Abstract
Transforming growth factor β (TGF-β) was found to inhibit differentiation of myogenic cells only when they were grown to high density. Inhibition also occurred when myogenic cells were cocultured with other types of mesenchymal cells but not when they were cocultured with epithelial cells. It is therefore possible that some density-dependent signaling mediates the intracellular response to TGF-β. Within 30 min of treatment, TGF-β induced translocation of MEF2, but not MyoD, myogenin, or p21, to the cytoplasm of myogenic cells grown to high density. Translocation was reversible on withdrawal of TGF-β. By using immune electron microscopy and Western blot analysis on subcellular fractions, MEF2 was shown to be tightly associated with cytoskeleton membrane components. To test whether MEF2 export from the nucleus was causally related to the inhibitory action of TGF-β, we transfected C2C12 myoblasts with MEF2C containing the nuclear localization signal of simian virus 40 large T antigen (nlsSV40). Myogenic cells expressing the chimerical MEF2C/nlsSV40, but not wild-type MEF2C, retained this transcription factor in the nucleus and were resistant to the inhibitory action of TGF-β. We propose a mechanism in which the inhibition of myogenesis by TGF-β is mediated through MEF2 localization to the cytoplasm, thus preventing it from participating in an active transcriptional complex.
Transforming growth factor β (TGF-β) exerts a variety of biological effects in different cells, inducing or inhibiting proliferation or differentiation. In the case of skeletal myogenesis, TGF-β was originally shown to reversibly inhibit terminal differentiation, without interfering significantly with cell proliferation (1). However, we and others (2, 3) reported that embryonic myoblasts are resistant to the inhibitory effect of TGF-β and postulated a model in which the differential response to this molecule could be instrumental in the formation of primary and secondary fibers.
More recently we have also shown that the differential expression of protein kinase Cθ (PKCθ) in embryonic and fetal myoblasts may mediate the differential response to TGF-β (4). However, the understanding of this process is hampered by the fragmentary information available on the intracellular transducers that mediate the inhibitory effect of TGF-β on myogenic cells (5–8). In the last 2 years much work has concentrated on a class of effector molecules, termed SMAD, which appear to transduce the TGF-β signal from the cell-membrane receptor to the nucleus (9). However, much of this work has focused on TGF-β-induced growth arrest and virtually nothing is known of the intracellular signaling that leads to inhibition of myogenesis.
In the course of experiments designed to produce expression of a dominant negative TGF-β receptor in adult mouse satellite cells, we observed that all selected clones were capable of differentiating in the presence of high concentrations of TGF-β, independent of the dominant negative function. To elucidate this unexpected result, we investigated the effect of cell density on the TGF-β-induced inhibition of myogenesis.
Here we report that myogenic cells do differentiate in the presence of TGF-β provided that no cell-to-cell contact is established at the time during which myoblasts are exposed to the molecule. Furthermore, we show that this phenomenon depends on density-dependent MEF2 translocation to a cytoplasmic compartment.
MATERIALS AND METHODS
Cell Cultures.
Genetically marked or wild-type satellite cells were isolated from MLC3F/nlacZ transgenic mice, expressing a transgene encoding nucleus-localized β-galactosidase under the transcriptional control of the myosin light-chain 1/3-fast promoter as described (3). In these mice, expression is restricted to skeletal and cardiac muscle (16, 17). Primary satellite cells; C2C12, DyB (18), and L5 myogenic cells; 3T3 and 10T1/2 fibroblasts; and C5 (19) epithelial cells were grown in DMEM supplemented with 15% fetal calf serum (FCS), 1% gentamycin, and 0.3 mM β-mercaptoethanol (growth medium, GM). Myogenic differentiation was induced by transfering the cells to DMEM supplemented with 2% FCS (differentiation medium, DM). Unless otherwise indicated, cells were transferred to DM and after 2 hr treated with TGF-β1 (5 ng/ml; Sigma) in DM for various times.
Plasmids and Cell Transfection.
Plasmid pMEF2C/nlsSV40 was obtained by cutting pMEF2C (20) with SphI and XbaI and inserting an SphI/XbaI-digested double-stranded oligonucleotide encoding the nuclear localization signal of simian virus 40 large T antigen (oligonucleotide coding strand sequence was 5′-GCATGCCACCAAAGAAGAAGAGAAAGGTTGAAGACCCAGCAACTTGAAGCTTTCTAGA-3′). The resulting plasmid encodes a human MEF2C protein in which the last eight C-terminal amino acids have been replaced with the simian virus 40 nuclear localization signal (21). C2C12 or primary satellite cells were transfected by using the polyethylenimine method as described (22). In all cases 105 cells were plated on 60-mm tissue culture dishes and transfected with 10 μg of the indicated plasmid. Cells were transferred to DM (±5 ng/ml TGF-β1) 24 hr after transfection and processed for immunofluorescence at various times thereafter.
Immunocytochemistry.
Immunofluorescence analysis was carried out as described (23) using the following antibodies: MF20, a monoclonal antibody that recognizes all sarcomeric myosins (24); an anti-MyoD polyclonal antibody (25); rabbit polyclonal antibodies directed to both MEF2A and C, or to MEF2B or MEF2D (26); commercial anti-MEF2C and TGF-β Receptor II and I antibodies (Santa Cruz Biotechnology); an antibody against β-catenin (Transduction Laboratories, Lexington, KY); and a monoclonal antibody against N-cadherin (27). Briefly, cell cultures were fixed with 4% paraformaldehyde for 10 min at 4°C, washed in PBS, and incubated at 4°C overnight with the primary antibodies in PBS containing 1% BSA. At the end of the incubation, the cells were washed with PBS and incubated for 1 hr at room temperature with rhodamine-conjugated goat anti-mouse IgG and with fluorescein-conjugated goat anti-rabbit IgG (1:100 dilution; Sigma). Finally, cultures were washed, mounted in 75% glycerol/PBS (pH 7.5), and observed under a Zeiss Axiophot epifluorescence microscope.
Immunogold Electron Microscopy.
Cultured cells were fixed in 2.5% glutaraldehyde in Tyrode’s solution (pH 7.4) for 30 min at room temperature, postfixed in 1% OsO4 in Tyrode’s, dehydrated in ethanol, embedded in Durcupan, and then sectioned on a Reichert–Jung ultramicrotome. Ultrathin sections were collected on uncoated nickel grids, floated for 10 min on 2% BSA in PBS, washed in 1% BSA in PBS, transferred onto droplets of primary antibody (polyclonal anti-MEF2 A/C, diluted 1:20), and incubated overnight at 4°C in a humid chamber. Control sections were incubated in BSA-PBS without primary antibody. The sections were washed six times for 5 min in BSA-PBS and then incubated with 10 nM gold-conjugated goat anti-rabbit IgG (diluted 1:30; EY Laboratories) for 2 hr at room temperature in a humid chamber. After several washes in PBS and water, the sections were stained with uranyl acetate and lead citrate, and examined with a Jeol 1200EX electron microscope.
Cell Fractionation and Immunoblotting.
Both control and TGF-β-treated cells were homogenized in sample buffer, heated at 100°C for 5 min, and subjected to SDS/PAGE on 8% polyacrylamide gels. After electrophoretic separation, proteins were transferred to nitrocellulose filters for 2 hr at 4°C (200 mA). The filters were blocked by incubation with TBST (50 mM Tris⋅HCl, pH 8/137 mM NaCl/0.1% Tween 20) containing 5% lowfat dry milk for 2–14 hr and then incubated overnight with the indicated antibodies in tris(hydroxymethyl)aminomethane (TBST). The filters were washed five times in TBST and incubated for 2 hr with horseradish peroxidase-conjugated secondary antibodies. After five further washes, antigen-antibody complexes were visualized with the enhanced chemiluminescence kit from Amersham. When specified, samples were incubated for 90 min at 30°C in the presence of 40 units of alkaline phosphatase. To separate the various subcellular fractions, control and TGF-β-treated cells were homogenized in 10 volumes of ice-cold buffer (10 mM Tris⋅HCl, pH 7.5/5 mM MgCl2/0.1 units aprotinin). KCl (100 mM) was then added, and nuclei were collected by centrifugation at 800 × g. The supernatant was centrifuged at 100,000 × g. The resulting supernatant was harvested and the pellet extracted with the same buffer supplemented with 1% Nonidet P-40 for 20 min on ice. The extract was centrifuged again at 100,000 × g and the resulting pellet extacted with RIPA buffer [50 mM Tris⋅HCl,pH 7.5/150 mM NaCl/10 mM KCl/1 mM EDTA/20 mM NaF/1 mM Na2MoO4/1 mM Na2VO4/5 mM DTT/0.25% sodium deoxycholate/1% Nonidet P-40/5 μg/ml leupeptin, pepstatin, and aprotinin/1 mM PMSF(phenylmethylsulfonyl fluoride)]. Aliquots of the different fractions, containing 30 μg of protein, were mixed with an equal volume of sample buffer and processed as described above.
RESULTS
TGF-β Inhibits Myogenic Differentiation only in the Presence of Cell-to-Cell Contacts.
Preliminary experiments (not shown) indicated that clones of murine satellite cells differentiate into multinucleated myotubes when exposed to TGF-β at concentrations up to 10 ng/ml, which efficiently inhibit differentiation of the same cells grown to high density.
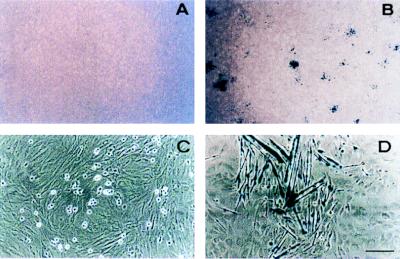
To investigate the basis of this phenomenon, genetically marked satellite cells from MLC3F-nlacZ mice were cultured at clonal density on a variety of substrates (collagen, laminin, fibronectin) or on feeder layers of 3T3, 10T1/2 fibroblasts, C2C12 or DyB myogenic cells, and C5 epithelial cells. After 5 days of clonal growth in GM, the cultures were transferred to DM, and half of them received 5 ng/ml TGF-β. After 3 additional days, all control cultures contained clones of similar size (50–100 cells) mostly (about 80%) composed of differentiated cells (revealed by expression of β-galactosidase and myotube formation). In the presence of TGF-β, differentiation was inhibited in clones growing on fibroblast (Fig. 1 A–C) or myogenic cell feeder layer but occurred in clones grown on epithelial cell feeder layers (Fig. 1 B–D) or on various substrates. A quantitative analysis of these data is reported in Fig. 2, which shows that TGF-β inhibits differentiation in a vast majority (>80%) of the cells grown on mesenchymal feeder layers (C2C12, 10T1/2) but in less than 30% of the cells grown on epithelial feeder layers or on plastic (C5, −). Thus, it appears that to inhibit myogenic differentiation in vitro, TGF-β requires high cell densities and that cell-to-cell contact needs to be established specifically among mesenchymal cells.
Figure 1.
Density-dependent inhibition of myogenesis by TGF-β. Morphology of satellite cell clones from MLC3F-nLacZ mice grown on 3T3 fibroblasts (A and C) or C5 epithelial cells (B and D) in the continuous presence of 5 ng/ml TGF-β for 14 days and then stained with 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) for β-galactosidase activity. At low magnification (A and B) no differentiated clones (i.e., β-galactosidase-positive) can be seen on a 3T3 feeder layer (A), whereas many clones are visible on C5 cells (B). At higher magnification, undifferentiated, round-shaped satellite cells are visible on the 3T3 fibroblasts (C). (Bar = 50 μm.)
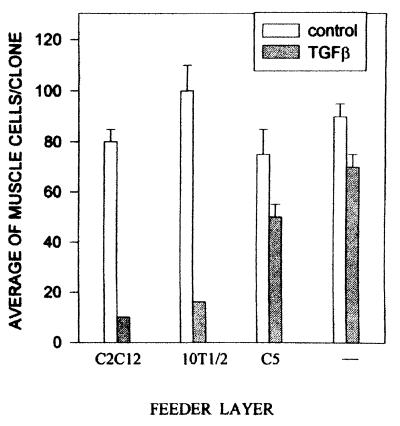
Figure 2.
Quantitative analysis of the differential inhibition of myogenesis by TGF-β in satellite-cell clones from MLC3F-nLacZ mice, grown on feeder layers of C2C12 myogenic cells (C2C12), 10T1/2 fibroblasts (10T1/2), C5 epithelial cells (C5) or on plastic (−). After 4 days of culture in the continuous presence of 5 ng/ml TGF-β, cells were stained for β-galactosidase activity, and the number of positive cells per clone was scored in 20 randomly selected microscopic fields of at least five different cultures. Error bars: SEM in control (open bars) and TGF-β-treated cultures (shaded bars).
TGF-β Induces Relocalization of MEF2 in the Cytoplasm of Myogenic Cells Grown to High Density.
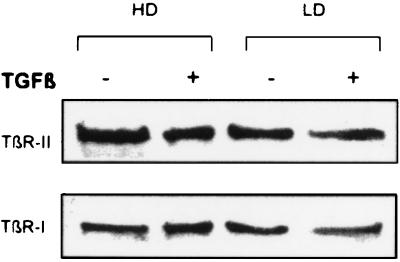
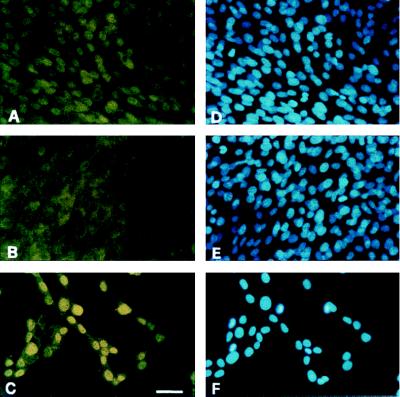
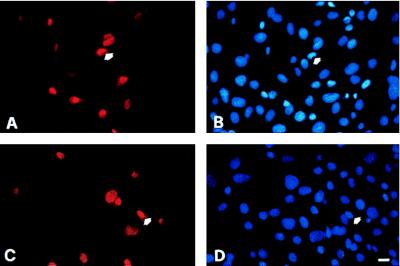
Because the possibility exists that the TGF-β receptor is down-regulated in myogenic cells grown at low density, we investigated the expression of TGF-β receptor II and I by Western blot analysis and immunofluorescence. No qualitative or quantitative changes were observed after the addition of TGF-β to myogenic cells grown at either high or low density (Fig. 3). Likewise, the levels of proteins such as N-cadherin involved in cell adhesion appeared unchanged(data not shown). We then investigated the presence of muscle-specific nuclear transcription factors, such as MEF2 or MyoD, which are known to be expressed in myogenic cells during proliferation or at the onset of differentiation (such as myogenin). C2C12 were transferred to DM and after 2 hr were treated with 5 ng/ml TGF-β. Fig. 4 shows that 30 min after exposure of C2C12 cells (grown to high density) to TGF-β, MEF2A and/or MEF2C were virtually absent from the nucleus, whereas immunoreactivity (unlocalized but stronger than background staining of fibroblasts) was found in the cytoplasm (Fig. 4B). Control cells grown to high density (Fig. 4A) and cells treated with TGF-β at low density (Fig. 4C) showed the expected nuclear localization of MEF2. The effect is specific, because myogenin (Fig. 5), MyoD, and p21 (not shown) persisted in the nucleus irrespective of TGF-β treatment. MEF2 relocalization was evident after 30 min but not after 15 min, lasted for at least 24 hr, and was reversible on removal of TGF-β. It was observed also with other members of the MEF2 family, such as MEF2D, although we could never detect MEF2B in either treated or untreated C2C12 cells (data not shown). Treatment of cells with TGF-β after the onset of differentiation (more than 8 hr after transfer to DM) had no effect. The lack of TGF-β effect is not unique to C2C12 because it was also observed in L5 rat and Dy8 murine myoblasts (not shown).
Figure 3.
Western blot analysis of TGF-β Receptor II and I in C2C12 myogenic cells grown at high(HD) or low density (LD) and exposed for 30 min to solvent (−) or 5 ng/ml TGF-β (+).
Figure 4.
Immunofluorescence analysis of MEF2 localization in control (A) and TGF-β-treated C2C12 myogenic cells, grown at high (B) or low (C) density. Hoechst staining of the nuclei in the corresponding fields is shown in D, E, and F. (Bar = 15 μm.)
Figure 5.
Immunofluorescence analysis of myogenin localization in control (A) and TGF-β-treated (C) C2C12 myogenic cells. Hoechst staining of the nuclei in the corresponding fields is shown in B and D. Note that after 2 hr in DM, only a minority of cells (arrow) have accumulated myogenin in the nucleus. This accumulation is, however, unchanged by treatment with TGF-β. (Bar = 15 μm.)
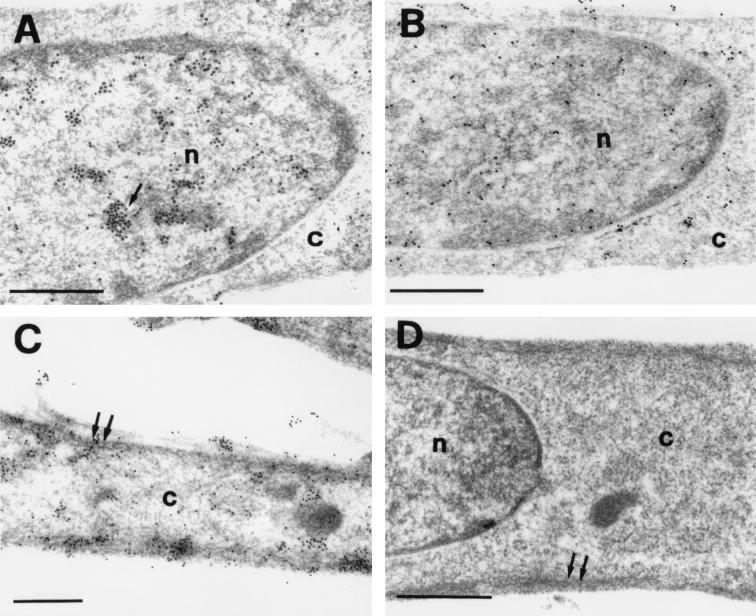
A better morphological definition of this phenomenon was achieved by using immune electron microscopy of myogenic cells treated as described above. The analysis confirmed the abundance of MEF2, as revealed by gold particles, in the chromatin of control cells (Fig. 6A), whereas very few particles persisted in the nuclei of TGF-β treated cells (Fig. 6B). Remarkably, most of the gold particles were detected in the cytoplasm of TGF-β-treated cells (Fig. 6C), often associated with either membranes or filamentous structures of a size compatible with that of intermediate filaments.
Figure 6.
Electron micrograph showing the immunogold localization of MEF2 in a control and TGF-β-treated C2C12 myogenic cells. The reaction products (shown by an arrow) accumulate in the chromatin of nuclei (n) in control (A) but not in TGF-β-treated (B) cells. In the latter cells, reaction products are mainly restricted to the cytoplasmic (c) cellular compartment (C). The gold particles (shown by double arrow in C) appear mainly associated with membranes and cytoskeletal filaments. Cells unreacted with primary antibody are shown in D. (Bar = 0.5 μm.)
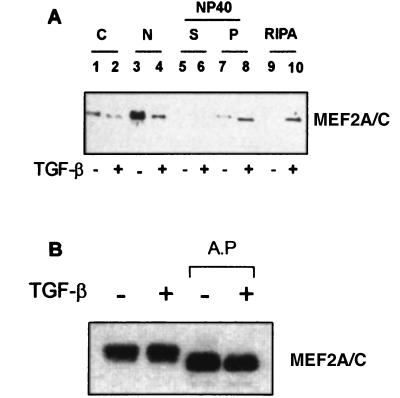
To confirm biochemically the relocalization of MEF2 after exposure of C2C12 cells to TGF-β, homogenates of both control and treated cells grown to high density were separated into cytosolic, nuclear, and insoluble (mainly membrane and cytoskeletal) fractions. The insoluble fraction was first extracted with 1% Nonidet P-40, to solubilize hydrophobic proteins, and then with RIPA buffer, to solubilize tightly bound proteins. Each fraction was then subjected to electrophoretic separation, transferred to nitrocellulose membranes, and reacted with antibodies against MEF2A/C. Fig. 7A shows that treatment with TGF-β induces a dramatic decrease in MEF2A/C from the nucleus (Fig. 7A, lanes 3 and 4) and a concomitant increase in the fractions insoluble in Nonidet P-40 (Fig. 7A, lanes 7 and 8) and solubilized by RIPA buffer (Fig. 7A, lanes 9 and 10). Thus, biochemical analysis confirmed the immunocytochemical data, indicating that after treatment of myogenic cells with TGF-β a nuclear transcription factor is specifically translocated out of the nucleus. Remarkably, TGF-β treatment does not change the overall amount of MEF2A/C protein, nor its overall phosphorylation status (Fig. 7B).
Figure 7.
Western blot analysis of expression, phosphorylation, and subcellular distribution of MEF2 in control and TGF-β-treated C2C12 myogenic cells. (A) Control and TGF-β-treated C2C12 myogenic cells were homogenized in hypotonic buffer, and nuclei (lanes 3 and 4) were isolated by low-speed centrifugation. The cytoplasmic fraction was further separated by high-speed centrifugation in a cytosolic soluble fraction (lanes 1 and 2), and a pellet that was then sequentially extracted with Nonidet P-40 (lanes 5 and 6) and with RIPA buffer (lanes 9 and 10). Lanes 7 and 8 represent the pellet of the Nonidet P-40 extraction before the final extraction with RIPA buffer. Thirty micrograms of protein for each fraction from control (−) and treated (+) cells were subjected to SDS/PAGE, transferred to nitrocellulose filters, and reacted with the anti-MEF2A/C polyclonal antibody. (B) Control (−) and TGF-β-treated (+) cells were homogenized in sample buffer; 30 μg of protein from control (−) and treated (+) cells were subjected to SDS/PAGE, transferred to nitrocellulose filters, and reacted with the anti-MEF2A/C polyclonal antibody. Other 30-μg aliquots of were pretreated with 40 units of alkaline phosphatase (AP)for 90 min at 30°C and similarly processed.
Inhibition of Myogenesis by TGF-β Can Be Prevented by Forcing the Expression of MEF2 into the Nucleus.
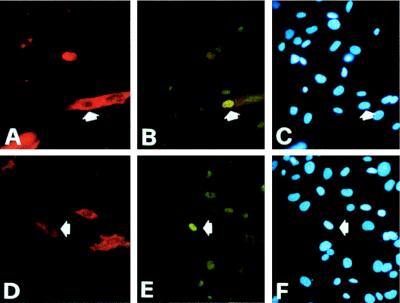
To test whether MEF2 relocalization was causally related to TGF-β-dependent inhibition of myogenesis, we constructed a plasmid expression vector carrying a MEF2C chimeric protein in which the nuclear localization signal of simian virus 40 large T antigen had been substituted for the eight C-terminal amino acids of wild-type MEF2C. Attempts to select stable transfectants with this vector, as well as with a control vector expressing wild-type MEF2C, were unsuccessful. However, efficient transient transfection could be achieved by the polyethylenimine transfection method (30% of C2C12 cells were transfected, based on the expression of a lacZ reporter gene; data not shown). Cells transfected with the MEF2C/nlsSV40 construct expressed the MEF2C protein in the nucleus both in the absence (Fig. 8 A–C) or in the presence (Fig. 8 D–F) of TGF-β and began to differentiate as revealed by accumulation of sarcomeric myosin; in contrast, cells transfected with wild-type MEF2C and treated with TGF-β translocated the protein to the cytoplasm essentially the same as control untransfected cells (data not shown). After 5 days in culture, about 70% of cells expressing MEF2C/nlsSV40 had differentiated (Fig. 9B), essentially at the same rate as control cultures (Fig. 9A). In the continuous presence of TGF-β, 30% of cells transfected with MEF2C/nlsSV40 still achieved a differentiated phenotype (Fig. 9 D), compared with less than 3% differentiated cells observed in control cultures (Fig. 9C). Cells transfected with a vector expressing wild-type MEF2C behaved the same as control untransfected cells (data not shown). Thus, myogenic cells in which the expression of MEF2C is forced into the nuclear compartment became resistant to the inhibitory action of TGF-β.
Figure 8.
Double immunofluorescence analysis of C2C12 myogenic cells transfected with the pMEF2/nlsSV40 vector. Twenty four hours after trasfection, cells were treated with solvent only (A–C) or 5 ng/ml TGF-β (D–F) for 24 hr and then stained with antisarcomeric myosin MF20 (A and D) and anti-MEF2A-C (B and E) antibodies. Hoechst staining of the nuclei is shown in C and F. Note nuclear staining of MEF2A-C in TGF-β-treated, transfected cells, part of which have already undergone differentiation, similar to untransfected control cells.
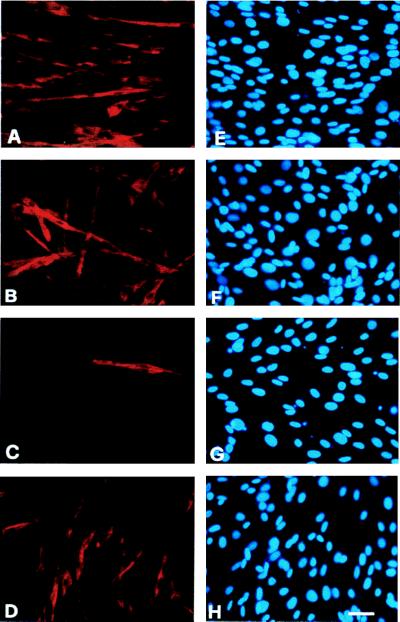
Figure 9.
Immunofluorescence analysis of untrasfected C2C12 myogenic cells (A and C) or C2C12 cells transfected with the pMEF2/nlsSV40 vector (B and D). Twenty-four hours after transfection, cells were treated with solvent only (A and B) or 5 ng/ml TGF-β (C and D) for 4 days and then stained with antisarcomeric myosin MF20. Hoechst staining of the nuclei is shown in E–H. (Bar = 15 μm.)
DISCUSSION
TGF-β-Mediated Inhibition of Myogenesis Is Density-Dependent.
The data reported in this work show that the inhibition of myogenesis caused by TGF-β is density-dependent. Inasmuch as TGF-β is thought to prevent premature differentiation of myoblasts in the developing embryo, it is not surprising that its action may require cell-to-cell contact. In this respect it is interesting to note that any mesenchymal cell, and not necessarily a committed myoblast, can provide this contact; in fact, during muscle histogenesis any single myoblast is surrounded by other myoblasts that may be at the same or at a different developmental stage, as well as by fibroblasts and endothelial cells. This is at variance with a community (28),in which a cell needs to be surrounded by identical sister cell to define a field of committed precursors. The requirement for interactions among mesenchymal cells suggests that the effect may be mediated by crosstalk between the TGF-β receptor and the N-cadherin complex (29). The idea of crossalk mediation is also supported by preliminary experiments with a peptide that reproduces the homophylic binding site of N-cadherin, suggesting that occupancy of this site is a prerequisite for the myoblasts to be inhibited by TGF-β (unpublished data). Attempts to coimmunoprecipitate the two molecules were unsuccessful, suggesting that the interaction is not direct but probably mediated by some as-yet-unknown intermediate molecule. One or more tyrosine phosphatases may be intermediates because several of these enzymes have been reported to be a target for the TGF-β receptor kinase activity (30). Perhaps more importantly, after 30 min of TGF-β treatment, at least two proteins of the N-cadherin complex become dephosphorylated, but only in myoblasts grown to high density (De Angelis et al., unpublished data). One of these proteins was identified as β-catenin but, as in this report, attempts to demonstrate a direct binding of β-catenin to MEF2 have been unsuccessful both in vivo and in vitro, again indicating that interaction may be indirect.
MEF2 Leaves the Nucleus After Exposure of Myoblasts to TGF-β.
Strikingly, it is MEF2, a transcription factor normally required for full transcriptional activation of myogenic genes, that is relocated from the nucleus to a membrane-cytoskeletal fraction after TGF-β treatment of myogenic cells grown at high density. Only denaturing detergents can solubilize MEF2 from the membrane-cytoskeletal fraction. This unexpected finding provides an explanation for the difficulties experienced in attempting to establish whether a direct binding of MEF2 to β-catenin may be induced by TGF-β binding to the membrane receptor; in fact, the conditions required for MEF2 solubilization would probably result in the disruption of any protein–protein interaction.
Recent evidence suggests that shuttling between the nucleus and the cytoplasm represents a rapid and efficient mechanism for regulating the activity of a transcription factor. For example, it was recently shown that the transcription factor NF-ATc, which plays a key role in the activation of many early response genes, translocates from the cytoplasm to the nucleus in response to a transient rise in intracellular calcium and immediately returns to the cytoplasm when the intracellular calcium level falls (31). A similar regulation has been reported for a number of nuclear proteins such as the Rev protein of HIV (32), the cAMP-dependent protein kinase inhibitor, pKI (33), or TFIIIA (34). Translocation to the cytoplasm depends on the presence of nuclear export signals consisting of highly divergent amino acid sequences that apparently have in common a short repeat of hydrophobic residues with defined spacing (35, 36). Therefore, it is not surprising that primary sequence analysis of MEF2s have not revealed reliable similarities to known nuclear export signals; functional assays will be needed to identify protein domains specifically involved in MEF2 nuclear export.
Expression of MEF2C containing a strong nuclear localization signal efficiently overcomes the inhibitory effect of TGF-β. This finding provides direct evidence for the causal relation between TGF-β inhibition of differentiation and MEF2 relocalization to the cytoplasm.
Regardless of the mechanism, once translocation of MEF2 out of the nucleus occurs, the transcription factor would then be unavailable for participation in an active transcriptional complex, thus preventing transactivation of myogenin and downstream genes once differentiation is initiated by serum removal. Our findings also help to explain previous reports showing that the inhibitory effect of TGF-β, at variance with fibroblast growth factor or serum, does not depend on the ability of basic helix–loop–helix transcription factors to bind their cognate DNA binding sites (6); indeed, it has been shown that fully efficient transcription of muscle-specific genes may require the coexistence of both basic helix–loop–helix and MEF2 proteins in the same transcriptional complex (37).
Acknowledgments
We thank M. Bouchè, M. Grossi, and F. Tatò for helpful discussion and M. Buckingham, M. Molinaro, and C. Nervi for critical reading of the manuscript. We also thank S. Alemà, M. Buckingham, R. Derinck, D. Fischman, R. Kelly, K. Knudsen, E. Olson, and R. Prywes, for the gifts of antibodies, plasmids, and transgenic mice. This work was supported by grants from Telethon, European Community BIOTECH-2, Fondazione Cenci Bolognetti, Associazione Italiana per la Ricerca sul Cancro, Ministero dell’Universitá e della Ricerca Scientifica e Tecnologica and CNR-P.F. Biotecnologie.
ABBREVIATIONS
- MEF2
Myocyte enhancer factor
- 2MLC3F
fast myosin light chain 3 gene
- TGF-β
transforming growth factor β
- GM
growth medium
- DM
differentiation medium
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1. Akhurst R J. In: Growth Factors and Signal Transduction in Development. Nilsen-Hamilton M, editor. New York: Wiley–Liss; 1994. pp. 97–122. [Google Scholar]
- 2.Schoffield P, Wolpert L. Exp Cell Res. 1992;191:144–148. doi: 10.1016/0014-4827(90)90048-f. [DOI] [PubMed] [Google Scholar]
- 3.Cusella De Angelis M G, Molinari S, Le Donne A, Coletta M, Vivarelli E, Bouchè M, Molinaro M, Ferrari S, Cossu G. Development (Cambridge, UK) 1994;120:925–933. doi: 10.1242/dev.120.4.925. [DOI] [PubMed] [Google Scholar]
- 4.Zappelli F, Willems D, Osada S, Onho S, Wetsel W C, Molinaro M, Cossu G, Bouchè M. Dev Biol. 1996;180:156–164. doi: 10.1006/dbio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 5.Vaidya T B, Rhodes S J, Taparowsky E J, Konieczny S F. Mol Cell Biol. 1989;9:3576–35779. doi: 10.1128/mcb.9.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan T J, Edmonson D G, Li L, Olson E N. Proc Natl Acad Sci USA. 1991;88:3822–3826. doi: 10.1073/pnas.88.9.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zentrella A, Massaguè J. Proc Natl Acad Sci USA. 1992;89:5176–5180. doi: 10.1073/pnas.89.11.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerber A N, Klesert T R, Bergstrom D A, Tapscott S J. Genes Dev. 1997;11:436–450. doi: 10.1101/gad.11.4.436. [DOI] [PubMed] [Google Scholar]
- 9.Baker J C, Harlan R M. Curr Opin Genet Dev. 1997;7:467–473. doi: 10.1016/s0959-437x(97)80072-x. [DOI] [PubMed] [Google Scholar]
- 10.Heldin C-H, Miyazono K, ten Dijke P. Nature (London) 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 12.Hartsough M T, Frey R S, Zipfel P A, Buard A, Cook S J, McCormick F, Mulder M. J Biol Chem. 1996;271:22368–22375. doi: 10.1074/jbc.271.37.22368. [DOI] [PubMed] [Google Scholar]
- 13.Mucsi I, Skorecki K L, Goldberg H J. J Biol Chem. 1996;271:16567–16572. doi: 10.1074/jbc.271.28.16567. [DOI] [PubMed] [Google Scholar]
- 14.Atfi A, Djelloul S, Chastre E, Davies R R, Gespach C. J Biol Chem. 1997;272:1429–1432. doi: 10.1074/jbc.272.3.1429. [DOI] [PubMed] [Google Scholar]
- 15.Frey R S, Mulder K M. Cancer Res. 1997;57:628–633. [PubMed] [Google Scholar]
- 16.Kelly R, Alonso S, Tajbakhsh S, Cossu G, Buckingham M. J Cell Biol. 1995;129:383–396. doi: 10.1083/jcb.129.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cossu G, Kelly R, Di Donna S, Vivarelli E, Buckingham M. Proc Natl Acad Sci USA. 1995;92:2254–2258. doi: 10.1073/pnas.92.6.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polimeni M, Giorgi S, De Gregorio L, Dragani T A, Molinaro M, Cossu G, Bouchè M. Mech Dev. 1996;54:107–118. doi: 10.1016/0925-4773(95)00465-3. [DOI] [PubMed] [Google Scholar]
- 19.Diaz-Guerra M, Haddow S, Bauluz C, Jorcano J L, Cano A, Balmain A, Quintanilla M. Cancer Res. 1992;52:680–687. [PubMed] [Google Scholar]
- 20.Martin J, Schwarz J J, Olson E N. Proc Natl Acad Sci USA. 1993;90:5282–5286. doi: 10.1073/pnas.90.11.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts B L, Richardson W D, Smith A E. Cell. 1987;50:465–475. doi: 10.1016/0092-8674(87)90500-9. [DOI] [PubMed] [Google Scholar]
- 22.Boussif O, Lezoualc’h F, Zanta M A, Mergny M D, Scherman D, Demeneix B, Behr J P. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tajbakhsh S, Vivarelli E, Cusella De Angelis G, Rocancourt D, Buckingham M, Cossu G. Neuron. 1994;13:813–821. doi: 10.1016/0896-6273(94)90248-8. [DOI] [PubMed] [Google Scholar]
- 24.Bader D, Masaki T, Fischman D A. J Cell Biol. 1982;95:763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koishi K, Zhang M, McLennan I S, Harris A J. Dev Dyn. 1995;202:244–254. doi: 10.1002/aja.1002020304. [DOI] [PubMed] [Google Scholar]
- 26.Han T H, Prywes R. Mol Cell Biol. 1995;15:2907–2915. doi: 10.1128/mcb.15.6.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.George-Weinstein M, Gerhart J, Blitz J, Simak E, Knudsen K. Dev Biol. 1997;185:12–14. doi: 10.1006/dbio.1997.8542. [DOI] [PubMed] [Google Scholar]
- 28.Gurdon J B. Nature (London) 1988;336:772–774. doi: 10.1038/336772a0. [DOI] [PubMed] [Google Scholar]
- 29.Takeichi M. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- 30.Greenwel P, Hu W, Kohanski R A, Ramirez F. Mol Cell Biol. 1995;15:6813–6819. doi: 10.1128/mcb.15.12.6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klemm J D, Beals C R, Crabtree G R. Curr Biol. 1997;7:638–644. doi: 10.1016/s0960-9822(06)00290-9. [DOI] [PubMed] [Google Scholar]
- 32.Fischer U, Huber J, Boelens W C, Mattaj I W, Luhrmann R. Cell. 1995;82:475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- 33.Wen W, Meinkoth J L, Tsien R Y, Taylor S S. Cell. 1995;82:463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- 34.Fridell R A, Fischer U, Luhrmann R, Meyer B E, Meinkoth J L, Malim M H, Cullen B R. Proc Natl Acad Sci USA. 1996;93:2936–2940. doi: 10.1073/pnas.93.7.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore M S. Curr Biol. 1996;6:137–140. doi: 10.1016/s0960-9822(02)00444-x. [DOI] [PubMed] [Google Scholar]
- 36.Golich D, Mattaj I W. Science. 1996;271:1513–1518. doi: 10.1126/science.271.5255.1513. [DOI] [PubMed] [Google Scholar]
- 37.Molkentin J D, Blek B L, Martin J F, Olson E N. Cell. 1995;83:1125–1136. doi: 10.1016/0092-8674(95)90139-6. [DOI] [PubMed] [Google Scholar]