar]
- 31.Klima H, Tanaka A, Schnabel D, Nakano T, Schröder M, Suzuki K, Sandhoff K. FEBS Lett. 1991;289:260–264. doi: 10.1016/0014-5793(91)81084-l. [DOI] [PubMed] [Google Scholar]