Abstract
To evaluate the immunogenicity of human immunodeficiency virus (HIV) type 1 p55gag virus-like particles (VLPs) released by budding from yeast spheroplasts, we have analyzed the effects of yeast VLPs on monocyte-derived dendritic cells (DCs). Yeast VLPs were efficiently incorporated into DCs via both macropinocytosis and endocytosis mediated by mannose-recognizing receptors, but not the mannose receptor. The uptake of yeast VLPs induced DC maturation and enhanced cytokine production, notably, interleukin-12 p70. We showed that yeast membrane components may contribute to DC maturation partly through Toll-like receptor 2 signaling. Thus, Gag particles encapsulated by yeast membrane may have an advantage in stimulating Gag-specific immune responses. We found that yeast VLPs, but not the control yeast membrane fraction, were able to activate both CD4+ and CD8+ T cells of HIV-infected individuals. We tested the effect of cross-presentation of VLP by DCs in two subjects recruited into a long-term nonprogressor-slow progressor cohort. When yeast VLP-loaded DCs of these patients were cocultured with peripheral blood mononuclear cells for 7 days, approximately one-third of the Gag-specific CD8+ T cells were activated and became perforin positive. However, some of the Gag-specific CD8+ T cells appeared to be lost during in vitro culture, especially in a patient with a high virus load. Our results suggest that DCs loaded with yeast VLPs can activate Gag-specific memory CD8+ T cells to become effector cells in chronically HIV-infected individuals, but there still remain unresponsive Gag-specific T-cell populations in these patients.
The cytotoxic T lymphocyte (CTL) is the primary specific effector that protects hosts from virus infection including human immunodeficiency virus (HIV). During acute HIV infection, CTL activity is temporally associated with the initial decline in plasma viral RNA (6, 21). Strong HIV type 1 (HIV-1)-specific CTL responses have also been demonstrated in several cohorts of individuals who were infected with HIV-1 long before they developed any clinical manifestations, i.e., long-term nonprogressors (LTNPs) (32). The importance of CTLs has been clearly demonstrated by the increase in virus load in macaques depleted of CD8+ T cells prior to simian immunodeficiency virus infection (17, 36). However, the role of CTLs in chronic HIV infection is still not clear.
When HIV-recombinant vaccinia-infected autologous B cells were used as antigen-presenting cells (APCs), gamma interferon-positive (IFN-γ+) CD8+ cells were frequently detected at all stages of HIV infection (13). However, several studies with the major histocompatibility complex (MHC) class I peptide tetramer complex combined with the analysis of peptide-specific IFN-γ production have suggested that the number of HIV-specific CD8+ T cells is maintained during progression to AIDS but that CTL function based on IFN-γ production is impaired (14, 19, 20, 37, 46; Y. Sun et al., submitted for publication). These results indicate that HIV-specific CD8+ T cells in chronic HIV infection have different degrees of anergy (25), and apparently, HIV-specific CD8+ T cells are not effective enough to control virus replication.
A hybrid HIV-1 V3 loop-yeast Ty virus-like particle (VLP) has been shown to induce antibody and CTL responses against HIV env without adjuvant (1, 23). It was assumed that viral proteins in particulate form facilitate uptake into APCs with subsequent access to the MHC class I processing pathway (5). Nonreplicating, self-assembled VLPs of Pr55gag can be released from various eukaryotic cells by budding (44). Because VLPs are noninfectious yet morphologically and antigenically similar to naive virions, they are candidates for a vaccine against HIV infection (45). Indeed, recent studies with macaques have shown that HIV-1 p55gag VLPs can induce strong and long-lived CTL activity against multiple HIV-1 p55gag epitopes (30). However, the precise mechanism of action by which these VLPs are able to elicit immune responses has not been fully elucidated.
Dendritic cells (DCs) are the professional APCs and play a crucial role in the initiation of immune responses. They ingest antigens by phagocytosis, macropinocytosis, and receptor-mediated endocytosis (40). When DCs mature, their antigen presentation capacity increases, which is accompanied by the upregulation of MHC antigens, CD83, and other costimulatory molecules such as CD40, CD80, and CD86. The maturation signal is triggered by a variety of microbes through Toll-like receptors (TLRs) (2). It has recently been shown that papillomavirus-like particles can induce maturation in a similar manner in human (33) and mouse DCs (24) in vitro. Therefore, particulate antigens may have a common effect on DCs, irrespective of the source of virus, which may explain the strong anti-HIV immune responses of p55gag VLPs without any adjuvant in vivo (7, 30, 44).
By utilizing the potent antigen-presenting activity of DCs, it may be possible to restore the function of CD8+ T cells in HIV-infected individuals. A novel method was recently developed, using yeast spheroplasts, to produce HIV-1 Gag VLPs that are encapsulated with the yeast plasma membrane (34). Since a whole recombinant yeast carrying the OVA antigen has been shown to activate DCs and to elicit protective cell-mediated immunity in mice (38), yeast-derived VLPs may have strong immunogenicity to activate Gag-specific CTLs. To address this issue, we have analyzed the interaction of these yeast VLPs with monocyte-derived DCs. We demonstrate here that yeast VLPs are capable of inducing maturation of DCs that is accompanied by a high level of interleukin-12 (IL-12) production. The yeast membrane component was partly responsible for DC maturation through TLR2 signaling. We also show that DCs loaded with yeast VLPs can activate CD8+ T cells in HIV-infected individuals. Moreover, even in individuals chronically exposed to HIV, at least some Gag-specific CD8+ T cells have the potential to terminally differentiate into perforin-positive effector T cells via stimulation of yeast VLP-loaded DCs.
MATERIALS AND METHODS
Reagents.
All cultures were carried out with RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, and antibiotics (Invitrogen Corp., Carlsbad, Calif.). Labeled monoclonal antibodies (MAbs) against CD40, CD83, CD86, HLA-ABC, and HLA-DR and unlabeled MAbs against mannose receptor, DC-LAMP, and DC-SIGN were all purchased from BD Bioscience (San Jose, Calif.). Alexa 660-labeled anti-mouse immunoglobulin G (IgG) (Molecular Probes, Inc., Eugene, Oreg.) was used as a secondary antibody. Anti-human Golgin-97 and Alexa 546-conjugated dextran (10 kDa) were purchased from Molecular Probes. HLA-A2.01-restricted Gag77-85 peptide (SLYNTVATL) or Pol309-317-peptide (ILKEPVHGV) MHC class I tetramer complex (Gag- or Pol-tetramer) and HLA-A2.01-restricted Epstein-Barr virus (EBV)-peptide (BMLF1:GLCTLVAML) tetramer (EBV-tetramer) were obtained from Immunotech (BD Bioscience).
Purified anti-tumor necrosis factor alpha (TNF-α) (IgG1) was purchased from Roche Diagnostics Co. Ltd. (Tokyo, Japan). Purified anti-TLR2 (TL2.1, IgG2a) and anti-TLR4 (HT125, IgG2a) were obtained from eBioscience (San Diego, Calif.). Cytokines, TNF-α and IL-4, were obtained from PeproTech Inc. (London, England). Granulocyte-macrophage colony-stimulating factor was a kind gift from M. Tatsumi, Department of Veterinary Science, National Institute of Infectious Diseases (NIID), Tokyo, Japan. Escherichia coli lipopolysaccharide (LPS), mannan, LY294002, and fluorescein isothiocyanate (FITC)-mannolysated bovine serum albumin (BSA) were purchased from Sigma-Aldrich (St. Louis, Mo.).
Study populations.
Fresh peripheral blood mononuclear cells (PBMCs) of 6 noninfected and 16 HIV-infected individuals were tested for reactivity to VLP. Four of the HIV-infected patients were not on therapy and had various virus loads ranging from 3,100 to 61,000 copies/mm3, and 12 patients were on highly active antiretroviral therapy, six of whom had a residual virus load (150 to 8,100 copies/mm3). None of these HIV-infected individuals were hospitalized, and they had either moderate or no depletion of CD4+ T cells (374 to 998 cells/mm3; average, 544 cells/mm3).
Frozen PBMCs of three subjects from the ALT (asymptomatique long temps) cohort were used for the study carried out in France. Patients in the ALT cohort of LTNPs and slow progressors (SPs) met the following criteria: an HIV seropositivity evolving for at least 8 years with stable CD4 cell counts above 600 cells/mm3 for the last 5 years, no clinical symptoms, and no antiretroviral therapy. These blood samples were collected with written informed consent and approved by the ethical committees in France and Japan.
Preparation of DCs and T cells.
PBMCs from healthy donors or HIV-infected individuals were separated by a Ficoll-Hypaque density gradient (Lymphosepal; IBL, Gunma, Japan) and enriched for CD14+ cells with magnetic anti-CD14 beads and a magnetic cell sorter (Miltenyi Biotec, Cologne, Germany). To obtain monocyte-derived DCs, CD14+ cells were cultured in the presence of IL-4 (100 U/ml) and granulocyte-macrophage colony-stimulating factor (500 U/ml) for 1 week, essentially as described previously (41).
CD14− cells were kept frozen until DCs were generated and used as a T-cell source. In some experiments, CD14− cells were reacted either with anti-CD4 or anti-CD8 beads, followed by the magnetic cell sorter column procedure to positively select CD4+ or CD8+ T cells. The purities of these enriched population were 85 to 90% (CD4) and 93 to 95% (CD8), as assessed by flow cytometry.
For the mixed-lymphocyte reaction (MLR), CD4+ T cells were negatively selected by depletion with anti-CD8, -CD14, -CD11b, -CD16, -CD20 and anti-HLA-DR MAbs followed by an anti-mouse IgG column (BIOTEX Inc., Edmonton, Canada) as described previously (41). In this case, the purity was >96%. Allogeneic CD4+ T cells (105) were cocultured in the presence or absence of DCs (104) for 5 days in a 96-well flat-bottom microplate. [3H]thymidine (0.5 μCi per well; Amersham Biosciences, Piscataway, N.J.) incorporation was measured after overnight incubation.
Production of yeast VLPs of HIV-1.
VLPs of HIV-1 were produced from yeast as described elsewhere (34). Briefly, a full-length HIV-1 (the IIIB strain) gag gene was amplified by PCR and subcloned into the yeast expression vector pKT10. Saccharomyces cerevisiae was transformed by this vector, and a spheroplast was formed by the standard procedure by using Zymolase (Seikagaku Kogyo, Co. Ltd., Tokyo, Japan). After two washings with 1 M sorbitol, spheroplasts were cultured overnight at 30°C in yeast extract-peptone-dextrose medium containing 1 M sorbitol. During the culture period, budding of Gag VLPs occurred from the transformed spheroplasts. The culture supernatant was clarified at 7,000 rpm for 30 min and centrifuged through 30% sucrose cushions in an SW28 rotor (Beckman Coulter, Inc., Palo Alto, Calif.) at 26,000 rpm for 1.5 h. The VLP pellets were resuspended and centrifuged on 20 to 70% sucrose gradients in an SW41 rotor (Beckman) at 35,000 rpm overnight. Following fractionation, the peak fractions containing VLPs (corresponding to 50 to 55% of the sucrose fraction) were collected, diluted with phosphate-buffered saline (PBS), and pelleted by centrifugation. Purified VLP pellets were resuspended with PBS and quantitated by Western blotting with the anti-HIV-1 capsid MAb (Advanced Biotechnologies Inc., Columbia, Md.) and by Coomassie brilliant blue staining. Serial threefold dilutions of the purified VLP suspension were compared with standard dilutions prepared with purified soluble Gag protein (Innogenetics, Alpharetta, Ga.) for Western blotting or BSA (Sigma-Aldrich) for Coomassie brilliant blue staining. As a control, the culture supernatant of empty-vector-transformed spheroplasts was purified from the equivalent culture volume by the same procedure as for VLPs (control culture supernatant [CS]).
For localization of VLPs in DCs, VLPs expressing the Gag-enhanced green fluorescent protein (EGFP) fusion protein (VLP-EGFP) were produced. The gag gene (truncated just before the termination codon) and the EGFP gene were amplified by PCR and ligated in frame. The DNA fragment encoding the Gag-EGFP fusion protein was cloned into pKT10. Spheroplast formation and purification of VLP-EGFP were carried out as described above.
Enzyme-linked immunospot (ELISPOT) assay.
Hemagglutinin-multiscreen plates (Millipore, Burlington, Mass.) were precoated with 100 μl of PBS containing 1 μg of anti-human IFN-γ MAb (clone 1-D1K; Mabtech, Cincinnati, Ohio)/ml, incubated overnight at 4°C, washed, and blocked with RPMI 1640-10% FBS for 2 to 3 h at 37°C. DCs were pulsed overnight with 10 μg of VLPs or CS/ml, washed, and plated with T cells at a DC/T cell ratio of 1:10 in duplicate or triplicate. The cultures were incubated for 40 h at 37°C. Cells were washed with tap water and followed by three washes with PBS-Tween 20 (0.05%). Wells were incubated with biotin-conjugated anti-human IFN-γ MAb (clone 7-B6-1; Mabtech) in PBS containing 0.5% Tween 20 and 0.5% BSA for 2 h at 37°C, washed extensively, and reacted with horseradish peroxidase-conjugated streptavidin (Boehringer-Roche) for 1 h at room temperature (RT). After washing, spots were stained with AEC (Sigma-Aldrich), and colored spots representing the IFN-γ-producing cells were counted under a microscope (Nikon Co. Ltd., Tokyo, Japan).
Confocal microscopy.
Immature DCs at day 7 were loaded with VLP-GFP for 20 min, washed twice with cold medium, and then incubated further for 1 or 4 h or overnight. Cells were fixed on a slide with 1% formalin-PBS for 20 min at RT, washed, and incubated with the first mouse MAb for 1 h in PBS containing 30% goat serum, 2% fetal calf serum, and 0.05% sodium azide in the presence or absence of 0.025% digitonin (Sigma-Aldrich). Slides were washed and reacted with Alexa 660-conjugated anti-mouse IgG for 1 h at RT. At the final wash with PBS-2% fetal calf serum-0.05% sodium azide, propidium iodide (PI) was added for nuclear staining. The samples were analyzed by confocal laser microscopy (Carl Zeiss Jene GmbH, Jena, Germany).
Fluorescence-activated cell-sorter (FACS) analysis.
Flow cytometric detection of surface and intracellular antigens was performed essentially as described previously (42). For in vitro stimulation with peptides, T cells were incubated with peptides (2 μg/ml) for 2 h and cultured further for 4 h in the presence of 10 μg of brefeldin A (Sigma-Aldrich)/ml. For long-term cultured cells, perforin secretion was blocked by adding 2 μM monensin (Sigma-Aldrich) during the final 6 h of culture. Cells were collected, incubated with 5 μg of EMA (Molecular Probes, Inc.)/ml under room light, washed, and then stained with phycoerythrin (PE)-labeled Gag or EBV tetramer or control tetramer in combination with APC anti-CD8 MAb for 40 min on ice. Samples were then washed and fixed with 4% paraformaldehyde in PBS for 20 min at RT, followed by washing with permeabilization buffer containing 0.2% saponin in the staining buffer (PBS containing 2% FBS or 0.5% BSA and 0.05% sodium azide). The fixed and permeabilized cells were incubated with FITC-conjugated anti-perforin, PE-conjugated anti-IFN-γ, or isotype-matched control MAbs for 30 min on ice. Stained cells were analyzed by FACScalibur (BD Bioscience) by using the Cell Quest program.
Detection of cytokines.
The culture supernatant of immature DC incubated with or without 10 μg of VLP/ml or in the presence of 100 ng of LPS/ml was collected at 2 days and kept frozen. The cytometric bead array (CBA) kit (BD Bioscience) was utilized to measure the level of cytokines (IFN-γ, IL-12 p70, IL-10, IL-6, TNF-α, IL-8, and IL-1β) in these supernatants.
Statistical analyses.
Intergroup comparisons were performed with the Mann-Whitney U test (for univariate nonparametric group analysis). All P values were two-tailed and considered significant if less than 0.05.
RESULTS
Uptake of yeast VLPs by DCs.
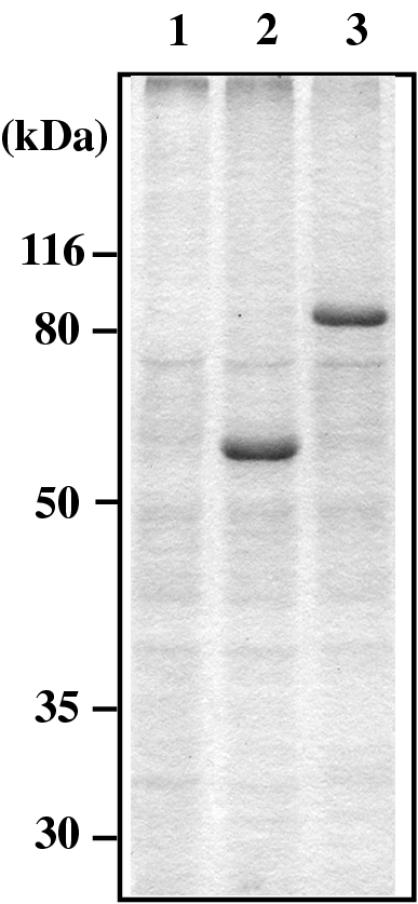
According to the production and purification methods described in the previous report (34), we prepared two forms of VLPs, one containing Gag protein and the other containing Gag-EGFP fusion protein from yeast spheroplasts (referred to as VLP and VLP-EGFP, respectively). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subsequent Coomassie brilliant blue staining (Fig. 1) revealed that each material contained one major protein, Gag or Gag-EGFP (confirmed by Western blotting) (data not shown) and several minor proteins also present in the sample prepared from the culture supernatant of spheroplasts transformed by an empty vector (referred to as CS). As Gag or Gag-EGFP was the only major and known protein in each VLP sample, the levels of VLPs were evaluated by Western blotting along with serially diluted Gag protein as a standard.
FIG. 1.
Purity of yeast VLPs analyzed by SDS-PAGE. Purified VLP (obtained by Gag expression) and VLP-EGFP (obtained by Gag-EGFP expression) were subjected to SDS-PAGE followed by Coomassie brilliant blue staining. The fraction purified from the control culture supernatant of yeast (CS) was similarly analyzed. The masses (in kilodaltons) of proteins were indicated based on the prestained molecular mass markers run in parallel. Lanes: 1, CS; 2, VLP; 3, VLP-EGFP.
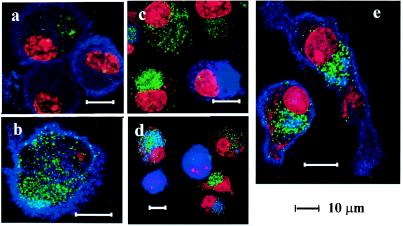
Monocyte-derived DCs are immature and efficiently internalize extracellular materials via mannose receptor-mediated endocytosis or macropinocytosis (35). We first examined the uptake of yeast VLPs by using confocal microscopy. We added VLP-EGFP (40 μg/ml) to DCs and incubated them at 37°C. Twenty minutes later, DCs were washed twice to remove extracellular VLP-GFP and were spotted onto glass slides immediately or after further incubation for 1 or 4 h or overnight at 37°C. Then, slides were fixed and stained with a MAb against the mannose receptor, DC-SIGN, DC-LAMP, or anti-Golgin-97, and subsequently with Alexa 660-conjugated anti-mouse IgG in the absence (for surface staining) or presence (for permeabilization) of digitonin. PI was used for nuclear staining. Dot-like EGFP signals were scattered on the cell surface 20 min after incubation (Fig. 2a and b), and then accumulated predominantly in the perinuclear region 1 h (Fig. 2c) and 4 h (Fig. 2d) later. Because yeast VLPs were budded from yeast membranes rich in mannose, a family of C-type lectins, including the mannose receptor and DC-SIGN, may mediate the internalization of yeast VLPs. The mannose receptor and DC-SIGN were highly expressed on the cell surface of immature DCs (Fig. 2a and b, respectively), as well as in the cytoplasm of permeabilized cells (data not shown). However, colocalization of VLP-EGFP with the mannose receptor was not obvious (Fig. 2a) while signals of VLP-EGFP and DC-SIGN overlapped partially (Fig. 2b), indicating that some VLP-EGFP binds to DC-SIGN. DCs expressing DC-LAMP, which is one of the DC maturation markers (9), were minimal at 20 min (data not shown) and 1 h later (Fig. 2c). Of note, DC-LAMP-positive DCs contained no or few VLPs at these time points. Four hours later, DC-LAMP-positive cells increased and some VLP-EGFP positive cells were also DC-LAMP positive (Fig. 2d). Taken together, the internalization of VLPs into DCs occured within 1 h of exposure, which directly or indirectly induced the maturation of DCs during the subsequent 4-h incubation. After incubation overnight, VLP-EGFP was still visible, mostly in the perinuclear region. As shown in Fig. 2e, anti-Golgin-97, which specifically recognizes the 97-kDa granin family protein on the cytoplasmic face of the Golgi apparatus (16), stained this region exclusively, supporting the notion that VLP-EGFP accumulated in the Golgi-rich region.
FIG. 2.
Uptake and localization of yeast VLP-EGFP by DCs. Monocyte-derived immature DCs were cultured in the presence of 40-μg/ml yeast VLP-EGFP for 20 min (a to c), 1 h (d), or overnight (e), washed, and spread onto glass slides. Slides were fixed with 1% formalin-PBS for 20 min at RT and then reacted with either anti-mannose receptor (a), anti-DC-SIGN (b), anti-DC-LAMP (c and d), or anti-Golgin 97 (e) MAbs for 1 h in the absence (a and b) or presence (c to e) of digitonin for permeabilization. Slides were then incubated with Alexa 660-conjugated anti-mouse IgG and washed, and PI was added for nuclear staining.
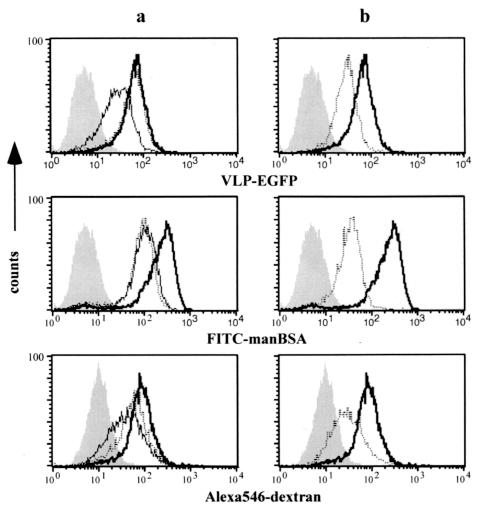
We then compared the mechanism of capture of yeast VLPs with other macromolecules such as dextran and mannosylated BSA (manBSA) by flow cytometry. Mannan and the anti-mannose receptor MAb were used to block mannose-mediated uptake. The phosphatidylinositol 3-kinase inhibitor LY294002 inhibits signal transduction essential in membrane ruffling (4) and was used as an inhibitor of macropinocytosis. As shown in Fig. 3a, we found that preincubation of DCs with mannan (2 mg/ml) for 30 min significantly reduced uptake of VLP-EGFP, FITC-labeled manBSA, and Alexa 546-labeled dextran, although the inhibition was not complete. Anti-mannose receptor MAb (10 μg/ml) inhibited the uptake of manBSA, as mannan did, and partially inhibited uptake of dextran while the uptake of VLP-EGFP was not inhibited at all by this MAb. When DCs were pretreated with LY294002, the uptake of all these molecules was substantially reduced (Fig. 3b). Taken together, we conclude that similar to manBSA and dextran, both macropinocytosis and receptor-mediated endocytosis mediate the uptake of yeast VLPs and that mannose-recognizing receptors, including DC-SIGN, but not the mannose receptor, may be involved in this process.
FIG. 3.
Mechanism of uptake of yeast VLP-EGFP. Immature DCs were not treated (bold line) or pretreated with 2 mg of mannan/ml (solid line) or 10 μg of anti-mannose receptor MAb/ml (dotted line) (a) or with 50 μM LY294002 (dotted line) (b) for 30 min. Then 10 μg of VLP-EGFP (upper), 100 μg of FITC-mannosylated BSA (middle), or 100 μg of Alexa 546-detran (lower) was added to DCs and incubated for 20 min. The cells were washed three times, incubated for another 15 min, and analyzed by flow cytometry. The background is shown as a shadow.
Yeast VLP induces a maturation phenotype of DCs.
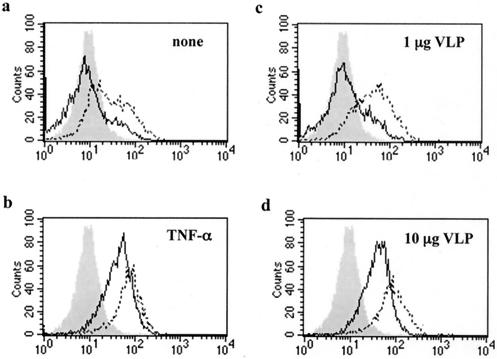
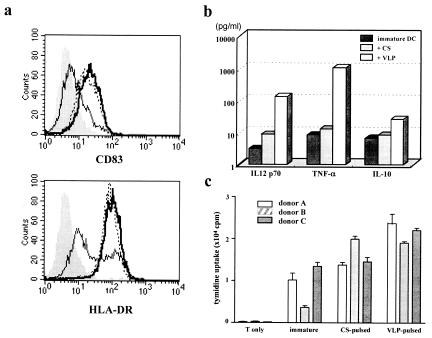
Since maturation of DCs can be induced by TNF-α, we compared VLP-induced maturation with TNF-α-induced maturation by flow cytometry. Immature DCs were incubated with 1 or 10 μg of VLPs/ml or with 10 ng of TNF-α/ml for 2 days, and the surface expression of CD83 and HLA-DR, the representative surface markers of mature DCs, was then examined. As shown in Fig. 4, the expression of both CD83 and HLA-DR was upregulated strongly with 10 μg and weakly with 1 μg of VLPs/ml. The expression level of these molecules induced by 10 μg of VLPs/ml was equivalent to that induced by TNF-α. The expression levels of CD1a and CD11c, which are specific markers of monocyte-derived DCs, were not altered. Interestingly, we found that a similar maturation phenotype was induced by yeast culture supernatant without Gag particle (CS) (Fig. 5a), suggesting that the cellular components of yeast, but not the Gag particle itself, contribute to the phenotypic maturation of DCs.
FIG. 4.
Maturation of DCs by yeast VLP. Immature DCs were incubated in the absence (a) or presence of 10 ng of TNF-α/ml (b) or 1 (c) or 10 (d) μg of yeast VLPs/ml for 2 days. The expression of CD83 (solid line) or HLA-DR (dotted line) was analyzed by FACSCalibur. The background staining with control IgG is shown as a shadow.
FIG. 5.
The effect of yeast VLPs on DC maturation is partly due to yeast membrane components. Immature DCs (solid line) were incubated in the presence of 10 μg of yeast VLPs/ml (bold line) or 10 μg of yeast membrane component control (CS)/ml (dotted line) for 2 days. (a) The surface expression of CD83 (upper panel) and HLA-DR (lower panel) was analyzed. The background staining with control IgG is shown as a shadow. (b) Cytokines produced by yeast VLP- or CS-stimulated DCs during cultivation were measured. (c) Allogeneic CD4+ T cells (105) of three donors were cocultured without DCs or with immature CS-pulsed or VLP-pulsed DCs (104 each) for 5 days in 96-well flat-bottom microplates. Then [3H]thymidine (0.5 μCi per well) was added to the culture, and cells were harvested after overnight incubation.
DC maturation is characterized by the production of cytokines that are important for the activation of T cells. We assessed the level of a variety of cytokines, including IFN-γ, TNF-α, IL-6, IL-12 p70, IL-1β, IL-8, and IL-10, in the supernatant of DCs cultured for 2 days with yeast VLPs or CS with a FACS-based CBA kit. The representative result of one of three experiments is shown in Fig. 5b. The production of IFN-γ and IL-1β was not induced in these cultures, and a high level of IL-8 was produced even in immature DCs without stimulation. Notably, VLP-stimulated DCs produced a higher level of IL-12 p70, TNF-α, IL-10, and IL-6 than did CS-stimulated DCs. These results indicate that DC maturation induced by yeast VLPs is coupled to the production of various cytokines and is not dependent on the cellular components of yeast.
Strong stimulation of MLR is considered to indicate functional maturation of DCs. Therefore, we compared the MLR stimulation capacity of phenotypically matured DCs by CS or VLP. We examined the proliferation of allogeneic CD4+ T cells of three donors by coculturing with immature CS-pulsed or VLP-pulsed DCs. As shown in Fig. 5c, VLP-pulsed DCs more effectively stimulated allogeneic T cells than immature or CS-pulsed DCs in two of three donors. The result indicates that yeast VLPs are able to induce functional maturation of DCs.
Maturation of DCs by VLPs is partially mediated by signaling through TLR2.
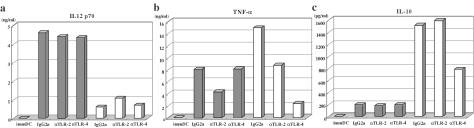
TLRs play an important role in DC maturation, probably through the production of TNF-α. Immature DCs express various types of TLRs (29, 43). Among them, TLR4 recognizes LPS of gram-negative bacteria while TLR2 recognizes a wide variety of infectious pathogens and their products, including lipoproteins, peptidoglycan, and a major component of the yeast cell wall, zymosan (2). Because TLR2 and TLR4 differentially activate DCs (31), we compared the level of cytokines produced by DCs stimulated with either VLP or LPS in the presence of blocking MAb against each TLR (11, 39). As shown in Fig. 6, VLP-stimulated DCs produced a higher level of IL-12 p70 than LPS-stimulated DCs while the level of IL-10 production was lower in VLP-stimulated DCs than in LPS-stimulated DCs. This preferential production of IL-12 over IL-10 could be important to support the Th1 type responses of Gag-specific T cells. When DCs were preincubated with a MAb against TLR4, the production of both TNF-α and IL-10 was strongly inhibited in LPS-stimulated DCs. In contrast, anti-TLR4 MAb did not affect IL-10 production, and TNF-α production was only partially inhibited by anti-TLR2 MAb in VLP-stimulated DCs. Representative results of three experiments are shown here. Taken together, these results suggest that DC maturation induced by yeast VLPs is not equivalent to that induced by LPS: yeast VLPs may utilize TLR2, but not TLR4 signaling for TNF-α production. The trace amounts of the yeast cell wall component zymosan remaining in the VLP preparation even after the treatment by zymolase may have been recognized by TLR2.
FIG. 6.
Yeast VLPs induce a higher level of IL-12 expression than LPS, which is independent of TLR2. Immature DCs (immDC) were stimulated with either yeast VLP (shaded bars) or LPS (100 ng/ml, open bars) in the presence of blocking MAbs or control IgG2a at 20 μg/ml for 2 days. The level of cytokines in the culture supernatant was measured by using a FACS-based CBA kit. The representative result of three experiments is shown. (a) IL-12 p70; (b) TNF-α; (c) IL-10. αTLR-2, anti-TLR2; αTLR-4, anti-TLR4.
Activation of T cells by yeast VLPs in HIV-infected individuals.
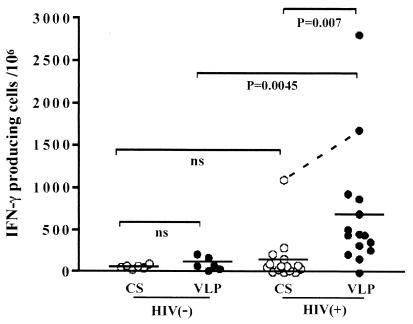
As yeast VLPs induce maturation and production of IL-12 in DCs, it is possible that yeast VLPs internalized by DCs may serve as potent antigens that activate Gag-specific T cells in HIV-infected individuals. To test this possibility, we generated immature DCs from monocytes of noninfected or HIV-infected individuals and pulsed them with either CS or VLPs at a concentration of 10 μg/ml overnight. These VLP-loaded DCs were cocultured with autologous PBMCs depleted of monocytes for 40 h, and the number of IFN-γ-producing cells was measured by ELISPOT assay. The results of ELISPOT are shown in Fig. 7. Two of the noninfected individuals showed a significant level of VLP response (177 and 214 IFN-γ+ cells per million PBMCs), but the overall response of noninfected individuals to VLPs (average, 101 IFN-γ+ cells per million PBMCs) was not statistically different from that of noninfected individuals to CS (average, 64 IFN-γ+ cells per million PBMCs). Although some HIV-infected patients exhibited a considerable response to CS compared to noninfected individuals, the differences were not significant. In contrast, the number of VLP-reactive T cells was higher than that of CS-reactive T cells in most of the HIV-infected patients (P = 0.008). Of note, one patient showed a high response to CS (1,097 cells) and an even higher response to VLPs (1,683 cells). This patient and one who did not respond to VLPs at all were not being treated with any kind of therapy despite their high virus loads (56,000 and 61,000 copies/mm3, respectively). The response to VLPs in HIV-infected patients was quite variable but still significantly higher than that of noninfected individuals to VLPs (P = 0.004). These results indicate that Gag-reactive T cells are activated by VLP-derived antigen-presenting DCs.
FIG. 7.
Yeast VLPs stimulate T cells of HIV-infected individuals to produce IFN-γ. Immature DCs were generated from monocytes and pulsed overnight with either yeast VLPs or CS. They were washed three times and mixed with autologous monocyte-depleted (CD14−) PBMCs, and the number of IFN-γ-producing cells was determined by ELISPOT analysis. The results for 6 noninfected [HIV(−)] and 16 HIV-infected [HIV(+)] individuals are shown by circles. A short horizontal bar represents the mean of each group. P values are noted for intergroup comparisons. The result of a remarkably high CS response observed in one patient is connected to that of the VLP response with a dotted line. ns, not significant.
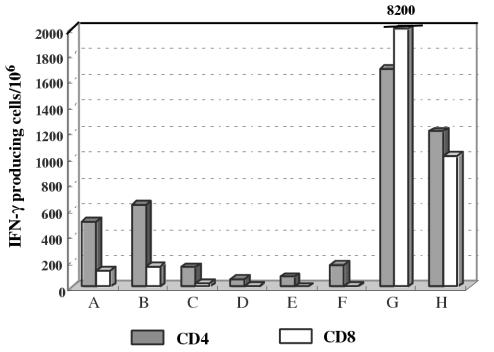
DCs are potent APCs which can present exogenous antigens not only to CD4+ T cells but also to CD8+ T cells by cross-presentation. To examine whether VLPs were cross-presented by DCs, CD4+ or CD8+ enriched T cells were cocultured with VLP-loaded DCs, and the number of IFN-γ-producing cells was measured by ELISPOT. As shown in Fig. 8, although CD4+ T cells were the major cell population that responded to yeast VLPs, CD8+ T cells were also activated to some extent. In some patients (G and H), whose responses to yeast VLPs were very high, CD8+ T cells were strongly activated. Thus, cross-presentation of yeast VLPs by DCs did occur. Considering that circulating Gag p24 antigen is frequently detectable during HIV infection, such cross-presentation may play some roles in priming and activation of CTL in vivo.
FIG. 8.
Cross-presentation of yeast VLPs by DCs. Monocyte-depleted (CD14−) PBMCs of 8 HIV-infected individuals were enriched for CD4+ or CD8+ T cells, and ELISPOT analysis was carried out as described in the legend to Fig. 7. The number of IFN-γ-producing T cells present after coculture with yeast VLP-pulsed DCs was determined by subtracting the number of those present following coculture with CS-pulsed DCs. Shadowed box, CD4+ enriched T cells (85 to 90%); open box, CD8+ enriched T cells (93 to 95%).
Gag-specific CD8+ T cells express perforin after coculture with yeast VLP-loaded DCs in LTNPs.
Whether Gag-specific memory CD8+ T cells of chronically HIV-infected individuals are capable of being terminally differentiated to perforin-positive effector T cells through cross-presentation of yeast VLPs by DCs was the next question. We selected three patients who had HLA-A2 in the French ALT cohort of LTNPs and SPs and analyzed A2-restricted Gag- or Pol-specific CD8+ T cells by flow cytometry. Their PBMCs have been frozen each year since 1996. The immunological and virological characteristics of these three patients are shown in Table 1. Gag-tetramer-positive CD8+ T cells were frequently detected, whereas the frequencies of Pol-tetramer-positive CD8+ T cells were very low in all patients. We found that about half of the total CD8+ T cells in PBMCs expressed perforin ex vivo in patient L2 and that most of his Gag-specific CD8+ T cells were perforin positive (Fig. 9). In this particular patient, most of the EBV-specific CD8+ T cells were also perforin positive. In contrast, the expression of perforin was low in the total CD8 population of patients L1 and L3, and perforin was expressed only in 0.06% and 2.5% of Gag-tetramer-positive cells, respectively. When PBMCs were stimulated with the same peptide as Gag tetramer, IFN-γ+ CD8+ T cells in patients L1, L2, and L3 were 0.8, 0.35, and 0.68% of the total CD8+ T cells, respectively, indicating that only a small population of Gag peptide-specific (tetramer positive) CD8+ T cells can be activated immediately after peptide stimulation.
TABLE 1.
Immunological and virological characteristics of LTNPs and SPs
| Patient no. | Year | No. of CD4+ cells/mm3 | Virus load (copies/mm3) | % CD8+ cellsa | % Perforin-positive cellsb | % Gag-specific IFN-γ+ cellsc | % Tetramer-positive cellsd
|
||
|---|---|---|---|---|---|---|---|---|---|
| Gag | Pol | EBV | |||||||
| L1 | 1996 | 603 | 1,980 | 60 | 3 | 0.8 | 2.8 | 0.14 | 0.19 |
| 1997 | 655 | 2,400 | |||||||
| 1998 | 465 | 1,239 | |||||||
| L2 | 1996 | 483 | 7,400 | 38 | 0.35 | 1.61 | 0.08 | ||
| 1997 | 364 | 8,800 | 40 | 50 | 1.72 | 0.30 | |||
| 1998 | 468 | 4,972 | |||||||
| L3 | 1996 | 776 | 110,000 | 29 | 0.68 | 2.5 | 0.04 | 0.32 | |
| 1997 | 647 | 480,000 | |||||||
| 1998 | 443 | 340,000 | 3.5 | 3.12 | 0.96 | ||||
Percentage of CD8+ T cells in CD3+ T cells.
Percentage of perforin-positive cells in CD8+ T cells.
Percentage of IFN-γ+ cells in CD8+ T cells after stimulation with Gag peptide (SLYNTVATL).
Percentage of tetramer-positive cells in CD8+ T cells.
FIG. 9.
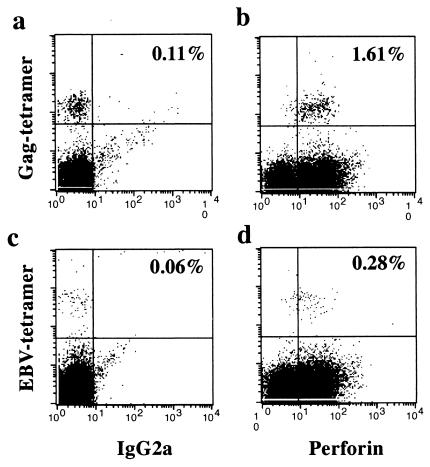
Most of the Gag- and EBV-peptide-specific T cells in patient L2 expressed perforin. Frozen PBMCs were thawed, directly stained with PE-labeled Gag-tetramer (a and b) or EBV-tetramer (c and d) in combination with APC-labeled anti-CD8, fixed, and permeabilized. Intracellular staining was carried out with FITC-labeled anti-perforin (b and d) or control IgG2a (a and c) MAb. Dead cells were excluded by EMA staining, and CD8+ T cells were gated. The percentages of Gag-tetramer-positive CD8+ T cells and EBV-tetramer-positive CD8+ T cells in the total CD8+ T cell population were 1.72 and 0.43%, respectively.
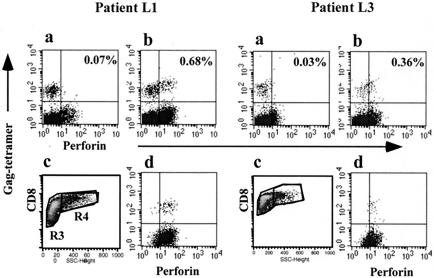
We then cocultured PBMCs of these patients with their own DCs that had been pulsed with CS or yeast VLPs. Frozen samples for patient L1 from 1996 and for patient L3 from 1998 were examined, but unfortunately, PBMCs were not available for patient L2 for further experimentation because of the shortage of the cell stock. Seven days after coculture of PBMCs with DCs in the presence of CS or VLPs, cells were collected and analyzed for the expression of perforin by FACS. As shown in Fig. 10, in patient L1, who maintained a low virus load (Fig. 10b), 0.68% of 2.24% Gag-specific CD8+ T cells expressed perforin after VLP stimulation while perforin expression was negligible following control CS stimulation (Fig. 10a). When CD8high SSChigh activated blast cells were gated (Fig. 10c), they were all found to be perforin positive (Fig. 10d). Similarly, in patient L3, 0.36% of 1.14% Gag-specific CD8+ T cells were perforin positive (Fig. 10b) following coculture with DCs pulsed with yeast VLPs, and as in patient L1, all CD8high SSChigh activated cells were perforin positive (Fig. 10d). These results indicate that approximately one-third of Gag-specific memory CD8+ T cells in chronically HIV-infected individuals can develop into effector T cells by cross-presentation of DCs, irrespective of the virus load (Table 1). However, in patient L3, who had a high virus load, the number of Gag-specific CD8+ T cells was markedly reduced from 3.12% before cultivation (Table 1) to 1.14% after cultivation, which did not occur in patient L1, who had a low virus load. Therefore, the potent stimulation of HIV-specific T cells may not always be beneficial for those patients who have been chronically exposed to a high level of viral antigens.
FIG. 10.
Perforin expression is induced by cross-presentation of DCs loaded with yeast VLPs. Immature DCs were cultured with monocyte-deprived PBMCs in the presence of CS or yeast VLPs for 7 days. The perforin expression of Gag-tetramer-positive T cells was analyzed by FACS. CD8+ T cells (R3) in the lymphocyte fraction (FSClow SSClow−high) were gated in panels a and b. In panels c and d, only activated CD8+ T cells (SSChigh, R4) were gated. The percentage of Gag-tetramer-positive CD8+ T cells in the total CD8+-T-cell population were 2.24 and 1.14% in patients L1 and L3, respectively.
DISCUSSION
We have shown here that yeast-derived VLPs are efficiently internalized by DCs via both macropinocytosis and endocytosis mediated by mannose-recognizing receptors but not by the mannose receptor. The uptake of yeast VLPs induced DC maturation, resulting in the production of cytokines, particularly IL-12 p70. Control supernatant containing yeast membrane alone induced a DC maturation phenotype similar to that of VLPs. However, cytokine production and MLR by DCs stimulated with CS was much less than that by DCs stimulated with VLP, suggesting that although cytokine production by DCs is accompanied by the upregulation of several maturation-associated markers, it requires a separate signal that is distinct from that for phenotypic maturation. Although TNF-α production by LPS-stimulated DCs has been reported to be mediated mostly by TLR4 signaling (reviewed in reference 2), our study showed that TNF-α production by VLP-stimulated DCs is mediated by TLR2 but not by TLR4 signaling. TLR2 is known to form a heterodimer with either TLR1 or TLR6 (2). Which of these molecules is involved in the signaling of yeast VLPs for DCs is currently unknown. It should be noted that VLP-stimulated DCs produce a much higher level of IL-12 p70 and a lower level of IL-10 than LPS-stimulated DCs and that the signaling through TLRs was not directly involved in this dominant IL-12 production by VLP. In line with this notion, Gartner et al. quite recently reported that dectin-1, rich in DCs and one of the C-type lectins that recognizes β-glucan-containing particles such as zymosan, collaborated with TLR2 for the signaling to enhance IL-12 production (12). Such collaborative signaling probably mediated the effect of yeast VLP observed in this study. Together, our results suggest that yeast VLPs, with a combination of virion-like structure and yeast membrane components, might preferentially target DCs to induce strong Th1-type immune responses.
Cross-priming is an important mechanism for the induction of CTL responses against infection. DCs are the primary APCs that can present exogenous antigens efficiently to CTLs (8). Quite recently, Jung et al., using CD11c-driven diphtheria toxin receptor-transgenic mice, elegantly demonstrated that DCs were the critical APCs for the cross-presentation of exogenous antigens or apoptotic cells to induce CTLs against Listeria monocytogenes or Plasmodium yoelii (18). Previous reports have shown that a noninfectious form of antigen such as VLP can be efficiently taken up by a murine DC line, and such an incorporated antigen can be presented via MHC class I molecules in vitro (5). Here, we demonstrated that DCs loaded with yeast VLPs are able to activate Gag-specific CD8+ T cells of HIV-infected individuals. As a high level of p24 antigenemia and subsequent anti-p24 antibody levels are the common features of HIV infection, it is highly likely that the Gag p24 and anti-p24 antibody immune complexes are cross-presented by DCs. Although the importance of cross-presentation of exogenous or apoptotic cell-associated HIV antigens during HIV infection is still unknown, at least some CD8+ T-cell responses against HIV may be induced by cross-presentation of such antigens by DCs (47). Circulating blood DCs are reduced in number in HIV-infected individuals (15), and it has also been shown that interdigitating DCs in lymphoid tissue express only a low level of costimulatory molecules during acute and chronic HIV infection (26). DCs are a heterogeneous population, and these blood or interdigitating DCs are phenotypically quite distinct from the monocyte-derived DCs we used here. However, any type of DC with impaired cross-presenting activity in vivo should be considered a major player in the progression of disturbed immune function during chronic HIV infection.
Perforin expression in circulating CD8+ T cells was low in two patients, but as illustrated by patient L2 in this study, it can be quite variable, a phenomenon that has also been described by others (3, 25). We observed that the perforin expression of HIV-specific CD8+ T cells in LTNPs and SPs is relatively low compared to that in progressors, despite the presence of a high level of activated HIV-specific memory CD8+ T cells in LTNPs and SPs. Furthermore, the level of perforin expression was well correlated with virus load (Sun et al., submitted). Therefore, perforin expression in vivo may reflect ongoing activation of HIV-specific CD8+ T cells against HIV but is not indicative of T-cell dysfunction.
It is commonly believed that IFN-γ production represents the immediate response of antigen-specific CD8+ T cells to peptide antigen stimulation (22). For example, based on the fact that many of the tetramer-positive cells of HIV-infected individuals do not produce IFN-γ upon peptide stimulation, the function of HIV-specific CD8+ T cells has been considered impaired (20, 37). However, although immediate IFN-γ production upon antigen stimulation is an important function of antigen-specific CD8+ T cells, the activated memory T cells must undergo further maturation to become effectors. Because perforin is an effector molecule of CD8+ T cells that is essential for killing target cells, we considered that the analysis of perforin expression would be crucial for the evaluation of the functional activation of memory CTLs following in vitro stimulation. In fact, it was previously shown that for the development of CD8+ T cells against Mycobacterium bovis bacillus Calmette-Guérin-infected DCs, the expression of IFN-γ and perforin was differentially regulated (42).
When HIV-specific CD8+ T cells in LTNPs were cultured with DCs loaded with VLPs, perforin expression was partially induced but still not complete. This could be due to the low level of antigen cross-presented by DCs or may reflect the different stages of CD8+ T-cell differentiation. Gea-Banacloche et al. reported that, by stimulating PBMCs with HIV-infected CD4+ T cells for 6 days in vitro, perforin expression was coupled with HIV-specific CD8+ T-cell proliferation and that this proliferation in progressors was specifically impaired while IFN-γ production was maintained (28). These findings may explain the partial lack of perforin expression in HIV-specific CD8+ T cells that we observed even after in vitro stimulation with DCs. Of note, it has also been reported that HIV-specific CD4+ T cells do not proliferate in viremic patients, despite the fact that they were frequently present and capable of producing IFN-γ (27). The clonal expansion of HIV-specific T cells during immune responses is absolutely crucial for controlling HIV infection, although why neither HIV-specific CD4+ nor CD8+ T cells are able to proliferate upon antigen stimulation is currently unknown. Because HIV-specific T cells are known to be highly activated by chronic exposure to viral antigens, the lack of proliferation may be explained by the fact that they are prone to die via apoptosis immediately following in vitro restimulation, as observed with patient L3.
We demonstrated here that it is possible to induce terminal differentiation of HIV-specific memory CD8+ T cells by cross-presentation of yeast VLP-loaded DCs but that this is only a partial effect. Cross-presentation of exogenous antigens may not be strong enough to activate unresponsive CD8+ T cells compared to internally expressed antigens during virus infection. However, even in chronically HIV-infected individuals, DCs generated in vitro were shown to be functionally competent and were able to restore anti-HIV responses after repeated in vitro stimulation with liposome-complexed p55 Gag protein (10). Taking as an advantage that yeast VLPs can induce a high level of IL-12 production accompanied by the maturation of DCs, we consider that, in combination with other vaccine regimens, yeast VLPs should be useful for stimulating Gag-specific T cells in HIV-infected individuals.
Acknowledgments
We thank Yoshiyuki Nagai (former president of the AIDS Research Center, NIID) for arrangement of Japanese-French international collaboration and Toshitada Takemori (director of the Department of Immunology, NIID) for kind help and discussions.
This work was supported by grants from the Ministry of Public Health and Labor of Japan and from the Human Science Foundation.
REFERENCES
- 1.Adams, S. E., K. M. Dawson, K. Gull, S. M. Kingsman, and A. J. Kingsman. 1987. The expression of hybrid HIV:Ty virus-like particles in yeast. Nature 329:68-70. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 3.Appay, V., L. Papagno, C. A. Spina, P. Hansasuta, A. King, L. Jones, G. S. Ogg, S. Little, A. J. McMichael, D. D. Richman, and S. L. Rowland-Jones. 2002. Dynamics of T cell responses in HIV infection. J. Immunol. 168:3660-3666. [DOI] [PubMed] [Google Scholar]
- 4.Araki, N., M. T. Johnson, and J. A. Swanson. 1996. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 135:1249-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachmann, M. F., M. B. Lutz, G. T. Layton, S. J. Harris, T. Fehr, M. Rescigno, and P. Ricciardi-Castagnoli. 1996. Dendritic cells process exogenous viral proteins and virus-like particles for class I presentation to CD8+ cytotoxic T lymphocytes. Eur. J. Immunol. 26:2595-2600. [DOI] [PubMed] [Google Scholar]
- 6.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. A. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deml, L., R. Schirmbeck, J. Reimann, H. Wolf, and R. Wagner. 1997. Recombinant human immunodeficiency Pr55gag virus-like particles presenting chimeric envelope glycoproteins induce cytotoxic T-cells and neutralizing antibodies. Virology 235:26-39. [DOI] [PubMed] [Google Scholar]
- 8.den Haan, J. M., and M. J. Bevan. 2001. Antigen presentation to CD8+ T cells: cross-priming in infectious diseases. Curr. Opin. Immunol. 13:437-441. [DOI] [PubMed] [Google Scholar]
- 9.de Saint-Vis, B., J. Vincent, S. Vandenabeele, B. Vanbervliet, J. J. Pin, S. Ait-Yahia, S. Patel, M. G. Mattei, J. Banchereau, S. Zurawski, J. Davoust, C. Caux, and S. Lebecque. 1998. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity 9:325-336. [DOI] [PubMed] [Google Scholar]
- 10.Fan, Z., X. L. Huang, L. Borowski, J. W. Mellors, and C. R. Rinaldo, Jr. 2001. Restoration of anti-human immunodeficiency virus type 1 (HIV-1) responses in CD8+ T cells from late-stage patients on prolonged antiretroviral therapy by stimulation in vitro with HIV-1 protein-loaded dendritic cells. J. Virol. 75:4413-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flo, T. H., O. Halaas, E. Lien, L. Ryan, G. Teti, D. T. Golenbock, A. Sundan, and T. Espevik. 2000. Human toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J. Immunol. 164:2064-2069. [DOI] [PubMed] [Google Scholar]
- 12.Gantner, B. N., R. M. Simmons, S. J. Canavera, S. Akira, and D. M. Underhill. 2003. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gea-Banacloche, J. C., S. A. Migueles, L. Martino, W. L. Shupert, A. C. McNeil, M. S. Sabbaghian, L. Ehler, C. Prussin, R. Stevens, L. Lambert, J. Altman, C. W. Hallahan, J. C. deq Uiros, and M. Connors. 2000. Maintenance of large numbers of virus-specific CD8+ T cells in HIV-infected progressors and long-term nonprogressors. J. Immunol. 165:1082-1092. [DOI] [PubMed] [Google Scholar]
- 14.Goepfert, P. A., A. Bansal, B. H. Edwards, G. D. Ritter, Jr., I. Tellez, S. A. McPherson, S. Sabbaj, and M. J. Mulligan. 2000. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J. Virol. 74:10249-10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grassi, F., A. Hosmalin, D. McIlroy, V. Calvez, P. Debre, and B. Autran. 1999. Depletion in blood CD11c-positive dendritic cells from HIV-infected patients. AIDS 13:759-766. [DOI] [PubMed] [Google Scholar]
- 16.Griffith, K. J., E. K. Chan, C. C. Lung, J. C. Hamel, X. Guo, K. Miyachi, and M. J. Fritzler. 1997. Molecular cloning of a novel 97-kd Golgi complex autoantigen associated with Sjogren's syndrome. Arthritis Rheum. 40:1693-1702. [DOI] [PubMed] [Google Scholar]
- 17.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung, S., D. Unutmaz, P. Wong, G.-I. Sano, K. D. Los Santos, T. Sparwasser, S. Wu, S. Vuthoori, K. Ko, F. Zavala, E. G. Pamer, D. R. Littman, and R. A. Lang. 2002. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 17:211-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kostense, S., G. S. Ogg, E. H. Manting, G. Gillespie, J. Joling, K. Vandenberghe, E. Z. Veenhof, D. van Baarle, S. Jurriaans, M. R. Klein, and F. Miedema. 2001. High viral burden in the presence of major HIV-specific CD8+ T cell expansions: evidence for impaired CTL effector function. Eur. J. Immunol. 31:677-686. [DOI] [PubMed] [Google Scholar]
- 20.Kostense, S., K. Vandenberghe, J. Joling, D. Van Baarle, N. Nanlohy, E. Manting, and F. Miedema. 2002. Persistent numbers of tetramer+ CD8+ T cells, but loss of interferon-gamma+ HIV-specific T cells during progression to AIDS. Blood 99:2505-2511. [DOI] [PubMed] [Google Scholar]
- 21.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lalvani, A., R. Brookes, S. Hambleton, W. J. Britton, A. V. S. Hill, and A. J. McMichael. 1997. Rapid effector function in CD8+ memory T cells. J. Exp. Med. 186:859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Layton, G. T., S. J. Harris, A. J. H. Gearing, M. Hill-Perkins, J. S. Cole, J. C. Griffiths, N. R. Burns, A. J. Kingsman, and S. E. Adams. 1993. Induction of HIV-specific cytotoxic T lymphocytes in vivo with hybrid HIV-1 V3:Ty-virus-like particles. J. Immunol. 151:1097-1107. [PubMed] [Google Scholar]
- 24.Lenz, P., P. M. Day, Y.-Y. S. Pang, S. A. Frye, P. N. Jensen, D. R. Lowy, and J. T. Schiller. 2001. Papillomavirus-like particles induce acute activation of dendritic cells. J. Immunol. 166:5346-5355. [DOI] [PubMed] [Google Scholar]
- 25.Lieberman, J., N. Manjunath, and P. Shankar. 2002. Avoiding the kiss of death: how HIV and other chronic viruses survive. Curr. Opin. Immunol. 14:478-486. [DOI] [PubMed] [Google Scholar]
- 26.Lore, K., A. Sonnerborg, C. Brostrom, L. E. Goh, L. Perrin, H. McDade, H. J. Stellbrink, B. Gazzard, R. Weber, L. A. Napolitano, Y. van Kooyk, and J. Andersson. 2002. Accumulation of DC-SIGN+CD40+ dendritic cells with reduced CD80 and CD86 expression in lymphoid tissue during acute HIV-1 infection. AIDS 16:683-692. [DOI] [PubMed] [Google Scholar]
- 27.McNeil, A. C., W. L. Shupert, C. A. Lyasere, C. W. Hallahan, J. Mican, J. R. T. Davey, and M. Connors. 2001. High-level HIV-1 viremia suppresses viral antigen-specific CD4+ T cell proliferation. Proc. Natl. Acad. Sci. USA 98:13878-13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Migueles, S. A., A. C. Laborico, W. L. Shupert, M. S. Sabbaghian, R. Rabin, C. W. Hallahan, D. V. Baarle, S. Kostense, F. Miedema, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, and M. Connors. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3:1061-1068. [DOI] [PubMed] [Google Scholar]
- 29.Muzio, M., D. Bosisio, N. Polentarutti, G. D'Amico, A. Stoppacciaro, R. Mancinelli, C. van't Veer, G. Penton-Rol, L. P. Ruco, P. Allavena, and A. Mantovani. 2000. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J. Immunol. 164:5998-6004. [DOI] [PubMed] [Google Scholar]
- 30.Paliard, X., Y. Liu, R. Wagner, H. Wolf, J. Baenziger, and C. M. Walker. 2000. Priming of strong, broad, and long-lived HIV type 1 p55gag-specific CD8+ cytotoxic T cells after administration of a virus-like particle vaccine in rhesus macaques. AIDS Res. Hum. Retrovir. 16:273-282. [DOI] [PubMed] [Google Scholar]
- 31.Re, F., and J. L. Strominger. 2001. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem. 276:37692-37699. [DOI] [PubMed] [Google Scholar]
- 32.Rowland-Jones, S., R. Tan, and A. McMichael. 1997. Role of cellular immunity in protection against HIV infection. Adv. Immunol. 65:277-346. [PubMed] [Google Scholar]
- 33.Rudolf, M. P., S. C. Fausch, D. M. Da Silva, and W. M. Kast. 2001. Human dendritic cells are activated by chimeric human papillomavirus type-16 virus-like particles and induce epitope-specific human T cell responses in vitro. J. Immunol. 166:5917-5924. [DOI] [PubMed] [Google Scholar]
- 34.Sakuragi, S., T. Goto, K. Sano, and Y. Morikawa. 2002. HIV type 1 Gag virus-like particle budding from spheroplasts of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 99:7956-7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sallusto, F., M. Cella, C. Danieli, and A. Lanzavecchia. 1995. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182:389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 37.Shankar, P., M. Russo, B. Harnisch, M. Patterson, P. Skolnik, and J. Lieberman. 2000. Impaired function of circulating HIV-specific CD8+ T cells in chronic human immunodeficiency virus infection. Blood 96:3094-3101. [PubMed] [Google Scholar]
- 38.Stubbs, A. C., K. S. Martin, C. Coeshott, S. V. Skaates, D. R. Kuritzkes, D. Bellgrau, A. Franzusoff, R. C. Duke, and C. C. Wilson. 2001. Whole recombinant yeast vaccine activates dendritic cells and elicits protective cell-mediated immunity. Nat. Med. 7:625-629. [DOI] [PubMed] [Google Scholar]
- 39.Tabeta, K., K. Yamazaki, S. Akashi, K. Miyake, H. Kumada, T. Umemoto, and H. Yoshie. 2000. Toll-like receptors confer responsiveness to lipopolysaccharide from Porphyromonas gingivalis in human gingival fibroblasts. Infect. Immun. 68:3731-3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thery, C., and S. Amigorena. 2001. The cell biology of antigen presentation in dendritic cells. Curr. Opin. Immunol. 13:45-51. [DOI] [PubMed] [Google Scholar]
- 41.Tsunetsugu-Yokota, Y., T. Kato, S. Yasuda, Z. Matsuda, Y. Suzuki, Y. Koyanagi, N. Yamamoto, K. Akagawa, M. W. Cho, and T. Takemori. 2000. Transcriptional regulation of HIV-1 LTR during antigen-dependent activation of primary T cells by dendritic cells. J. Leukoc. Biol. 67:432-440. [DOI] [PubMed] [Google Scholar]
- 42.Tsunetsugu-Yokota, Y., H. Tamura, M. Tachibana, K. Ogata, M. Honda, and T. Takemori. 2002. Selective expansion of perforin-positive CD8+ T cells by immature dendritic cells infected with live Bacillus Calmette-Guerin mycobacteria. J. Leukoc. Biol. 72:115-124. [PubMed] [Google Scholar]
- 43.Visintin, A., A. Mazzoni, J. H. Spitzer, D. H. Wyllie, S. K. Dower, and D. M. Segal. 2001. Regulation of Toll-like receptors in human monocytes and dendritic cells. J. Immunol. 166:249-255. [DOI] [PubMed] [Google Scholar]
- 44.Wagner, R., L. Deml, R. Schirmbeck, M. Niedrig, J. Reimann, and H. Wolf. 1996. Construction, expression, and immunogenicity of chimeric HIV-1 virus-like particles. Virology 220:128-140. [DOI] [PubMed] [Google Scholar]
- 45.Wagner, R., Y. Shao, and H. Wolf. 1999. Correlates of protection, antigen delivery and molecular epidemiology: basics for designing an HIV vaccine. Vaccine 17:1706-1710. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe, N., M. Tomizawa, A. Tachikawa-Kawana, M. Goto, A. Ajisawa, T. Nakamura, and A. Iwamoto. 2001. Quantitative and qualitative abnormalities in HIV-1-specific T cells. AIDS 15:711-715. [DOI] [PubMed] [Google Scholar]
- 47.Zhao, X. Q., X. L. Huang, P. Gupta, L. Borowski, Z. Fan, S. C. Watkins, E. K. Thomas, and C. R. Rinaldo, Jr. 2002. Induction of anti-human immunodeficiency virus type 1 (HIV-1) CD8+ and CD4+ T-cell reactivity by dendritic cells loaded with HIV-1 X4-infected apoptotic cells. J. Virol. 76:3007-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]