Abstract
Hepatitis C virus (HCV) infection is associated with immune-mediated abnormalities and B-cell lymphoproliferation. Recently, CD81 was identified as an HCV receptor on B lymphocytes, providing a mechanism by which B cells are infected and activated by the virus. It has recently been shown that peripheral B-cell CD81 overexpression and CD5+ subpopulation expansion correlate with HCV viral load and are associated with the development of HCV-related autoimmunity. In the present study, we assessed the effects of combination antiviral therapy (alfa interferon and ribavirin) on peripheral B-cell CD81 expression and CD5 expansion and the presence of autoimmune markers. Peripheral B-cell CD5 expression and the mean fluorescence intensity of CD81 were assessed by flow cytometry before and after treatment in 15 HCV-infected patients, in 10 untreated patients, and in 25 healthy controls. A significant posttreatment decrease in peripheral B-cell CD81 expression and disappearance of CD5+ B-cell expansion were observed in all nine patients in whom a complete and sustained virological response was achieved (P < 0.01) (comparable to those for healthy controls). The decrease in CD81 overexpression and CD5 expansion in these patients was associated with a decrease and/or disappearance of autoimmune markers. In contrast, in nonresponders overexpression of CD81 and expansion of the CD5+ B-cell subpopulation were not significantly changed and were comparable to those for untreated patients. In conclusion, antiviral therapy down-regulates peripheral B-cell CD81 expression and the CD5+ population, either directly or by its effect on HCV RNA load. The overexpression of CD81 and the expansion of the population of CD5+ peripheral B cells in HCV-infected patients may possibly play a role in the development of HCV-associated autoimmunity and lymphoproliferation.
Hepatitis C virus (HCV) infection is considered to be one of the most common important known causes of chronic liver disease worldwide, affecting between 0.5 and 2% of the population of the Western world (12). In addition to its being hepatotropic, HCV is a lymphotropic virus (34). This peculiar lymphotropism may be responsible, at least in part, for the multiple immune-mediated extra hepatic manifestations of HCV infection, such as mixed cryoglobulinemia (1, 19, 20), Sjögren-like syndrome (10), the presence of rheumatoid factor (RF) in serum, the production of autoantibodies (2, 9, 11, 24, 28), and B-cell non-Hodgkin lymphoma (5, 31, 36). The pathogenetic link between HCV and the immune system in inducing both autoimmunity and lymphoproliferation is unclear. The persistence of HCV in peripheral blood mononuclear cells, preferentially in B cells (33), results in chronic stimulation of B cells, leading to polyclonal and later to monoclonal proliferation of RF immunoglobulin M kappa (IgMκ)-producing cells, which eventually may result in malignant transformation and development of overt lymphoma (7, 13, 23, 30). The recent identification of CD81 protein as one of the HCV receptor candidates on B lymphocytes (26), providing a mechanism by which B cells are infected with or activated by HCV, may raise a wide spectrum of interesting issues regarding the pathogenetic link between HCV infection, autoimmunity, and lymphoproliferative disorders. CD81 is expressed on the surfaces of a variety of cell types and has a diverse spectrum of biological activities. The second extracellular loop of CD81 has recently been shown to interact with HCV envelope 2 glycoprotein in vitro (26). On B cells, CD81 is a member of a signaling complex that includes CD19 and CD21 (17). Cross-linking these complexes using antibodies to either CD81 or CD19 lowers the threshold for B-cell activation and proliferation. Similarly, binding of HCV particles to a CD81-containing complex might facilitate B-cell activation, possibly explaining at least in part the association between HCV, B-cell activation, and lymphoproliferative diseases. In addition, it has recently been shown that the peripheral blood CD5+ B-cell subpopulation is expanded in patients with chronic HCV infection (3). These cells are characterized by the production of low-affinity IgM with RF activity, arise early in ontogeny, and are considered to represent the bridge linking innate and acquired immune responses (32). The production of circulating autoantibodies coupled with their increased frequency in rheumatoid arthritis and Sjögren's syndrome has implicated them in the development of autoimmune diseases (4, 27). In addition, the numbers of CD5+ B cells are also increased in patients with essential mixed cryoglobulinemia (25) and were identified as monoclonal- and polyclonal-IgM-producing cells in the hepatic lymphoid follicles of HCV-infected patients (22). We have recently shown that in chronic HCV infection there are peripheral B-cell CD81 overexpression and CD5+ B-cell subpopulation expansion which correlate with HCV RNA load and are associated with the development of HCV-related autoimmunity (35). The present study was conducted to assess the effect of combined antiviral treatment on peripheral B-cell CD81 overexpression and CD5+ B-cell expansion in chronic HCV infection and to investigate the relationship between this overexpression, the decline of viral load, and autoimmune markers.
MATERIALS AND METHODS
Patients.
Twenty-five patients with chronic HCV infection in whom peripheral B-cell CD81 and CD5 expression was assessed in our previous study (35) were enrolled in the present study. Of these, 15 patients were treated with antiviral treatment consisting of alfa interferon and ribavirin, while in 10 patients antiviral treatment was avoided for one of the following reasons: decompensated cirrhosis or age older than 70 years (3 patients), significant cytopenia (i.e., a concentration of less than 10 g of hemoglobin/dl of plasma, a concentration of less than 3,000 leukocytes/mm3 of plasma, or a concentration of less than 75,000 platelets/mm3 of plasma) (n = 3), severe depression (n = 1), or refusal to take treatment (n = 3). A liver biopsy was performed for all treated patients and for five members (60%) of the nontreated group. All HCV-infected patients tested positive for anti-HCV antibody (ELISA II; Abbot Laboratories, North Chicago, Ill.), had detectable HCV RNA (Amplicor PCR assay; Roche Molecular Systems, Somerville, N.J.), and had histological evidence of chronic hepatitis with or without cirrhosis. Excluded were patients who had positive serological tests for HIV and/or hepatitis B surface antigen. All HCV-positive patients underwent a baseline evaluation consisting of the following: (i) a liver panel (which measured levels in serum of alanine aminotransferase [ALT], aspartate aminotransferase, albumin, globulin, bilirubin, alkaline phosphatase, and lactic dehydrogenase and prothrombin time); (ii) HCV genotyping and viral load analysis; (iii) immunological screening, including protein electrophoresis and screening for RF, cryoglobulin, and a panel of autoantibodies including antinuclear (ANA), anti-smooth muscle (aSMA), and anticardiolipin (aCLA) antibodies; and (iv) assessment of peripheral B-cell CD5 expression and the mean fluorescence intensity (MFI) of CD81. In addition, a control group consisting of 25 healthy individuals (recruited from Bnai Zion Medical Center staff; mean age ± standard deviation [SD], 45 ± 15; male to female ratio, 14:11) was also assessed for peripheral B-cell CD5 expression and the MFI of CD81.
In all patients, liver and immunological panel assessments, HCV RNA, B-cell CD5 expression, and the MFI of CD81 were reexamined at the end of treatment (in the treated patients) or 12 months after baseline evaluation (in the nontreated patients). Disease severity was assessed by the level of ALT in serum and liver histology (inflammation and fibrosis).
Informed consent was obtained from each patient. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the Institutional Review Board of the Bnai Zion Medical Center.
Antiviral treatment.
Antiviral treatment consisted of (i) alfa interferon (3 million U, three times a week, subcutaneously) combined with ribavirin, 800 mg to 1,200 mg daily for 12 months (for eight patients with HCV genotype 1b); (ii) alfa interferon (3 million U, three times a week) in combination with ribavirin, 800 to 1,200 mg daily for 6 months (for three patients with HCV genotype 2 or 3); (iii) pegylated alfa interferon 2a, 180 μg once weekly combined with ribavirin, 800 mg daily for 12 months (for four patients with HCV genotype 1a or 1b).
Definition of response.
Biochemical response was defined as normalization of ALT at the end of treatment. Virological response was defined as undetectable HCV RNA in serum at the end of treatment. Sustained virological response was defined as undetectable HCV RNA at 6 months after completion of antiviral treatment.
HCV quantification.
The concentration of HCV RNA in serum was measured by using the Amplicor Monitor test kit (Roche Diagnostic Systems, Basel, Switzerland). The test includes an RNA quantification standard of known copy number that is coamplified with the target and is used to calculate the copy level of a sample by colorimetric assay following hybridization to a specific probe.
HCV genotyping.
HCV genotypes were assayed using a commercial assay (INNO-Lipa HCV II test; Innogenetics N.V., Zwijndrecht, Belgium) based on amplification with biotinylated oligonucleotide primers for the 5′ untranslated region of HCV. This assay identifies six genotypes and major subtypes.
Histological analysis.
The histological score was graded from 0 to 22 by a modification of the method of Knodell et al. (15). For the Knodell histological activity index (HAI), an inflammation score was obtained by combining scores of periportal injury (interface hepatitis and/or bridging necrosis) (0 to 10), parenchymal injury (0 to 4), portal inflammation (0 to 4), and fibrosis (0 to 4). The degree of inflammation and fibrosis was scored by an independent histopathologist unaware of the patients' clinical status. The Knodell HAI was calculated for only the noncirrhotic patients.
B-cell separation.
Peripheral blood mononuclear cells were isolated by Ficoll-Hypaque density gradient separation. B lymphocytes were positively selected with magnetic microbeads conjugated to antihuman CD22 (MAC's System; Melteni Biotech) according to the manufacturer's instructions. Following isolation, the CD19+ cell population (and CD81+ cells) comprised >95% of the resulting population. The purified B cells were assayed for the CD81 MFI and for the expression of CD5 on resting B cells.
Flow cytometry.
Purified B cells were immunostained directly (one step) with phycoerythrin- and fluorescein isothiocyanate-conjugated monoclonal antibodies for CD81 and CD5 (Ancell, Bayport, Minn.). Flow cytometry was carried out with a fluorescence-activated cell scan operated with Cellquest software (Becton Dickinson, Mountain View, Calif.). The total population of viable cells was gated according to their typical forward and right-angle light scatter. The fluorescence of cells treated with fluorescent-isotype monoclonal antibody was evaluated in each experiment to determine the level of background fluorescence of negative cells. Data were acquired on the flow cytometer, and the number of CD5+ B cells was expressed as a percentage of the number of CD81+ cells. Only the positive cells were included in the percentages of stained cells used to calculate results. Data were displayed on a logarithmic scale of increasing MFI for CD81, and each histogram plot was generated from at least 104 events.
Cryoglobulin detection and analysis.
Venous blood samples were collected into prewarmed tubes after the patients had fasted overnight, and samples were allowed to clot at 37°C. After being centrifuged, the sera were incubated at 4°C for 3 days. The cryocrit was evaluated by centrifugation of the serum in hematocrit tubes at 4°C. The cryoprecipitates were further analyzed and characterized by immunofixation electrophoresis (Immunofix kit; Helena Laboratories, Beaumont, Tex.).
Statistical analysis.
Comparison of the percentages of CD5+ B cells and the expression of B-cell CD81 before and after treatment (for the treated group) or at baseline and at the end of follow-up (for the nontreated group) was performed using the Wilcoxon test for pairs. Comparison of the above variables among the different groups (treated responders, treated nonresponders, and nontreated patients) and healthy controls was performed by using Kruskal-Wallis analysis of variance followed by Dunn's post-hoc test. Comparison between two noncategorical groups was performed by using the Mann-Whitney U test. Association between categories was analyzed using the chi-square test or Fisher's exact test. Two-tailed P values of 0.05 or less were considered to be statistically significant.
RESULTS
Table 1 summarizes the baseline clinical characteristics of the treated and untreated HCV-infected patients. The two groups were comparable with respect to age, gender, and disease severity, i.e., ALT levels, presence of cirrhosis, and HAI score. Genotype 1 was the most frequent genotype found in both groups: 66% in treated and 60% in nontreated patients. A relatively high prevalence of RF, mixed cryoglobulin, and non-organ-specific autoantibodies was found in both groups. Analysis of the cryoglobulin in 4 of 6 treated patients and 2 of 3 nontreated patients revealed type II mixed cryoglobulin with monoclonal IgMκ in all patients. Nine of the 15 patients who received antiviral treatment had a complete and sustained biochemical and virological response, while the other 6 were nonresponders.
TABLE 1.
Baseline clinical characteristics of the treated and untreated HCV-infected patients
| Patient status | Age (y)b | Sex | HAI scorec | ALT level (IU/liter) | Mean HCV RNA titerd (105 copies/ml) | No. positive for:
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cirrhosis (%) | HCV genotype 1 (%) | RF (%) | Cryo- globulins (%) | Auto- antibodiese (%) | ||||||
| Treated (n = 15) | 49.4 ± 13.1 | 10 females, 5 males | 9 ± 3 | 123 ± 95 | 5.32 ± 3 | 4 (26) | 10 (66) | 6 (40) | 6 (40) | 8 (53) |
| Untreated (n = 10) | 50.9 ± 17.3 | 7 females, 3 males | 7.2 ± 2.7 | 79 ± 25 | 5.73 ± 2.4 | 5 (50) | 6 (60) | 4 (40) | 3 (30) | 6 (60) |
P values were not significant for any of the characteristics listed in the table.
Ages are expressed as means ± SDs.
HAI scores are expressed as means ± SDs.
HCV RNA levels represent the pretreatment viral load.
The autoantibodies present may include anti-nuclear, anti-smooth muscle, and/or anti-cardiolipin autoantibodies.
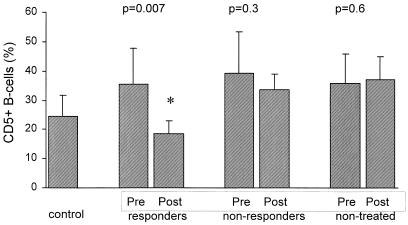
A significant posttreatment decline in peripheral CD5+ B-cell expansion was observed in all nine sustained responders (Fig. 1 and 2) compared with the pretreatment proportion of CD5+ cells (means ± SDs, 18.5 ± 4.4% versus 35.5 ± 12.2%, respectively; P = 0.007), and in fact, the posttreatment proportion was comparable to the proportion of CD5+ cells in the healthy controls (18.5 ± 4.4% versus 24.4 ± 7.3%, respectively; P = 0.12) (Fig. 2). The posttreatment CD5+ B-cell subpopulation in the sustained responders was significantly decreased compared with that of the nonresponders and nontreated patients (18.5 ± 4.4% versus 33.5 ± 5.4% and 37.1 ± 7.9%, respectively; P < 0.01). In contrast, in nonresponders the expansion of the CD5+ B-cell subpopulation was not significantly changed and was comparable to that for untreated patients.
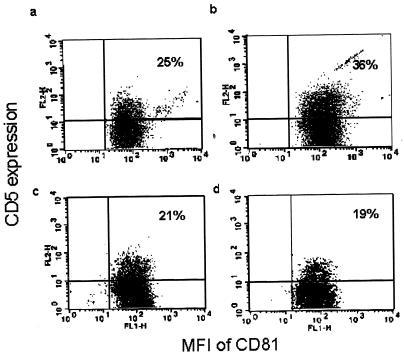
FIG. 1.
Representation of the results of fluorescence-activated cell scans of CD81/CD5 expression on peripheral B lymphocytes from an HCV-infected patient before and after antiviral treatment and a healthy control. Results shown are for the baseline evaluation for the control (a), the patient before treatment (b), the control 12 months after the baseline assessment (c), and the patient after treatment (after achieving sustained virological response) (d).
FIG. 2.
CD5 expression on peripheral B cells in HCV-infected patients before and after antiviral treatment. “Pre” and “Post” refer to pre- and posttreatment results for treated patients. For nontreated patients, “Pre” indicates results for the baseline assessment and “Post” refers to results 12 months after the baseline evaluation.
Similarly, posttreatment B-cell CD81 expression was significantly lower in the patients who achieved a sustained virological response than pretreatment CD81 expression (means ± SDs for CD81 MFI, 135 ± 40.3 versus 202 ± 47.2., respectively; P = 0.007) and was actually similar to that observed in the healthy controls (135 ± 40.3 versus 142.7 ± 34.2, respectively; P = 0.8) (Fig. 3). A significant posttreatment decline in B-cell CD81 overexpression was observed in the sustained responders compared with that of the nonresponders and nontreated patients (135 ± 40.3 versus 180.7 ± 20 [P = 0.04] and 200 ± 42 [P = 0.016], respectively). In contrast, in nonresponders posttreatment CD81 expression remained similar to CD81 expression in untreated patients (Fig. 3).
FIG. 3.
MFI of CD81 on peripheral B cells in HCV-infected patients before and after antiviral treatment. “Pre” and “Post” refer to pre- and posttreatment results for treated patients. For nontreated patients, “Pre” indicates results for the baseline assessment and “Post” refers to results 12 months after the baseline evaluation.
Interestingly, in addition to a decrease in CD5+ B-cell expansion and CD81 overexpression, virological response to antiviral treatment was associated with the disappearance of cryglobulin, RF, and autoantibodies in all but one of the sustained responders (Table 2). Four responders had aCLA (two with IgM and two with IgG) and one had ANA. After successful treatment, aCLA (in 3 patients) and ANA disappeared. In nonresponders and in nontreated patients, cryoglobulin, RF, and non-organ-specific autoantibodies remained unchanged from the baseline assessment to the follow-up period. Of the nonresponders, two patients had aCLA (both of IgM isotype) and one had aSMA, while of the nontreated patients, four had aCLA (IgM in three and IgG in one), two had ANA, and one had aSMA. However, the number of patients analyzed in each subgroup (i.e., responders, nonresponders, and nontreated) was too small to reach statistically significant conclusions.
TABLE 2.
Results of antiviral treatment on the presence of autoimmune markers
| Autoimmune marker | No. of patients positive for indicated autoimmune marker who were:
|
|||||
|---|---|---|---|---|---|---|
| Nontreated (n = 10)
|
Nonresponsive (n = 6)
|
Responsive (n = 9)
|
||||
| Prea | Postb | Prea | Postb | Prea | Postb | |
| Cryoglobulin | 3 | 3 | 3 | 2 | 3 | 0 |
| RF | 4 | 4 | 2 | 2 | 4 | 0 |
| Autoantibodyc | 6 | 7 | 3 | 3 | 5 | 1 |
“Pre” indicates the results of baseline evaluations made for all patients whether or not they received treatment.
For nontreated patients, “Post” indicates the results for assessments made 12 months after the baseline evaluation. For treated patients, “Post” refers to the results of assessments made after treatment.
Autoantibodies may include anti-nuclear, anti-smooth muscle, and/or anti-cardiolipin antibodies.
DISCUSSION
Chronic HCV infection is associated with a host of extrahepatic manifestations, including autoimmune phenomena, benign clonal expansion of B cells, and B-cell non-Hodgkin lymphoma, which suggest B-cell activation and proliferation. However, the exact mechanism linking HCV infection with autoimmunity and lymphoproliferation is unknown. The interaction between the HCV envelope 2 glycoprotein of HCV and the CD81-containing complex on B cells may provide one of these missing links. Of particular interest are the activation and proliferation of a specific B-cell subpopulation, namely, the CD5+ cells. These cells proliferate in patients with essential mixed cryoglobulinemia and various other autoimmune disorders (4, 22, 25, 27, 35). In the present study, we have demonstrated that combined antiviral treatment led to a significant decrease in peripheral B-cell CD81 expression and the disappearance of CD5+ B-cell expansion in all patients in whom a complete and sustained virological response was achieved. The decrease in CD81 overexpression and CD5 expansion in these patients was strongly associated with a decrease or disappearance of autoimmune markers (i.e., RF, cryoglobulin, or autoantibodies), whereas in nonresponders overexpression of CD81 and expansion of the CD5+ B-cell subpopulation did not significantly change and were comparable to those for untreated patients.
B-cell proliferation in HCV-infected patients is probably enhanced by HCV-specific properties, including the ability of HCV proteins to bind to CD81 on the B-cell surface and to influence intracellular regulatory functions following viral entry into B cells. Thus, it is possible that viral factors enhance CD81 up-regulation, possibly leading to the enhanced binding of HCV proteins to this receptor. In support of this hypothesis is the recent finding that lower levels of cell surface-associated CD81 are associated with HCV genotype 3 and the initial decline of HCV RNA after initiation of antiviral therapy (16). In respect to that observation, we have not found a significant difference in pretreatment CD81 expression between sustained responders and nonresponders. The mechanism by which antiviral treatment lowers CD81 expression on the surfaces of peripheral B cells, as found in the present study, is not clear. Recently, peripheral B-cell CD81 expression was shown to be increased in HCV-infected patients (16, 35) and was down-regulated by antiviral therapy to a level comparable to that for normal controls. This effect may be directly related to antiviral therapy or indirectly to decrease of viral load. As suggested by Kronenberger and colleagues (16), the up-regulation of CD81 mRNA in this study indicates that alfa interferon-induced down-regulation of CD81 protein probably occurs at the posttranscriptional or translational level. The decrease in cell surface-associated CD81 expression induced by alfa interferon could therefore reduce the attachment of HCV, and if CD81 is required for uptake of HCV virions, down-regulation of CD81 could also diminish de novo infection of susceptible cells. Additionally, mice lacking CD81 have enhanced T-cell proliferation and immune responses following T-cell antigen receptor engagement (18, 21). The decreased expression of CD81 may thus enhance the immune response and facilitate virus elimination. Another possible effect of antiviral treatment on B-cell CD81 expression is an indirect effect via reducing the viral load. In support of this assumption are our previous observations that pretreatment B-cell CD81 expression is increased and that this increase is correlated with the HCV RNA level (35). In addition, patients with a rapid initial decline of HCV RNA after initiation of antiviral therapy showed a decline of cell surface-associated CD81 and had lower levels of CD81 than patients without initial virological response (16). Interestingly, in our six nonresponders, some decline of B-cell CD81 expression was observed, although it did not reach statistical significance. It is possible that antiviral treatment induced some decrease in HCV RNA load during treatment, leading to down-regulation of B-cell CD81. With all these factors taken together, it is yet unclear whether HCV facilitates the expression of CD81 on B cells or whether high levels of CD81 may confer a selective advantage for HCV either by modulation of the immune response or by increasing attachment of viral particles.
The other important finding in the present study is the reversal of CD5+ B-cell expansion followed by the disappearance of RF and cryoglobulins and diminished autoantibody production in all but one of the treated patients who achieved sustained virological response but not in nonresponders. Supported by data from several studies, it has been suggested that HCV infection may induce antigen-driven benign proliferation of selected B cells producing IgM monoclonal RF, namely, the CD5+ cells (8, 14, 30). Additionally, there are recent data indicating that HCV infection and clonal expansion of B cells within the liver preferentially involve RF-producing cells (29) and that in selected cases of patients with type II mixed cryoglobulinemia, B-cell subsets expressing IgM RF are the prevalent cell type targeted by HCV (6). Based on these data and our previous finding that the expansion of CD5+ B cells correlates with the HCV RNA level and the production of RF, cryoglobulin, and other autoantibodies (35), it is reasonable to speculate that CD5+ B-cell activation and proliferation are facilitated by HCV binding to the cell surface-associated CD81-containing complex. In support of this hypothesis is the recent evidence showing that CD81 on B cells plays an important role in generating antibody responses to T-cell-dependent Th2 antigens (32). The effect of antiviral therapy on the expansion of CD5+ cells is presumably caused by decreasing HCV RNA load and cessation of the chronic antigenic stimulus by HCV virions to these cells, thus reducing the production of RF, cryoglobulin, and other autoantibodies. The small but not significant decrease in CD5+ cell expansion in treated patients who did not achieve sustained virological response may reflect some decrease in HCV virions, thus decreasing the antigenic stimulation to peripheral B cells.
In conclusion, in HCV-infected patients who achieved sustained virological response, decreased B-cell CD5+ expansion and CD81 overexpression were observed. This effect of antiviral therapy was associated with diminished production of RF, cryoglobulin, and autoantibodies. These findings may indicate that B-cell CD81 and CD5+ subpopulations play an important role in the development of HCV-associated autoimmunity and lymphoproliferation. Therefore, down-regulation of B-cell CD81 overexpression and reversal of CD5+ expansion by antiviral treatment may be a desirable therapeutic goal in preventing these phenomena. However, the results of the present study are too preliminary to support a clear role for CD81-HCV interaction in the expansion of the CD5+ cell subset, and therefore, further studies are needed to investigate the immunomodulatory role of decreasing B-cell CD81 expression by antiviral treatment in patients with chronic HCV infection.
REFERENCES
- 1.Angello, V., R. T. Chung, and L. M. Kaplan. 1992. A role for hepatitis C virus infection in type II cryoglobulinemia. N. Engl. J. Med. 327:1490-1495. [DOI] [PubMed] [Google Scholar]
- 2.Clifford, B. D., D. Donahue, L. Smith, E. Cable, B. Luttig, M. Manns, and H. L. Bonkovsky. 1995. High prevalence of serological markers of autoimmunity in patients with chronic hepatitis C. Hepatology 21:613-619. [PubMed] [Google Scholar]
- 3.Curry, M. P., L. Golden-Mason, N. Nolan, N. A. Parfrey, J. E. Hegarty, and C. O'Farrelly. 2000. Expansion of peripheral blood CD5+ B cells is associated with mild disease in chronic hepatitis C virus infection. J. Hepatol. 32:121-125. [DOI] [PubMed] [Google Scholar]
- 4.Dauphinee, M., Z. Tovar, and N. Talal. 1988. B cells expressing CD5 are increased in Sjogren's syndrome. Arthritis Rheum. 31:642-647. [DOI] [PubMed] [Google Scholar]
- 5.Ferri, C., F. Caracciolo, A. L. Zignego, L. La Civita, M. Monti, G. Longombardo, F. Lombardini, F. Greco, E. Capochiani, and A. Mazzani. 1994. Hepatitis C virus infection in patients with non-Hodgkin's lymphoma. Br. J. Haematol. 88:392-394. [DOI] [PubMed] [Google Scholar]
- 6.Fornasieri, A., P. Bernasconi, M. L. Ribero, R. A. Sinico, M. Fasola, L. Zhou, G. Portera, A. Tagger, A. Gibelli, and G. D'amoco. 2000. Hepatitis C virus (HCV) in lymphocyte subset and in B lymphocytes expressing rheumatoid factor cross-reacting idiotype in type II mixed cryoglobulinemia. Clin. Exp. Immunol. 122:400-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franzin, F., D. G. Efremov, G. Pozzato, P. Tulissi, F. Batista, and O. R. Burrone. 1995. Clonal B-cell expansion in peripheral blood of HCV-infected patients. Br. J. Haematol. 90:548-552. [DOI] [PubMed] [Google Scholar]
- 8.Galli, M. 1995. Viruses and cryoglobulinemia. Clin. Exp. Rheumatol. 13(Suppl.):S63-S70. [PubMed] [Google Scholar]
- 9.Gumber, S. C., and S. Chopra. 1995. Hepatitis C: a multifaceted disease. Ann. Intern. Med. 123:615-620. [DOI] [PubMed] [Google Scholar]
- 10.Haddad, J., P. Deny, C. Munz-Gotheil, J. C. Ambrosini, C. Trinchet, D. Pateron, F. Mal, P. Callard, and M. Beaugrand. 1992. Lymphocytic sialadenitis of Sjorgen's syndrome associated with chronic hepatitis C virus liver disease. Lancet 339:321-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadziyannis, S. J. 1997. The spectrum of extrahepatic manifestations in hepatitis C virus infection. J. Viral Hepat. 4:9-28. [DOI] [PubMed] [Google Scholar]
- 12.Hoofnagle, J. H., and T. S. Tralka. 1997. Management of hepatitis C virus. Proceedings of the National Institutes of Health Consensus Development Conference. Hepatology 26(Suppl. 1):S1-S2. [DOI] [PubMed] [Google Scholar]
- 13.Ivanovski, M., F. Silversti, G. Pozzato, S. Anand, C. Mazzaro, O. R. Burrone, and D. G. Efremov. 1998. Somatic mutation, clonal diversity, and preferential expression of the VH 51p1/VL kv325 immunoglobulin gene combination in hepatitis C virus-associated immunocytomas. Blood 91:2433-2442. [PubMed] [Google Scholar]
- 14.Kipps, T. 1989. The CD+ B cell. Adv. Immunol. 47:117-185. [DOI] [PubMed] [Google Scholar]
- 15.Knodell, R. G., K. G. Ishak, W. C. Black, T. S. Chen, R. Craig, N. Kaplowitz, T. W. Kiernan, and J. Wollman. 1981. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1:431-435. [DOI] [PubMed] [Google Scholar]
- 16.Kronenberger, B., B. Roster, R. Elez, S. Weber, A. Piiper, J-H. Lee, W. K. Roth, and S. Zeuzem. 2001. Interferon alfa down-regulates CD81 in patients with chronic hepatitis C. Hepatology 33:1518-1526. [DOI] [PubMed] [Google Scholar]
- 17.Levy, S., S. C. Todd, and H. T Maecker. 1998. CD81 (TAPA-1): a molecule involved in signal transduction and cell adhesion in the immune system. Annu. Rev. Immunol. 16:89-106. [DOI] [PubMed] [Google Scholar]
- 18.Maecker, H. T., M. S. Do, and S. Levy. 1998. CD81 on B cells promotes interleukin 4 secretion and antibody production during T helper type 2 immune response. Proc. Natl. Acad. Sci. USA 95:2458-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcellin, P., V. Descamps, M. Marinot-Peignoux, D. Larzul, L. Xu, N. Boyer, B. N. Pham, B. Cricks, L. Guillevin, and S. Belaich. 1993. Cryoglobulinemia with vasculitis associated with hepatitis C virus infection. Gastroenterology 104:272-277. [DOI] [PubMed] [Google Scholar]
- 20.Misiani, R., P. Bellavita, D. Fenili, G. Borelli, D. Marchesi, M. Massazza, G. Vendramin, B. Comotti, E. Zanzi, and G. Scudeller. 1992. Hepatitis C infection in patients with essential mixed cryoglobulinemia. Ann. Intern. Med. 117:573-577. [DOI] [PubMed] [Google Scholar]
- 21.Miyazaki, T., U. Muller, and K. S. Campbell. 1997. Normal development but differentially altered proliferative responses of lymphocytes in mice lacking CD81. EMBO J. 16:4217-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monteverde, A., M. Ballare, and S. Pileri. 1997. Hepatic lymphoid aggregates in chronic hepatitis C and mixed cryoglobulinemia. Springer Semin. Immunopathol. 19:99-110. [DOI] [PubMed] [Google Scholar]
- 23.Monteverde, A., E. Sabattini, S. Poggi, M. Ballare, M. C. Bertoncelli, A. De Vivo, A. Briskomatis, G. Roncador, B. Falini, and S. A. Pileri. 1995. Bone marrow findings further support the hypothesis that essential mixed cryoglobulinemia type II is characterized by monoclonal B-cell proliferation. Leuk. Lymphoma 20:119-124. [DOI] [PubMed] [Google Scholar]
- 24.Pawlotsky, J. M., F. Roudot-Thoraval, P. Simmonds, J. Mellor, M. B. Ben Yahia, C. Andre, M. C. Voisin, L. Intrator, E. S. Zafrani, and J. Duval. 1995. Extrahepatic manifestations in chronic hepatitis C and hepatitis C virus serotypes. Ann. Intern. Med. 122:169-173. [DOI] [PubMed] [Google Scholar]
- 25.Pietrogrande, M., M. Corona, S. Milani, A. Rosti, M. Ramella, and G. Tordato. 1995. Relationship between rheumatoid factor and the immune response against hepatitis C virus in essential mixed cryoglobulinemia. Clin. Exp. Rheumatol. 13(Suppl. 13):109-113. [PubMed] [Google Scholar]
- 26.Pileri, P., Y. Uematsu, S. Campagnoli, G. Galli, F. Falugi, R. Petracca, A. J. Weiner, M. Houghton, D. Rosa, G. Grandi, and S. Abrignani. 1998. Binding of hepatitis C virus to CD81. Science 282:938-941. [DOI] [PubMed] [Google Scholar]
- 27.Plater Zybrec, C., R. N. Maini, K. Lam, T. D. Kennedy, and G. Janossy. 1985. A rheumatoid arthritis B cell subset expresses a phenotype similar to that in chronic lymphocytic leukemia. Arthritis Rheum. 28:971-976. [DOI] [PubMed] [Google Scholar]
- 28.Prieto, J., J. R. Yuste, O. Beloqui, M. J. Civeira, J. Riezu, B. Aguirre, and B. Sangro. 1996. Anticardiolipin antibodies in chronic hepatitis C: implication of hepatitis C virus as the cause of the antiphospholipid syndrome. Hepatology 23:199-204. [DOI] [PubMed] [Google Scholar]
- 29.Sansonno, D., S. De Vita, A. R. Iacobelli, V. Cornacchiulo, M. Boiocchi, and F. Dammacco. 1998. Clonal analysis of intrahepatic B cells from HCV-infected patients with and without mixed cryoglobulinemia. J. Immunol. 160:3594-3601. [PubMed] [Google Scholar]
- 30.Sasso, E. H. 2000. The rheumatoid factor response in the etiology of mixed cryoglobulinemia associated with hepatitis C virus infection. Ann. Med. Interne 15(1):30-40. [PubMed] [Google Scholar]
- 31.Silvestri, F., C. Pipan, G. Barillari, F. Zaja, R. Fanin, L. Infanti, D. Russo, E. Falasca, G. A. Botta, and M. Baccarani. 1996. Prevalence of hepatitis C virus infection in patients with lymphoproliferative disorders. Blood 87:4296-4301. [PubMed] [Google Scholar]
- 32.Tsuji, R. F., G. P. Geba, Y. Wang, K. Kawamoto, L. A. Matis, and P. W. Askenase. 1997. Required early complement activation in contact sensitivity with generation of local C5-dependent chemotactic activity, and late T cell interferon gamma: a possible initiating role of B cells. J. Exp. Med. 186:1015-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zehender, G., L. Meroni, C. De Maddalena, S. Varchetta, G. Monti, and M. Galli. 1997. Detection of hepatitis C virus RNA in CD19 peripheral mononuclear cells of chronically infected patients. J. Infect. Dis. 176:1209-1214. [DOI] [PubMed] [Google Scholar]
- 34.Zignego, A. L., D. Macchia, M. Monti, V. Thiers, M. Mazzetti, M. Foschi, E. Maggi, S. Romagnani, P. Gentilini, and C. Brechot. 1992. Infection of peripheral mononuclear blood cells by hepatitis C virus. J. Hepatol. 15:382-386. [DOI] [PubMed] [Google Scholar]
- 35.Zuckerman, E., G. Slobodin, A. Kessel, E. Sabo, D. Yeshurun, K. Halas, and E. Toubi. 2002. Peripheral B-cell CD5 expansion and CD81 overexpression and their association with disease severity and autoimmune markers in chronic hepatitis C virus infection. Clin. Exp. Immunol. 128:353-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zuckerman, E., T. Zuckerman, A. M. Levine, D. Douer, K. Gutekunst, M. Mizokami, D. G. Qian, M. Velankar, B. N. Nathwami, and T. L. Fong. 1997. Hepatitis C infection in patients with non-Hodgkin B-cell lymphoma. Ann. Intern. Med. 127:423-428. [DOI] [PubMed] [Google Scholar]