Abstract
Sera from 17 patients with primary and secondary liver tumors who had been administered oncolytic adenovirus (Ad) mutant Addl1520 were analyzed for anti-Ad neutralization titers and antibodies to the Ad major capsid proteins hexon, penton base (Pb), and fiber. The antibodies recognized mainly conformational epitopes in hexon and both linear and conformational epitopes in Pb and fiber. Pb-specific antibodies were isolated from serum samples that had been obtained prior to and during the course of the treatment of four of these patients. We found that the Pb antibodies had a significant contribution toward anti-Ad neutralization, and this mainly occurred at the step of virus internalization. The Pb antigenic epitopes were determined by phage biopanning and were mapped to 10 discrete regions, which made up three major immunodominant domains within residues 51 to 120, 193 to 230, and 311 to 408, respectively. One of these domains (residues 311 to 408) overlapped the highly conserved, integrin-binding RGD (Arg-Gly-Asp) motif. The contribution of antibodies directed to RGD and other epitopes in Ad neutralization activity was determined indirectly by using a phage-mediated depletion assay. Our results suggested that circulating RGD antibodies were not prevalent and were poorly neutralizing and that other peptide motifs within residues 51 to 60, 216 to 226, and 311 to 408 in Pb sequence represented major target sites for neutralizing antibodies.
Currently, there are several ongoing clinical gene therapy trials with adenoviruses (Ads) as therapeutic agents. There are several reasons for such a wide-spread usage of Ad-derived vectors or viruses. (i) Ads are easy to grow to high titers and in a suitable clinical grade. (ii) Ads are biologically safe compared to other vectors such as retroviruses or lentiviruses since they are mostly responsible for benign infections in immunocompetent individuals, and their lack of genomic integration reduces the risk of genomic recombination and oncogenic transformation. (iii) Ads are able to transduce both dividing and nondividing cells and are particularly appropriate in clinical applications such as cancer therapy due to their transient expression. Several phase I and II studies have shown that Ads are well tolerated at reasonable doses, and only low-toxicity effects such as fever, rigors, and chills have been reported (8-10, 16, 31).
Despite these advantages, large numbers of the public acquire respiratory tract infections caused by various Ads, and host immune response due to the immunogenicity of Ad capsid components remains a major impediment to efficient Ad-mediated gene transfer and persistence of transgene expression. The relative inefficacy of Ad-mediated gene transfer has been attributed to the humoral response and to the presence of preexisting neutralizing antibodies. A majority of human adults have antibodies against Ad type 5 (Ad5), but it has been reported that only 57% of adults have neutralizing antibodies (28). Recent evidence has shown that the primary form of neutralization (NA) by serum was due to the inhibition of virus binding to cells (34). A detailed characterization of Ad antibodies from patients with lung cancer has shown that the NA effect was obtained mainly from sera containing antibodies against both fiber and penton base (Pb) proteins of the virus (7). Another study involving antibodies from ascitic fluid of ovarian cancer patients identified similarly that the antibodies were primarily directed toward the fiber and Pb proteins (29). Studies on mouse models showed that the depletion of antibodies against the major capsid proteins of Ad from serum facilitated gene transfer efficiency (4, 25).
In the present study, we analyzed the circulating antibodies against Ad from patients with primary and secondary liver tumors who underwent a clinical trial with the conditionally replicative Addl1520 (9, 10). Addl1520 is an E1B-55K-defective mutant of Ad2 that replicates in certain tumor cells and has been renamed ONYX-015 (1, 2, 15). The sera of the patients before and after Ad administration were analyzed for their NA effect on Ad infection, and their reactivity against the three major Ad capsid proteins: hexon, fiber, and Pb. Several research groups have shown Ad capsid epitopes in hexon (5, 32) and fiber (6, 19-21) proteins that were responsible for the immune response. However, the immunogenicity in humans and the characterization of neutralizing and nonneutralizing epitopes in the third major Ad capsid component, the Pb, has not been systematically studied.
We focused our study on the anti-Pb response and show here that the human Pb antibodies had a major contribution in Ad NA. We isolated Pb-specific human antibodies and found that they blocked Ad-mediated gene delivery at the step of virus internalization. Using the method of phage biopanning, we could identify 10 antigenic epitopes that make up three major immunodominant domains in Pb. One of the discrete epitopes identified was the highly conserved RGD motif, which has been described to be involved in integrin recognition (35). Phage-mediated depletion of patients' sera from antibodies against some of these epitopes significantly reduced Ad-neutralizing activity. Our results suggest that RGD antibodies are not prevalent and are poorly neutralizing. In contrast, we identified several other potential epitopes and motifs in the N-terminal and central domains of Pb that could represent major target sites for neutralizing antibodies.
MATERIALS AND METHODS
Patients and clinical trial.
A clinical study was performed with mutant Addl1520 to assess its toxicity and efficacy in patients. The patients included in the studies had advanced primary or secondary liver cancer that had either failed to respond or was not suitable for conventional therapy, including surgery, chemotherapy, and radiotherapy (9, 10). Addl1520 was given as an intravenous injection in the arm vein (vena mediana cubiti) for the first dose (day 1) and then by direct intratumoral injection under ultrasound guidance on days 2, 15, 16, 29, and 30 (9). All given doses were 1 ml in volume and contained 3 × 1011 PFU of Addl1520, which had been found to be well tolerated in a previous phase I study (10).
Viruses and mammalian cells.
The Ad mutant Addl1520 was donated by A. J. Berk (Molecular Biology Institute, University of California at Los Angeles). Addl1520 carries a 827-bp deletion in the E1B-55-kDa protein coding region, in combination with a stop codon to ensure no expression of the 55-kDa gene product. Ad recombinant Ad5Luc3 (22), a kind gift from F. Graham (McMaster University, Hamilton, Ontario, Canada), is a replication-competent Ad that carries the luciferase reporter gene under the control of the simian immunodeficiency virus early promoter inserted into the E3 region of the Ad5 genome. Ad5GFP vector (Adeno-CMV5-GFP) was obtained from Quantum Biotechnologies, Inc. (Montreal, Quebec, Canada). HeLa and 293 cells were grown as monolayers in Dulbecco modified Eagle medium (Invitrogen) supplemented with 10% fetal calf serum (FCS), l-glutamine, and antibiotics.
Insect cells and baculoviruses.
Spodoptera frugiperda (Sf9) cells were maintained as monolayers at 28°C in Grace insect medium supplemented with 10% FCS and antibiotics. The recombinant baculoviruses expressing Ad2 capsid proteins hexon, Pb, and fiber have been described previously (14, 23). Recombinant Ad capsid proteins were produced in Sf9 cells and purified as described in previous studies (3, 23).
Gel electrophoresis and Western blotting.
Sodium dodecyl sulfate (SDS)-denatured proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) in 10% acrylamide gels by using a discontinuous buffer system (17). Native proteins were separated in 8% polyacrylamide gels in the same discontinuous buffer system but without SDS and twice the normal buffer concentration. Transfer of proteins onto nitrocellulose membranes (Hybond ECL; Amersham Biosciences) was carried out by using a semidry blotting system. Blots were blocked with skimmed milk in TBS-T (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.05% Tween 20) and then successively reacted with patient sera, followed by the addition of phosphatase-labeled anti-human immunoglobulin M (IgM) and IgG conjugate (Sigma-Aldrich).
Isolation of Pb antibody from serum samples.
Recombinant Pb protein was electrophoresed in a preparative SDS-PAGE gel and transferred onto a nitrocellulose membrane. The protein band was then localized by Ponceau red staining and excised. Membrane strips with immobilized Pb protein were incubated with human sera overnight at 4°C. After several rinses with phosphate-buffered saline (PBS), the antibodies were eluted with 5 mM glycine-HCl (pH 2.3)-500 mM NaCl-5% FCS for 30 s and immediately neutralized with 0.5 M Na2HPO4 (pH 9.5) to a final concentration of 50 mM. Two successive elutions were performed to maximize the antibody recovery.
Normalization of Pb antibody in serum samples and Pb-specific antibody preparations.
The quantity of Pb antibodies eluted from the nitrocellulose membranes were determined in comparison to their corresponding original sera by using dot blot analysis. Aliquots of Pb antibody samples and of the patients' sera from which the Pb antibodies were isolated (at dilutions of 1:500, 1:1,000, 1:2,000, and 1:3,000) were spotted onto the same nitrocellulose membrane. The membrane was then reacted with peroxidase-conjugated anti-human IgG antibody and developed by chemiluminescence by using the SuperSignal substrate (Pierce). The amounts of antibodies recovered from the patients'sera were quantitatively determined by densitometric analysis of the dots by using the VersaDoc image analyzer and the Quantity One program (Bio-Rad).
Circulating anti-Ad antibodies. (i) Anti-Ad antibody titer.
The whole anti-Ad antibody titers of patients' sera obtained at preinjection and 10 days after Addl1520 administration were determined by enzyme-linked immunosorbent assay (Virolab, Berkeley, Calif.) (9), and the titers were expressed as the reciprocal of the serum dilution.
(ii) Neutralizing antibodies and virus NA assays.
Aliquots of Ad5GFP virus were preincubated with serial dilutions of heat-inactivated patient sera for 1 h at 37°C before the mixture was added to HeLa cells (104 cells/sample). After a further 1-h incubation at 37°C, the supernatants were removed and replaced with fresh culture medium. The cells were observed 48 h after infection for GFP expression. The NA titer was expressed as the dilution of serum that gave a 50% GFP expression compared to the control. Ad5Luc3 virus was used according to the protocol described above, except that the cells were harvested at 18 h after infection and the cell lysates were analyzed for luciferase activity by using luciferin substrate and a Lumat LB 9501 luminometer (Berthold, Bad Wildbad, Germany).
Epitope mapping of Pb antibodies by phage biopanning.
The filamentous phage hexapeptide library was a kind gift of G. Smith (University of Columbia, Columbia, Mo.). Affinity selection of phages bound to immobilized Pb antibodies was carried out according to published protocols (11-13). The phagotopes carried by the phages were identified by DNA sequencing of the pIII gene of the phage.
Phage-mediated depletion of specific antibodies.
The principle of this method is based on the normal content of IgG in human serum (ca. 15 mg/ml). Thus, 1 μl of serum would contain 15 μg of total IgG molecules, and among them probably fewer than 10% would consist of Pb antibodies, and 1% of antibodies specific for a particular Pb epitope, i.e., 150 ng at most. Since the average molecular mass of an IgG molecule is 150 kDa, incubation of 0.1 μl of serum in 100 μl of PBS (1:1,000 dilution, 6 × 1010 moles of IgG) with 1011 phages (five pIII proteins per phage, carrying one epitope each) would provide a 10-fold excess of epitope over their corresponding antibody molecules. Phage and serum were incubated overnight at 4°C with gentle mixing and then phage-antibody complexes were removed by centrifugation at 10,000 × g for 5 min. The supernatant was then assayed for virus NA as described above.
RESULTS
Characterization of circulating antibodies from patients who had been administered Addl1520.
Human sera used in the present study were issued from 17 patients who underwent a clinical trial with the conditionally replicative Addl1520 (9, 10). The serum samples were collected before (D0; pretreatment) and at 10 days (D10) after Ad administration and then assayed for their whole-antibody response against Ad5 virus and for their NA titer against Ad5. Their reactivity against the major Ad capsid proteins hexon, Pb, and fiber was also analyzed. For comparison purposes, sera from four healthy blood donors were included in the present study (Table 1).
TABLE 1.
Characteristics of serum samples from healthy blood donors and from patients before or after administration of Addl1520a
| Sample source and no. | Time after Ad administration | Anti-Ad titerb | NA titerc | Reactivity againstd:
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Hexon
|
Pb
|
Fiber
|
|||||||
| D | N | D | N | D | N | ||||
| Patient | |||||||||
| 1 | D0 | ND | 256 | − | + | +++ | +++ | +/− | ++ |
| D10 | ND | 512 | − | + | +++ | +++ | +/− | ++ | |
| 2 | D0 | ND | 2,048 | − | + | ++ | +++ | − | + |
| D10 | ND | 4,096 | − | + | +++ | +++ | − | + | |
| 3 | D0 | 256 | 256 | +/− | +++ | − | − | − | − |
| D10 | 8,192 | 2,048 | +/− | +++ | ++++ | ++ | + | +/− | |
| 4 | D0 | 512 | 1,024 | + | + | − | − | − | − |
| D10 | 8,192 | 8,192 | +/− | ++ | ++++ | +++ | + | +++ | |
| 5 | D0 | 256 | 256 | − | − | − | +++ | − | + |
| D10 | 8,192 | 4,096 | − | + | − | +++ | +++ | +++ | |
| 6 | D0 | 1,024 | 128 | − | + | − | + | − | + |
| D10 | 4,096 | 512 | − | + | + | + | − | +++ | |
| 7 | D0 | 512 | 8 | − | + | − | + | − | + |
| D10 | 16,384 | 512 | − | + | − | ++ | − | ++ | |
| 8 | D0 | 128 | 8 | − | − | − | ++ | − | + |
| D10 | 8,192 | 4,096 | + | ++ | ++ | +++ | ++ | ++ | |
| 9 | D0 | ND | 256 | − | ++ | +++ | + | ++ | +++ |
| D10 | ND | 512 | − | ++ | ++++ | +++ | ++ | +++ | |
| 10 | D0 | ND | 8 | − | − | − | ++ | + | + |
| D10 | ND | 8 | − | − | − | ++ | + | + | |
| 11 | D0 | ND | 64 | − | − | ++++ | +++ | + | +++ |
| D10 | ND | 128 | − | − | ++++ | +++ | + | +++ | |
| 12 | D0 | ND | 128 | − | − | ++++ | +++ | +++ | +++ |
| D10 | ND | 256 | − | − | ++++ | +++ | +++ | +++ | |
| 13 | D0 | ND | 32 | − | − | − | + | + | + |
| D10 | ND | 32 | − | − | − | + | + | + | |
| 14 | D0 | ND | 256 | − | + | ++++ | +++ | +++ | +++ |
| D10 | ND | 256 | − | +++ | ++++ | +++ | +++ | +++ | |
| 15 | D0 | ND | 128 | − | +++ | − | − | − | − |
| D10 | ND | 128 | − | +++ | − | − | − | − | |
| 16 | D0 | ND | 2,048 | − | ++ | − | ++ | + | +++ |
| D10 | ND | 4,096 | − | ++ | + | ++ | + | +++ | |
| 17 | D0 | ND | 16 | − | ++ | − | − | − | − |
| D10 | ND | 16 | − | ++ | − | − | − | − | |
| Healthy blood donors | |||||||||
| 18 | D0 | ND | >1,024 | − | +/− | +++ | ++++ | + | ++++ |
| 19 | D0 | ND | >1,024 | − | − | +++ | +++ | + | + |
| 20 | D0 | ND | >1,024 | − | + | +++ | ++++ | ++++ | ++++ |
| 21 | D0 | ND | >1,024 | − | − | +++ | ++++ | ++ | +++ |
Serum samples were taken before (D0) or 10 days after (D10) Ad administration. ND, not determined.
Sera were assayed for anti-Ad antibody by enzyme-linked immunosorbent assay, and the titers are expressed as the reciprocal of the serum dilution.
The NA titer is expressed as the reciprocal of the serum dilution and was indirectly determined by the inhibition of green fluorescent protein expression in HeLa cells infected with Ad5GFP vector.
The reactivity against the native (N) or SDS-denatured (D) Ad capsid proteins hexon, Pb, and fiber was evaluated by Western blot analysis.
(i) Ad5 antibodies and neutralizing antibodies.
The whole-antibody titer against Ad5, which was assayed for six patients (patients 3 to 8, D0 and D10 samples; Table 1), ranged between 128 to 1,024 in the pretreatment samples, whereas by 10 days after Ad administration there was an overall augmentation in antibody titer for all six patients: either 4-fold (patient 6), 16-fold (patient 4), 32-fold (patients 3, 5, and 7), or 64-fold (patient 8). The anti-Ad5 NA titers of the serum samples were analyzed, and 14 sera of 21 (67%) showed low titers of preexisting NA (titers of ≤256), whereas 7 sera (33%) had high titers of ≥1,024 (Table 1). At 10 days after Ad treatment, there was an increase in NA titers for 12 patients (71%), whereas there was no change for the five other patients. The increase in NA titers ranged from 2- to 16-fold, with one patient (patient 8) showing a 512-fold increase. These results indicated that preexisting NA antibodies were low in most of the patients; however, after Ad administration their titers augmented rapidly and significantly.
(ii) Antibody reactivity profile against Ad capsid proteins.
The patients' sera were assayed for IgM and IgG subclass antibodies against native or denatured hexon, Pb, and fiber proteins. However, the reactivity of IgM antibodies was found to be very low and negligible compared to IgG antibodies (data not shown), and thus only IgG reactivity are described here. The antibody reactivity profile of the patients' sera are summarized in Table 1. In the samples taken before Ad injection, 13 samples (62%) had hexon antibodies, 17 samples (81%) had Pb antibodies, and 17 samples (81%) had fiber antibodies. At 10 days after virus injection, most of the patients' sera showed either an increase in reactivity or acquired antibodies to hexon (4 of 17 patients [24%]), Pb (8 of 17 patients [45%]), or fiber (7 of 17 patients [41%]) proteins.
In the majority of the samples, hexon antibodies were detected mainly against the native protein and very few were detected against SDS-denatured hexon, implying that most of the antibodies were directed against SDS-sensitive, conformational epitopes on the protein. In contrast, fiber and Pb antibodies were detected against both the native and the SDS-denatured proteins, suggesting that these antibodies recognized both linear and conformation-dependent epitopes. Taken together, these observations suggested that among the three capsid components of the virus, antibodies to both Pb and fiber appeared to be better induced compared to antibodies to hexon.
There was no clear correlation between the NA titers and antibody reactivity against the capsid proteins. However, it is noteworthy that, for two serum samples (sera 15 and 17) that had uniquely hexon antibodies, the NA titers were low.
Isolation of Pb-specific antibodies.
We selected sera from two patients who had preexisting Pb antibodies prior to the Ad treatment (D0 sera of patients 1 and 2) and from two other patients with newly acquired Pb antibodies (D10 sera of patients 3 and 4) (refer to Table 1 and Fig. 1a and b; patients 1, 2, 3, and 4) for further studies. Antibodies recognizing the Ad Pb protein were isolated by adsorption of the patients' sera on electrophoretically purified Pb protein immobilized on a nitrocellulose membrane. The adsorbed antibodies were then acid eluted from the membrane, immediately neutralized, and analyzed for their recovery and reactivity.
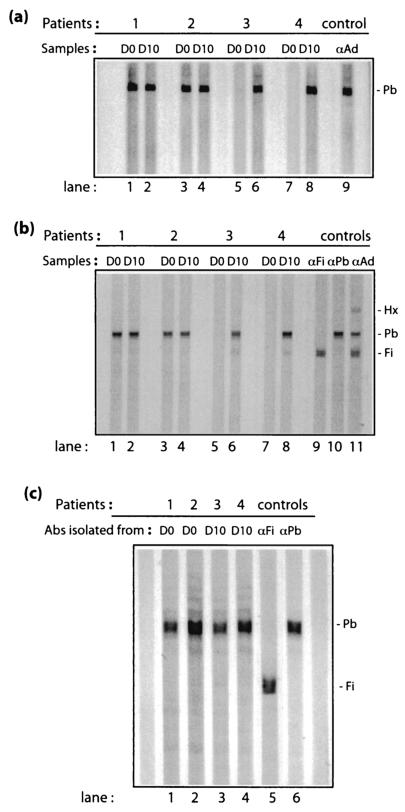
FIG. 1.
Western blot analysis of sera from patients 1, 2, 3 and 4 taken before (day 0 [D0]) or after (day 10 [D10]) Addl1520 administration. Aliquots of serum dilutions were reacted with membrane strips transferred with recombinant Ad Pb protein electrophoresed under native conditions (a) or a mixture of SDS-denatured hexon, Pb, and fiber proteins separated by conventional SDS-PAGE (b and c). Control samples (lane 9 in panel a; lanes 9 to 11 in panel b; lanes 5 and 6 in panel c) consisted of strips that were reacted with rabbit anti-fiber (αFi), anti-Pb (αPb), or anti-whole Ad virion (αAd) antibodies, respectively.
To ensure their functionality and monospecificity against Pb proteins, the isolated antibodies were tested for their reactivity against Pb protein by Western blot analysis (Fig. 1c). The positive reactivity of all four different Pb antibodies suggested that the brief acid treatment during the elution process was apparently not detrimental to their antigen-binding capacity and to their functionality as antibodies. Their nonreactivity against fiber protein that was present on the same blot showed that the antibodies recovered were specific toward Pb proteins (Fig. 1c). The amounts of IgG antibodies recovered were determined by dot blotting, followed by quantitative densitometric analysis. The Pb antibodies recovered from serum samples 1, 2, 3, and 4 contained 1/2,500, 1/2,000, 1/1,428, and 1/1,666 of the total IgG content of their original sera, respectively.
Neutralizing activity of isolated Pb antibodies.
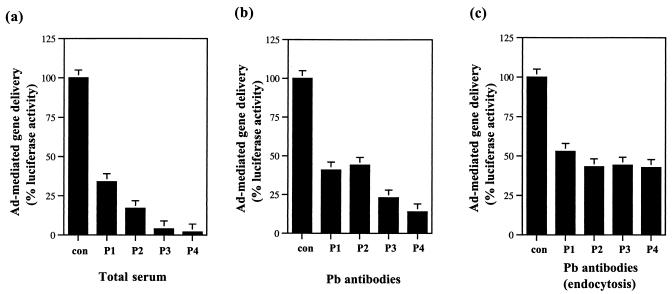
The Pb of the Ad virion has been shown to react with cell surface integrin molecules which subsequently result in the internalization of the virion (18, 35). Pb antibodies could therefore have a neutralizing effect on Ad infection by interfering with the step of virion endocytosis and internalization. We therefore tested whether the isolated Pb antibodies had any neutralizing activity against Ad virus: first, in the course of a normal viral infection and, second, at the step of virus internalization. The Ad5Luc3 virus used was a replication-competent virus that carried the luciferase reporter gene in its deleted E3 region (22). Ad5Luc3 was preincubated for 1 h at 37°C with (i) mock serum, (ii) total serum, or (iii) the corresponding isolated Pb antibodies. The total sera were diluted accordingly to normalize their IgG content to that of their corresponding Pb antibody preparations. After the preincubation period, the virus-antibody samples were added to HeLa cells, and infection was allowed to occur for 18 h at 37°C. At 18 h postinfection, the cells were harvested and assayed for luciferase activity as a measure of Ad infection efficiency. The four serum samples from patients 1, 2, 3, and 4 blocked Ad-mediated gene delivery by 65, 83, 96, and 98%, respectively (Fig. 2a). Their corresponding Pb antibodies inhibited Ad-mediated gene transfer by 59, 56, 77, and 86%, respectively (Fig. 2b). This result suggested that a significant proportion of the Ad NA activity from the serum samples could be attributed to Pb antibodies.
FIG. 2.
Virus NA activity of Pb antibodies from human sera. (a) Global effect of whole sera; (b) global effect of Pb-specific antibodies; (c) effect of Pb-specific antibodies at the endocytotic step. In panel a, Ad5Luc3 recombinant was preincubated without (control, no antibody [con]) or with total serum samples from patients 1 (P1), 2 (P2), 3 (P3), or 4 (P4) (using the D0 sera of patients 1 and 2 and the D10 sera of patients 3 and 4) and then incubated with HeLa cells. In panel b, Ad5Luc3 was preincubated with or without isolated Pb antibodies. In panel c, isolated Pb antibodies were added to virus-cell monolayers after preattachment of Ad5Luc3 to HeLa cells at 4°C for 30 min. In the three sets of experiments (i.e., panels a to c), virus infection was allowed to proceed for 18 h at 37°C, and the cells were processed for luciferase assays. The NA effect was indirectly assayed by the level of luciferase activity expressed as arbitrary units and then normalized to the percentage of the control samples.
We next addressed the question as to whether virus NA by the Pb antibodies occurred at the step of virus internalization. The entry of Ad into cells is a two-step process that first involves virus attachment on the cell surface, followed by endocytosis and internalization of the virus (35). The virus attachment step can occur at 4°C, but virus internalization can only occur at 37°C. To study the effect of Pb antibodies at the level of virus internalization, Ad5Luc3 virus was first allowed to attach to HeLa cells at 4°C for 30 min. Unattached viruses were then removed, Pb antibodies were added to the virus-cell monolayer for another 30 min at 4°C, and the cells were warmed to 37°C for virus internalization to occur. The infection was then allowed to proceed for 18 h at 37°C, and the cells were collected and assayed for luciferase activity. As shown in Fig. 2c, the presence of Pb antibodies at the step of virus internalization significantly reduced Ad-mediated gene delivery. The percent inhibitions observed were 47, 57, 55, and 57% for the Pb antibodies isolated from the serum samples of patients 1, 2, 3, and 4, respectively.
Mapping of the Pb epitopes by phage biopanning of Pb antibodies.
In order to determine the epitopes recognized by the Pb antibodies in the Pb sequence, we used the technique of phage biopanning with a phage-displayed hexapeptide library. Bacteriophages bound to immobilized Pb antibodies were selected, enriched, and sequenced after three rounds of adsorption and elution. The amino acid sequences (or phagotopes) thus obtained were compared to the Pb protein sequence. As shown in Table 2, the different phagotopes were tentatively aligned with corresponding regions of the Pb sequence according to amino acid and peptide motif identity or homology. A sequence alignment could be proposed with a reasonable degree of probability for the majority of the phagotopes isolated, since there were at least three to four contiguous amino acids out of six that were identical or had a strong homology with corresponding residues in Pb.
TABLE 2.
Phagotopes isolated by phage biopanning of Pb antibodiesa
| Class | Sequence | Patient no. |
|---|---|---|
| I (epitopes identified only in D0 | 51GRNSIRYSEL60 | |
| samples [preexisting Ab]) | NVRYSW | 1 |
| GRYAES | 1 | |
| RVRYFI | 1 | |
| 216RLGFDPVTGLV226 | ||
| LGLDPG | 2 | |
| AVTGID | 2 | |
| 336DHAIRGDTFA345 | ||
| RGDVTF | 1 | |
| II (epitopes identified only in | 69VYLVDNKST79 | |
| D10 samples [newly acquired | SLVDNS | 3 |
| Ab]) | RNLFVD | 4 |
| YMSVNV | 3 | |
| 193LKVGRQNG200 | ||
| KGQRFR | 3 | |
| VNKGQR | 3 | |
| LMRVGS | 3 | |
| PHVRGS | 4 | |
| 223TGLVMPGV230 | ||
| VMLPWL | 4 | |
| VMLPRR | 3 | |
| SHPGAV | 3 | |
| 551CPYVYKALGI560 | ||
| KVYKYF | 4 | |
| PYVIWK | 3 | |
| EGIYKT | 3 | |
| III (epitopes common to D0 and | 110TINLDDRSHWG120 | |
| D10 samples [preexisting and | FIYLDD | 3 |
| newly acquired Ab]) | LDD-S-YS | 2 |
| ASFDRS | 4 | |
| YTLLDR | 4 | |
| LDD----WGV | 3 | |
| DRRWHR | 4 | |
| MSD--HWR | 4 | |
| NRSMWQ | 1 | |
| NRSMWQ | 1 | |
| 311NNSGSGAEEN320 | ||
| YMSGSG | 3 | |
| YMSGSG | 3 | |
| HSGAEF | 2 | |
| 398STFTQYRSWYL408 | ||
| NFTQVG | 2 | |
| NFTQVG | 2 | |
| TAGGQY | 1 | |
| TAGGQY | 1 | |
| QTNYRG | 3 | |
| GQYSSS | 2 | |
| RYGWYL | 3 |
The phagotopes recovered from immobilized Pb-specific Pb antibodies (Ab) were grouped according to conserved residues and peptide motifs and then aligned with homologous regions of the Ad Pb sequence (underlined, with residue numbers as indicated). Homologous or identical residues at similar locations are in boldface. Patient numbers correspond to the patient identification numbers in Table 1. Note that motif LDD-WGV likely corresponded to a discontinuous epitope.
Ten individual different antigenic epitopes within the Pb protein sequence could be identified. They were separated into three different classes (Table 2). Class I included epitopes unique to D0 samples containing preexisting Pb antibodies. Class II epitopes were unique to D10 samples recovered from patients' sera after Ad administration (newly acquired Pb antibodies). Class III epitopes were common to both types of Pb antibodies, since they were found in samples from patients both before and after Ad treatment.
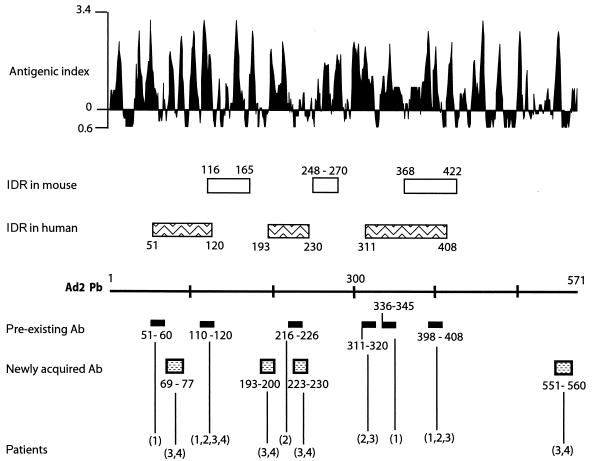
Most of the epitopes corresponded to regions of high antigenic index, according to the predictive method of Jameson-Wolf (Fig. 3). This was the case for the three major epitopes of the first class, mapped to positions 53NSIRYSE59, 217LGFDPVTGL225, and 338AIRGDTF344 in the Pb sequence. Interestingly, one of the epitopes contained the tripeptide sequence, RGD (underlined), which is responsible for interacting with cell surface integrins (35). Likewise, the three epitopes of class III that mapped within the regions 113LDDRSHWG121, 313SGSGAE318, and 400FTQYRSWYL408 showed a high probability of accessibility and antigenicity (Fig. 3). In the class II epitopes, 70YLVDNKS76, 193LKVGRQ198, and 553PYVYK556 corresponded to regions of high antigenic index; however, the epitope 224GLVMPGV230 had a relatively low probability of antigenicity.
FIG. 3.
Epitope mapping in Ad Pb protein. The Pb sequence is represented linearly. Under the Pb line are shown the positions of the epitopes defined in Table 2. The patient sera reacting with the different epitopes are indicated in parentheses. Above the Pb line are shown the major IDRs in humans, as defined in the present study, and in mice, after immunization with recombinant Pb (11). The probability of immunogenicity (as evaluated by the antigenic index of Jameson-Wolf) is presented at the top of the figure.
Phage-mediated depletion of RGD- and other epitope-directed Pb antibodies.
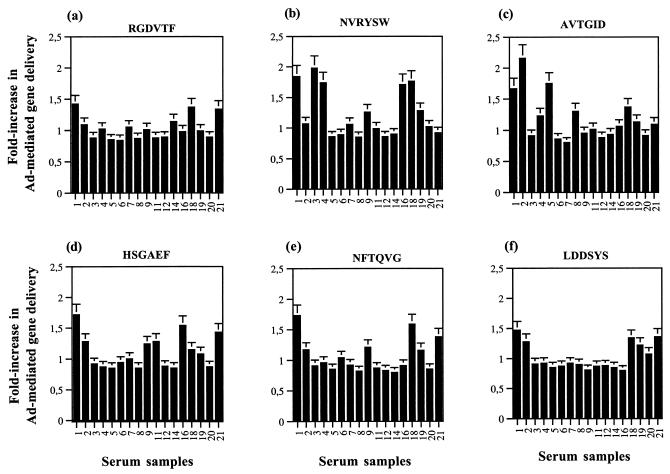
In order to determine the contribution of anti-RGD antibody in Ad vector NA, the unique phage carrying the epitope RGDVTF was amplified and incubated with 17 different serum samples which had Pb antibodies and moderate to high Ad NA titers (D10 samples from patients 1, 2, 3, 4, 5, 6, 7, 8, 9, 11, 12, 14, and 16 and samples 18, 19, 20, and 21 from healthy donors). After removal of the phage (RGD)-antibody complexes by centrifugation, the serum supernatants were tested for Ad NA, which were indirectly assayed by their effect on gene transduction by using the Ad5Luc3 vector. As a control for nonspecific depletion of antibodies, the phage library carrying random peptides was used. As shown in Fig. 4a, depletion of RGD-antibodies resulted in an approximately 1.5-fold increase in Ad-mediated gene transfer for only three serum samples, whereas there was no significant change for the 14 other samples, suggesting that RGD antibodies were present in negligible amounts, if any, in these serum samples.
FIG. 4.
Phage-mediated depletion of Pb epitope-specific antibodies. Phages carrying the Pb epitopes indicated at the top of each panel were amplified and incubated with D10 serum samples from patients 1, 2, 3, 4, 5, 6, 7, 8, 9, 11, 12, 14, and 16, as well as samples 18, 19, 20, and 21 from healthy donors. After removal of the phage-antibody complexes by centrifugation, the serum supernatants were assayed for their effect on Ad-mediated gene transfer by using the replication-competent Ad5Luc3 vector. Luciferase expression was compared in cells infected with Ad5Luc3 treated with depleted or nondepleted sera. Theoretically, values greater than 1.0 for the ratio of luciferase levels between depleted and nondepleted serum samples indicated a decrease in NA activity after phage depletion but, once the experimental errors are taken into account, only values of >1.3 were considered significant.
We performed the same antibody depletion assays by using phages carrying the epitopes NVRYSW (Fig. 4b), AVTGID (Fig. 4c), HSGAEF (Fig. 4d), NFTQVG (Fig. 4e), and LDDSYS (Fig. 4f), representing the different class I and class III epitopes mapped on the Pb protein. The class II epitopes YLVDNKS, LKVGRQ, GLVMPGV, and PYVYK were not included in the present study since we were not able to resuscitate the phages carrying these epitopes. The strongest effect observed after antibody depletion with the different phages was in the order NVRYSW, AVTGID, HSGAEF, NFTQVG, RGDVTF, and LDDSYS, based on the fold increase in Ad-mediated gene transfer efficiency and the number of serum samples that induced a significant increase in luciferase expression. These data implied (i) that the RGD antibodies were poorly neutralizing and (ii) that the motifs identified on Pb, i.e., 53NSIRYS58, 222VTGL225, 315SGAE318, and 401FTQ403, which were homologous to the phage sequences NVRYSW, AVTGID, HSGAEF, and NFTQVG, respectively, represented potential major epitopes of neutralizing antibodies.
DISCUSSION
Ads are the cause of common upper respiratory infections, and thus most people have preexisting immunity. Preexisting immunity to Ad is an important issue in clinical trials with Ad as vectors because these vectors are often used in direct in vivo delivery, and one of the limiting factors to Ad-mediated gene transfer had been attributed to preexisting neutralizing antibodies. In the present study, sera from 4 healthy individuals and 17 patients who underwent a clinical trial with Ad were characterized according to their NA activity against Ad and their reactivity toward the major capsid proteins of Ad. We observed that all of the patients had preexisting neutralizing antibodies to Ad, although most of the neutralizing titers were low. However, after Ad administration, the titers rose rapidly for 71% of these patients. It has been known for some time that the administration of Ad vectors to patients stimulates the immune system due to the immunogenicity of the viral capsid proteins. Analysis of the antibody reactivity profile after Ad administration showed that antibodies to the Pb and fiber capsid proteins were induced to a greater extent than antibodies to the hexon protein. The shedding of fibers from virions has been shown to occur upon Ad-cell attachment (24), which would effectively expose more surface of both fiber and Pb proteins to the host immune system. This early event of dismantling could explain the higher immunogenicity of fiber and Pb.
We then further analyzed the patients' sera and Pb antibodies for their NA activity and epitope specificity. The rationale for this choice was based on the strong reactivity profile for Pb protein obtained with most of the patient sera tested (Table 1) and on the possibility of following the evolution of the neutralizing activity and of the antigenic epitopes recognition by Pb antibodies during Addl1520 administration. We selected a pair of human sera that showed a particularly strong reactivity to the Pb protein before Ad treatment (preexisting Pb antibodies; D0 samples from patients 1 and 2) and another pair of sera from patients who had developed a strong Pb reactivity just after Ad administration (newly acquired Pb antibodies; D10 samples from patients 3 and 4; refer to Fig. 1a and b). We isolated the Pb-specific antibodies from serum samples and found that a significant proportion of the neutralizing activity present in the patient sera was due to Pb antibodies (Fig. 2b). Pb antibodies had been postulated to act in Ad NA by blocking the infection process at the step of virus endocytosis or vesicular release (36). Indeed, these Pb antibodies were capable of reducing significantly the Ad-mediated gene delivery at the step of virus internalization (Fig. 1c).
The endocytosis and internalization process of Ad5 had been shown to be triggered via the interaction of the RGD motif in the Pb capsomers with cell surface integrins, i.e., αvβ5 (35). In support of the importance of the highly conserved RGD motif in Pb protein, one of the phagotopes identified from the Pb antibodies indeed carried the RGD motif (Table 2). The contribution of the RGD-specific antibodies present in total sera to Ad NA activity was determined indirectly by using an RGD-phage-mediated depletion assay. The depletion of RGD-specific antibodies from serum samples increased Ad-mediated gene delivery in only 3 of 17 sera tested, implying that circulating RGD-recognizing antibodies were not prevalent in serum samples, and poorly neutralizing, an observation that has been reported earlier and interpreted by structural characteristics of the apex capsomer (30). However, the results of phage-mediated depletion of patients' sera from antibodies against other Pb antibody epitopes suggested that the motifs identified on the Pb protein—53NSIRYS58, 222VTGL225, 315SGAE318, and 401FTQ403—could represent major targets of neutralizing antibodies.
By using human sera, the combination of all epitopes identified from antibodies recognizing Pb allowed us to draw a map of immunodominant regions (IDR) (Fig. 3). Three major regions, potentially accessible in the Ad capsid, were identified at positions 51 to 120, 193 to 230, and 311 to 408, respectively. We had previously reported that immunization of mouse with recombinant Ad2 Pb elicited three IDR, which were mapped within positions 116 to 165, 248 to 270, and 368 to 422 (11). With the exception of region 368 to 422, which overlapped our epitope from positions 398 to 408 (class III, Table 2), the other regions elicited by the mouse monoclonal antibodies were apparently different from the major immunogenic regions defined here. This could be due to the fact that certain regions of the Pb capsomer are buried within the capsid and thus inaccessible to circulating antibodies. On the other hand, reactive epitopes that are common to free and virion-incorporated Pb would probably correspond to epitopes accessible in the virion. This was likely the case for the class III epitope mapped to positions 398 to 408. The finding that newly acquired antibodies recognize an epitope situated within residues 551 to 560 suggested that the C-terminal domain of the Pb protein is probably accessible at the surface of the capsid. Comparison of preexisting and newly acquired Ad-neutralizing Pb antibodies showed that four epitopes seemed to correlate with a strong increase in the virus NA activity after 10 days of Ad vector administration. These epitopes were mapped to positions 69 to 77 near the N terminus, to positions 193 to 200 and 223 to 230 in the central domain of the Pb, and to positions 551 to 560 at the C terminus, respectively (Table 2 and Fig. 3). Again, it is very likely that these three regions are accessible on the Ad capsid. The third IDR defined above, within residues 311 to 408, included the conserved RGD motif. Since this motif is involved in the integrin recognition (35) and has been assigned to a protruding structural domain of the Pb (27), the region from positions 311 to 408 in the Pb capsomers is most likely a very accessible and highly reactive domain of the Ad capsid and responsible for the induction of long-lasting (compare D0 and D10 serum samples), virus-neutralizing Pb antibodies.
Genetic modification of the corresponding gene sequence to alter Pb immunogenicity and the induction of neutralizing antibody response, if theoretically possible, is hardly conceivable if one considers that Ad vector viability depends in part upon some critical functions carried by the Pb capsomers. For example, the RGD motif is required for efficient cell endocytosis and entry of the virus. Likewise, a conservative mutation (W to H) made in recombinant Pb at positions 119 or 406, which belong to the first and third IDRs, respectively (Fig. 3), have been shown to have a deleterious effect on Pb pentamerization (mutant W119H) (14, 27) or on the assembly of Pb with fiber (mutant W406H) (14, 27). However, one could envisage the simultaneous administration of Ad vector with a mixture of synthetic peptides mimicking the Pb NA epitopes in order to block the neutralizing antibodies and help Ad to escape the NA effect.
Unlike hexon and fiber proteins, which have been crystallized and whose structures have been determined (26, 33), the structure of the Ad Pb protein has not yet been elucidated, and only indirect information is available (14, 27). Our mapping of accessible, neutralizing epitopes in Ad-encapsidated Pb molecule should help in defining its three-dimensional structure as a pentameric capsomer, as well as its sites of insertion in the viral capsid and of binding to its neighboring capsomers. In addition, modification or masking of highly reactive Pb epitopes, when feasible, or swapping Pb capsomers between Ad serotypes from different subgroups should improve the efficacy of Ad virions or Ad-derived vectors used as therapeutic agents in cancer or gene therapy.
Acknowledgments
The work in Lyon, France, was financially supported by a research grant from the French Cystic Fibrosis association Vaincre la Mucoviscidose (VLM TG-0203). The work in London, United Kingdom, was supported by The Charitable Pedersen Family Foundation.
We thank Arnold Berk (University of California at Los Angeles) for providing us with the Addl1520 clone and Frank Graham (McMaster University, Toronto, Ontario, Canada) for the Ad5-luciferase vector Ad5Luc3.
REFERENCES
- 1.Barker, D. D., and A. J. Berk. 1987. Adenovirus proteins from both E1B reading frames are required for the transformation of rodent cells by viral infection and DNA transfection. Virology 156:107-121. [DOI] [PubMed] [Google Scholar]
- 2.Bischoff, J. R., D. H. Kirn, A. Williams, C. Heise, S. Horn, M. Muna, L. Ng, J. A. Nye, A. Sampson-Johannes, A. Fattaey, and F. McCormick. 1996. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science 274:373-376. [DOI] [PubMed] [Google Scholar]
- 3.Boulanger, P., and F. Puvion. 1973. Large-scale preparation of soluble adenovirus hexon, penton and fiber antigens in highly purified form. Eur. J. Biochem. 39:37-42. [DOI] [PubMed] [Google Scholar]
- 4.Chen, Y., D.-C. Yu, D. Charlton, and D. R. Henderson. 2000. Pre-existent adenovirus antibody inhibits systemic toxicity and antitumor activity of CN706 in the nude mouse LNCaP xenograft model: implications and proposals for human therapy. Hum. Gene Ther. 11:1553-1567. [DOI] [PubMed] [Google Scholar]
- 5.Crawford-Miksza, L., and D. P. Schnurr. 1996. Analysis of 15 adenovirus hexon proteins reveals the location and structure of seven hypervariable regions containing serotype-specific residues. J. Virol. 70:1836-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fender, P., A. H. Kidd, R. Brebant, M. Oberg, and J. Chroboczek. 1995. Antigenic sites on the receptor-binding domain of human adenovirus type 2 fiber. Virology 214:110-117. [DOI] [PubMed] [Google Scholar]
- 7.Gahéry-Ségard, H., F. Farace, D. Godfrin, J. Gaston, R. Lengagne, T. Tursz, P. Boulanger, and J.-G. Guillet. 1998. Immune response to recombinant capsid proteins of adenovirus in humans: anti-fiber and anti-penton base antibodies have a synergistic effect on neutralizing activity. J. Virol. 72:2388-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganly, I., D. Kirn, S. G. Eckhardt, G. I. Rodriguez, D. S. Soutar, R. Otto, A. G. Robertson, O. Park, M. L. Gulley, C. Heise, D. Von Hoff, and S. B. Kaye. 2000. A Phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin. Cancer Res. 6:798-806. [PubMed] [Google Scholar]
- 9.Habib, N., H. Salama, A. Abd El Latif Abu Median, I. Isac Anis, R. A. Abd Al Aziz, C. Sarraf, R. Mitry, R. Havlik, P. Seth, J. Hartwigsen, R. Bhushan, J. Nicholls, and S. Jensen. 2002. Clinical trial of E1B-deleted adenovirus (dl1520) gene therapy for hepatocellular carcinoma. Cancer Gene Ther. 9:254-259. [DOI] [PubMed] [Google Scholar]
- 10.Habib, N. A., C. E. Sarraf, R. R. Mitry, R. Havlik, J. Nicholls, M. Kelly, C. C. Vernon, D. Gueret-Wardle, R. El-Masry, H. Salama, R. Ahmed, N. Michail, E. Edward, and S. L. Jensen. 2001. E1B-deleted adenovirus (dl1520) gene therapy for patients with primary and secondary liver tumors. Hum. Gene Ther. 12:219-226. [DOI] [PubMed] [Google Scholar]
- 11.Hong, S. S., M. Bardy, M. Monteil, B. Gay, C. Denesvre, J. Tournier, G. Martin, M. Eloit, and P. Boulanger. 2000. Immunoreactive domains and integrin-binding motifs in adenovirus penton base capsomer. Viral Immunol. 13:353-371. [DOI] [PubMed] [Google Scholar]
- 12.Hong, S. S., and P. Boulanger. 1995. Protein ligands of the human adenovirus type 2 outer capsid identified by biopanning of a phage-displayed peptide library on separate domains of wild-type and mutant penton capsomers. EMBO J. 14:4714-4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong, S. S., L. Karayan, J. Tournier, D. T. Curiel, and P. Boulanger. 1997. Adenovirus type 5 fiber knob binds MHC class I a2 domain at the surface of human epithelial and B lymphoblastoid cells. EMBO J. 16:2294-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karayan, L., S. S. Hong, B. Gay, J. Tournier, A. Dupuy d'Angeac, and P. Boulanger. 1997. Structural and functional determinants in adenovirus type 2 penton base recombinant protein. J. Virol. 71:8678-8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirn, D. 2001. Clinical research results with dl1520 (ONYX-015), a replication-selective adenovirus for the treatment of cancer: what have we learned? Gene Ther. 8:89-98. [DOI] [PubMed] [Google Scholar]
- 16.Kirn, D., R. L. Maurtuza, and J. Zwiebel. 2001. Replication-selective virotherapy for cancer: biological principles, risk management and future directions. Nat. Med. 7:781-787. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 18.Li, E., S. L. Brown, D. G. Stupack, X. S. Puente, D. A. Cheresh, and G. R. Nemerow. 2001. Integrin αvβ1 is an adenovirus coreceptor. J. Virol. 75:5405-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liebermann, H., K. Lotz, R. Mentel, U. Bauer, and W. Seidel. 2001. Mapping of linear epitopes on fibre knob of human adenovirus serotype 5. Virus Res. 73:145-151. [DOI] [PubMed] [Google Scholar]
- 20.Liebermann, H., K. Lotz, and W. Seidel. 2002. Mapping of epitopes on the fiber knobs of human adenovirus serotypes 8 and 15. Intervirology 45:59-66. [DOI] [PubMed] [Google Scholar]
- 21.Mei, Y. F., and G. Wadell. 1996. Epitopes and hemagglutination binding domain on subgenus B:2 adenovirus fibers. J. Virol. 70:3688-3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mittal, S. K., M. R. McDermott, D. C. Johnson, L. Prevec, and F. L. Graham. 1993. Monitoring foreign gene expression by a human adenovirus-based vector using the firefly luciferase gene as a reporter. Virus Res. 28:67-90. [DOI] [PubMed] [Google Scholar]
- 23.Molinier-Frenkel, V., R. Lengagne, F. Gaden, S. S. Hong, J. Choppin, H. Gahery-Ségard, P. Boulanger, and J.-G. Guillet. 2002. Adenovirus hexon protein is a potent adjuvant for activation of a cellular immune response. J. Virol. 76:127-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakano, M. Y., K. Boucke, M. Souomalainan, R. P. Stidwill, and U. F. Greber. 2000. The first step of adenovirus type 2 disassembly occurs at the cell surface, independently of endocytosis and escape to the cytosol. J. Virol. 74:7085-7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahman, A., V. Tsai, A. Goudreau, J. Y. Shinoda, S. F. Wen, M. Ramachandra, R. Ralson, D. Maneval, D. LaFace, and P. Shabram. 2001. Specific depletion of human anti-adenovirus antibodies facilitates transduction in an in vivo model for systemic gene therapy. Mol. Ther. 3:768-778. [DOI] [PubMed] [Google Scholar]
- 26.Rux, J. J., and R. M. Burnett. 2000. Type-specific epitope locations revealed by x-ray crystallographic study of adenovirus type 5 hexon. Mol. Ther. 1:18-30. [DOI] [PubMed] [Google Scholar]
- 27.Schoehn, G., P. Fender, J. Chroboczek, and E. A. Hewat. 1996. Adenovirus 3 penton dodecahedron exhibits structural changes of the base on fibre binding. EMBO J. 15:6841-6846. [PMC free article] [PubMed] [Google Scholar]
- 28.Schulick, A. H., G. Vassalli, P. F. Dunn, G. Dong, J. J. Rade, C. Zamarron, and D. A. Dichek. 1977. Established immunity precludes adenovirus-mediated gene transfer rat carotid arteries: potential for immunosuppression and vector engineering to overcome barriers of immunity. J. Clin. Investig. 99:209-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stallwood, Y., K. D. Fisher, P. H. Gallimore, and V. Mautner. 2000. Neutralization of adenovirus infectivity by ascitic fluid from ovarian cancer patients. Gene Ther. 7:637-643. [DOI] [PubMed] [Google Scholar]
- 30.Stewart, P. L., C. Y. Chiu, S. Huang, T. Muir, Y. Zhao, B. Chait, P. Mathias, and G. R. Nemerow. 1997. Cryo-EM visualization of an exposed RGD epitope on adenovirus that escapes antibody neutralization. EMBO J. 16:1189-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sung, M. W., H.-C. Yeh, S. N. Thung, M. E. Schwartz, J. P. Mandeli, S.-H. Chen, and S. L. Woo. 2001. Intratumoral adenovirus-mediated suicide gene transfer for hepatic metastases from colorectal adenocarcinoma: results of a Phase I Clinical Trial. Mol. Ther. 4:182-190. [DOI] [PubMed] [Google Scholar]
- 32.Toogood, C. I., J. Crompton, and R. T. Hay. 1992. Antipeptide antisera define neutralizing epitopes on the adenovirus hexon. J. Gen. Virol. 73:1429-1435. [DOI] [PubMed] [Google Scholar]
- 33.van Raaij, J. M., A. Mitraki, G. Lavigne, and S. Cusack. 1999. A triple beta-spiral in the adenovirus fiber shaft reveals a new structural motif for a fibrous protein. Nature 401:935-938. [DOI] [PubMed] [Google Scholar]
- 34.Vincent, T., B.-G. Harvey, S. M. Hogan, C. J. Bailey, R. G. Crystal, and P. L. Leopold. 2001. Rapid assessment of adenovirus serum neutralizing antibody titer based on quantitative, morphometric evaluation of capsid binding and intracellular trafficking: population analysis of adenovirus capsid association with cells is predictive of adenovirus infectivity. J. Virol. 75:1516-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wickham, T. J., P. Mathias, D. A. Cheresh, and G. R. Nemerow. 1993. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell 73:309-319. [DOI] [PubMed] [Google Scholar]
- 36.Wohlfart, C. 1988. Neutralization of adenoviruses: kinetics, stoichiometry, and mechanisms. J. Virol. 62:2321-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]