Abstract
In addition to reference measurement procedures and reference materials, reference or calibration laboratories play an integral role in the implementation of measurement traceability in routine laboratories. They provide results of measurements using higher-order methods, e.g. isotope dilution mass spectrometry and may assign values to materials to be used for external quality assessment programs and to secondary reference materials. The requirements for listing of laboratories that provide reference measurement services include a statement of the metrological level or principle of measurement, accreditation as a calibration laboratory according to ISO 15195 and the participation in a proficiency testing system (regular inter-laboratory comparisons) for reference laboratories. Ring trials are currently conducted for thirty well-defined measurands and the results are made available to all laboratories. Through the use of reference laboratory services that are listed by the Joint Committee for Traceability in Laboratory Medicine there is the opportunity to further promote traceability and standardisation of laboratory measurements.
Traceability
The concept of measurement traceability provides probably the most important strategy to achieve standardisation in laboratory medicine and is aimed at accurate and comparable measurement results regardless of the method, the measurement procedure (test kit) and of the laboratory where analyses are carried out.
According to the ‘Vocabulary in Metrology (VIM)’ and the ‘Guide to the Expression of Uncertainty in Measurement (GUM)’ measurement traceability is defined as:
“The property of the result of a measurement or the value of a standard whereby it can be related to stated references, usually national or international standards, through an unbroken chain of comparisons all having stated uncertainties”.1,2
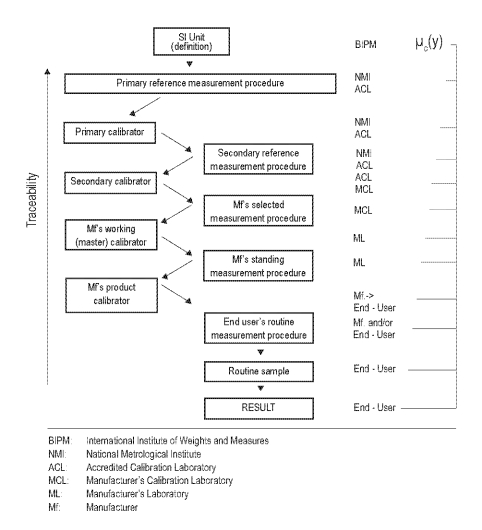
As demonstrated in Figure 1, traceability of a value attributed to a routine sample, a calibrator or a control material is established by a series of comparative measurements using measurement procedures and reference materials in a chain of increasing hierarchical order. Since each link in the traceability chain contributes to the uncertainty of the result it is advisable to omit as many steps as possible. In terms of metrology it would be ideal to omit all in-between steps of the traceability chain and to measure the routine sample directly by use of a primary reference procedure; this of course is not feasible.
Figure 1.
Calibration hierarchy and traceability according to EN ISO 17511.
The complete traceability chain as presented here is valid only for those measurable quantities which can have a value expressed in SI units. When primary or secondary calibrators are not available the traceability chain for many measurands in laboratory medicine ends at a lower level, e.g. at the manufacturer’s standing measurement procedure. When a manufacturer detects a new diagnostic marker and defines the measurable quantity by establishing a measurement procedure for this marker, the manufacturer’s measurement procedure will form the top of the traceability chain. Nevertheless, even in this simple situation, the principles of the traceability concept are applicable.
An inevitable precondition for establishing traceable results to calibrators and control materials is the specificity of the measurement procedures applied. Results of measurement cannot be traceable if the procedure applied partially detects components which are not consistent with the definition of the measurand.
Consequently, the In Vitro Diagnostics (IVD) Directive of the European Union stipulates that values assigned to calibrators and control materials must be traceable to reference materials and/or reference methods of a higher metrological order.3 To this end, the Joint Committee for Traceability in Laboratory Medicine (JCTLM) was established in 2002. Three organisations contribute to the JCTLM: the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC), the Bureau International des Poids et Mesures (BIPM) and the International Laboratory Accreditation Cooperation (ILAC). Within the JCTLM, two working groups (WG) have been established: WG1 deals with reference materials and reference measurement procedures. Since 2004, such materials and procedures have been listed on the BIPM website.4 The task of WG2 is to list reference measurement laboratories. The first list of reference measurement services provided by reference (calibration) laboratories was published in 2007. The lists will be reviewed annually.
Reference Laboratories
Hierarchical structure
Establishing networks of reference laboratories is – in addition to reference measurement procedures and reference materials - one of the biggest challenges in implementing the concept of measurement traceability. The implementation of the concept of traceability requires a measurement infrastructure consisting of three distinct hierarchical levels, namely:
National Metrology Institutes
Reference (Calibration) Laboratories
Routine (Testing) Laboratories.
This hierarchical infrastructure requires further explanation. At the top are the National Metrology Institutes which have to demonstrate their competence in the CIPM MRA (International Committee on Weights and Measures Mutual Recognition Arrangement) Key Comparisons and which are listed in Appendix C of the KCDB (Key Comparison Database). They may certify values in reference materials, e.g. of the National Institute of Standards and Technology (NIST, USA), the Institute of Reference Materials and Measurements (IRMM, EU), or they may provide results for measurement comparisons with Reference Laboratories thereby establishing a link to this group of intermediate level laboratories. These in turn may assign values to control material for External Quality Assessment Schemes (EQAS) used by routine laboratories.
Laboratory requirements for listing reference measurement services
The particular requirements for listing of reference measurement services offered by reference (calibration) laboratories are described in the procedure manual of JCTLM WG2 which may be downloaded from the BIPM website.4 There is general agreement that reference laboratories will be identified according to:
The metrological level or principle of measurement;
Accreditation according to ISO 15195 as calibration laboratory;5 and
Participation in a proficiency testing system (regular inter-laboratory comparisons) for reference laboratories.
With respect to the metrological level of the reference measurement procedure, it should be emphasised that this usually requires dedicated and highly specific techniques and equipment which usually is not available to basic testing laboratories. One of the most important analytical principles for establishing reference measurement procedures is isotope dilution mass spectrometry (IDMS). This technique, which is used today in many fields of chemical metrology as the high level ‘primary method’, was initially developed in a clinical chemical reference laboratory as early as 1970 for the measurement of steroid hormones in human body fluids, long before the concept of traceability became popular.6 Ever since, this technique has provided one of the most powerful tools for establishing reference method values for many substrates and metabolites in reference materials, calibrators and control materials, e.g. for external quality assessment.
Reference methods for a large number of metabolites and substrates, steroid hormones, thyroid hormones and therapeutic drugs have been developed using the analytical principle of the so-called ‘primary method’ IDMS in the reference laboratories of the German Society for Clinical Chemistry and Laboratory Medicine (DGKL) and in many other academic and commercial institutions. These include IDMS methods for creatinine, urea, cholesterol, total glycerol, uric acid, as well as the steroid hormones, thyroxine and therapeutic drugs.
1. Reference measurement procedure - IDMS
The analytical principle of IDMS as a reference measurement procedure is demonstrated using the measurement of estradiol-17β in human serum as an example. To a serum sample containing about 250 pg estradiol-17β, 250 pg estradiol-17β labelled with 14C or 13C is added. The two steroids are extracted and cleaned by column chromatography on Sephadex LH-20. The purified and derivatised samples are injected into a capillary column for gas chromatography. They are transported through the column by helium as carrier gas and eluted into the mass spectrometer after a retention time, which is characteristic for the substances under investigation. In the ion source of the mass spectrometer, the substances are transformed to positively charged molecular ions and to smaller fragment ions. These are separated in a magnetic or a quadrupole field. With the conventional technique of gas chromatography mass spectrometry, complete mass spectra can be recorded, showing the molecular ions and fragment ions in a substance-characteristic pattern.
For the quantitative application used here, a different technique applies. The ion separation system of the instrument is adjusted to two masses, one characteristic for the nonlabelled and one characteristic for the labelled substance under investigation. As a result, two chromatograms are recorded simultaneously after processing a serum sample. Although the sample, extracted and chromatographically cleaned, contains hundreds of accompanying components from the biological matrix in addition to estradiol-17β and the labelled steroid, it is almost exclusively these two that show up during gas chromatography when the mass spectrometric detector is adjusted to these specific masses as shown in Figure 2.
Figure 2.
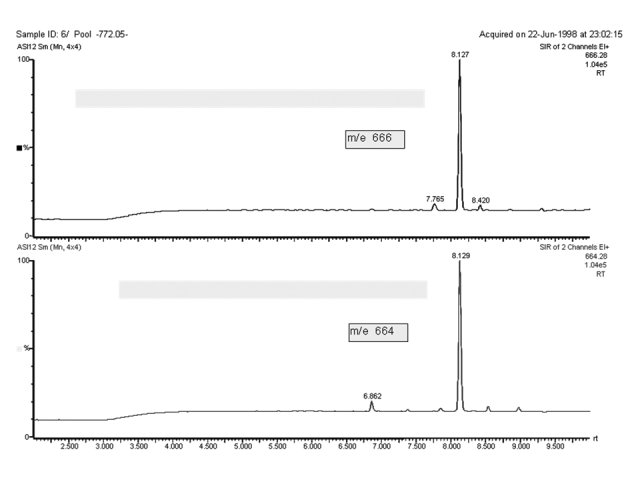
IDMS, the selected ion recording at m/z 664 and 666 after processing a serum sample and formation of the heptafluorobutyric ester derivative for measurement of estradiol-17β.
The two peaks are quantified by computer-assisted integration. The isotope ratio of the non-labelled and the labelled estradiol-17β derivatives is measured by IDMS. The analytical results are then calculated from the isotope ratios determined in each sample and in a series of standards containing defined mixtures of the labelled and the non-labelled steroid. The accuracy of this analytical process is achieved by means of the high specificity of mass spectrometry in combination with capillary gas liquid chromatography and the exact control of recovery that underlies isotope dilution.
Of course, IDMS is only one of several specific measurement principles, such as flame emission or atomic absorption spectrophotometry, coulometry and ion chromatography. In some cases, the measurands are defined by exactly defined reference measurement procedures, e.g. the IFCC enzyme reference procedures for ALT, AST, GGT, CK and amylase for which all individual steps are decribed in detail.
The JCTLM data base on the BIPM JCTLM website provides easy access to the approved reference methods for many measurands in laboratory medicine.4 This list is updated annually. Interested readers are referred to this data base which includes all relevant literature references not provided in the context of this article.
2. Accreditation requirements
Accreditation according to ISO 15195 as a calibration laboratory is the second criterion for listing reference laboratory services.5 This includes all management and technical requirements for reference measurement (calibration) laboratories in laboratory medicine. ISO 15195 encompasses also ISO 17025 as a normative reference which is a general standard describing the requirements of testing and calibration laboratories.7 In this context, ISO 15195 exclusively refers to the requirements for calibration laboratories in laboratory medicine, which are referred to as reference laboratories.
Currently, only a very few laboratories in laboratory medicine are accredited as reference laboratories according to ISO 15195. This will change soon as more accreditation bodies provide this service. During a transition period of two years, the JCTLM will also accept laboratories that are preparing for accreditation according to ISO 15195.
Although ISO 17025 covers all types of laboratories, routine (testing) and calibration laboratories, it includes separate paragraphs dedicated specifically to calibration laboratories. In the accreditation process, it should be emphasised that compliance with the calibration laboratory requirements is essential and JCTLM will ask for accreditation according to ISO 15195. While the management system requirements formulated in ISO 15195 are similar to those requested for any type of laboratory, the technical requirements, however, are dedicated to the metrological aspects that have to be observed by laboratories responsible for ‘calibration’.
3. Proficiency testing requirements
Participation in a proficiency testing system dedicated to reference (calibration) laboratories is the third requirement for listing reference measurement laboratory services by the JCTLM. According to the requirements formulated in the JCTLM WG2 procedure manual, such external quality assessment schemes must be dedicated to reference laboratories. The identity of the participating laboratories has to be disclosed and the results must be made publicly available.
Up until 2003, the candidate reference laboratories only occasionally performed comparative measurements, e.g. in the International Measurement Evaluation Programme (IMEP) of the IRMM or in national networks conducted by a National Metrology Institute. To date, the Consultative Committee on Amount of Substance (CCQM) has provided a small number of ring trials (cholesterol, glucose and creatinine) where only National Metrology Institutes were accepted as participants. In view of this, in 2003, the IFCC launched an external quality assessment scheme for reference laboratories in collaboration with the Reference Institute of Bioanalysis of the DGKL.
The rules for conducting this external quality assessment scheme dedicated to reference (calibration) laboratories are described in a procedure manual. All information is available on the DGKL website including the procedure manual as well as the results of participating laboratories and all technical and organisational details.8 The laboratories are requested to report results from reference measurement procedures - preferably those listed by the JCTLM. In these ring trials for reference laboratories, results and addresses will no longer be considered confidential. However, the participants have the possibility to withdraw individual laboratory results for particular measurands one month after a preliminary report has been sent only to the participating laboratories. After this time, each laboratory must agree that its results and identity are disclosed, which is not the general procedure for other ring trials where the identity of participants usually is strictly confidential. The evaluation of the ring trial is then published on the website.
Ring trials for reference laboratories are now available for some thirty different measurands according to the following groups of measurands:
metabolites and substrates
electrolytes
enzymes
hormones
proteins
therapeutic drugs.
The number of measurands will be extended on request by participants and the JCTLM.
In each ring trial key measurands will be selected for each group of measurands. This is necessary to collect statistically sound information from the ring trials and is explained by the following example. If an EQAS organisation offers ring trials for five enzyme activity measurements according to the IFCC 37 °C reference procedures it is likely that due to the workload required, not all laboratories will participate for all measurands. At the worst, laboratory A will analyse ALT, laboratory B, AST, laboratory C, GGT, laboratory D, CK and laboratory E, LDH. Due to the limited number of participating laboratories it will then be difficult to collect a statistically relevant number of results for each of the measurands necessary to demonstrate comparability of results from different laboratories. Therefore, the EQAS organiser will select one key measurand from each group of measurands for every ring trial and request results for this key measurand are provided. Participation for all other measurands is voluntary. The selected key measurand for each group of measurands will change from one ring trial to the next. As expected, the majority of results are reported for the key measurands.
For the procedure, two different samples are distributed in each ring trial and results reported by the laboratories are evaluated in Youden plots.9 An example is given in Figure 3 for the results of four laboratories that participated in 2005 for the measurement of calcium in serum. Each dot in the Youden plot indicates the two results from a laboratory with the result for sample A read from the abscissa and that for sample B from the ordinate. The relative standard deviations calculated from the laboratory results for both samples are lower than 1%, indicating the high performance of measurement by these laboratory services.
Figure 3.
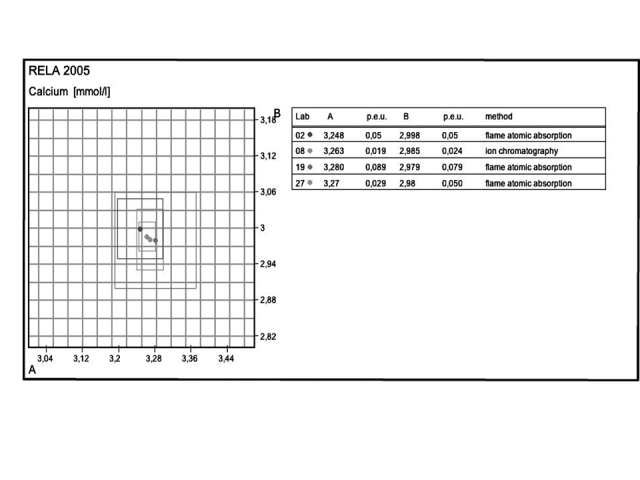
Youden plot from a ring trial for the measurement of calcium in two serum control materials by four reference laboratories. The grid size in the diagram reflects 1% distances. The two results for each laboratory are indicated by a grey scale dot. The individual uncertainty limits are given as rectangles in the same grey scale. The table lists the laboratory codes, the results and uncertainties (p.e.u.) for sample A and B, and the method applied. The identity of the laboratories can be obtained from the website (www.dgkl-rfb.de)8 using the laboratory code numbers.
Another example is shown in Figure 4 where the results for cortisol measurement from four reference laboratories are displayed on the Youden plot. In relation to the routine laboratory results, the reference laboratories show a very small dispersion. This justifies the use of reference method values as targets for the evaluation of routine laboratory results in proficiency testing.
Figure 4.
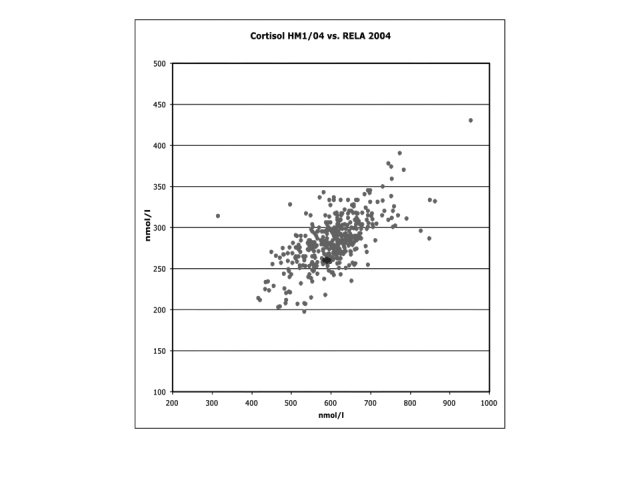
Youden plot from a routine laboratory external quality assessment ring trial for cortisol performed in Germany. The four dots in the middle highlighted in dark black indicate the results of four reference laboratories versus the dots in grey indicate the results for routine laboratories.
To date, the concept of traceability to the SI international system of units has been applicable only to well-defined measurands. For many groups of substances in laboratory medicine, the measurands are not exactly known or different measurement procedures determine different measurands despite the fact that the same name is used, e.g. in the fields of proteo hormones and tumour markers. Before the concept of traceability to SI units can be established here, scientific work is necessary to define the measurands in terms of their molecular structure.
For many well characterised measurands, the global agreement on reference materials, methods and reference laboratory services approved by the JCTLM will improve accuracy in laboratory medicine by providing a rational basis for standardisation – which will benefit patient care.
Footnotes
Competing Interests: None declared.
References
- 1.International Organization for Standardization. International Vocabulary of Basic and General Terms in Metrology. 2. Geneva, Switzerland: ISO; 1993. [Google Scholar]
- 2.International Organization for Standardization. Guide to the Expression of Uncertainty in Measurement. 1. Geneva, Switzerland: ISO; 1993. [Google Scholar]
- 3.Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices. Official Journal of the European Communities 1998; L 331.
- 4.BIPM: Bureau International des Poids et Mesures. [Accessed 14 September 2007]; doi: 10.1364/ao.42.001019. www.bipm.org. [DOI] [PubMed]
- 5.ISO 15195:2003. Laboratory medicine – Requirements for reference measurement laboratories. 1. Geneva, Switzerland: ISO; 2003. [Google Scholar]
- 6.Siekmann L, Hoppen HO, Breuer H. Zur gaschromatographisch-massenspektrometrischen bestimmung von steroidhormonen in körperflüssigkeiten unter verwendung eines multiple ion detectors (fragmentographie) Z Anal Chem. 1970;252:294–8. [Google Scholar]
- 7.ISO/IEC 17025:2005. General requirements for the competence of testing and calibration laboratories. Geneva, Switzerland: ISO; 2005. [Google Scholar]
- 8.RELA-IFCC. External quality assessment scheme for reference laboratories in laboratory medicine. [Accessed 14 September 2007]; doi: 10.1515/cclm-2012-0722. www.dgkl-rfb.de:81. [DOI] [PubMed]
- 9.Youden WJ. Graphical diagnosis of interlaboratory test results. Industrial Quality Control. 1959;15:24–8. [Google Scholar]