Abstract
In filamentous fungi, het loci (for heterokaryon incompatibility) are believed to regulate self/nonself-recognition during vegetative growth. As filamentous fungi grow, hyphal fusion occurs within an individual colony to form a network. Hyphal fusion can occur also between different individuals to form a heterokaryon, in which genetically distinct nuclei occupy a common cytoplasm. However, heterokaryotic cells are viable only if the individuals involved have identical alleles at all het loci. One het locus, het-c, has been characterized at the molecular level in Neurospora crassa and encodes a glycine-rich protein. In an effort to understand the role of this locus in filamentous fungi, we chose to study its evolution by analyzing het-c sequence variability in species within Neurospora and related genera. We determined that the het-c locus was polymorphic in a field population of N. crassa with close to equal frequency of each of the three allelic types. Different species and even genera within the Sordariaceae shared het-c polymorphisms, indicating that these polymorphisms originated in an ancestral species. Finally, an analysis of the het-c specificity region shows a high occurrence of nonsynonymous substitution. The persistence of allelic lineages, the nearly equal allelic distribution within populations, and the high frequency of nonsynonymous substitutions in the het-c specificity region suggest that balancing selection has operated to maintain allelic diversity at het-c. Het-c shares this particular evolutionary characteristic of departing from neutrality with other self/nonself-recognition systems such as major histocompatibility complex loci in mammals and the S (self-incompatibility) locus in angiosperms.
Self/nonself-recognition is essential for sexual reproduction, defense against pathogen invasion, and maintenance of individuality for an organism. In vertebrates, self/nonself-recognition relies on the major histocompatibility complex (MHC), which is an array of genetic loci that generate proteins important in pathogen recognition and activation of defense mechanisms. The human MHC consists of over 100 highly polymorphic genes that fall into two classes, MHC I and II. Allelic polymorphisms at the MHC II DRB1 locus, represented by 58 alleles in humans, have existed for at least 30 million years and are shared by humans, apes, and other primates (1). Self/nonself-recognition during sexual reproduction in many plant species is mediated by the gametophytic or sporophytic self-incompatibility locus, S, which elicits recognition and rejection of self-pollen (2). Similar to alleles at the DRB1 locus, different alleles at the S locus show long-term persistence, such that an allele from one species is often more closely related to an allele in a different species, rather than to another S allele in the same species (3, 4). Thus, alleles have been passed from ancestral to descendent species and are shared among contemporary species, a phenomenon referred to as “trans-species polymorphism” (5). Allele polymorphisms at loci in the MHC and S locus are thought to be maintained by balancing selection, either by overdominance (heterozygotes have a higher fitness than homozygotes) or by frequency dependent selection (rare alleles are at a selective advantage, but become disadvantageous when common) (4, 6, 7).
In filamentous fungi, the requirement for and biological significance of self/nonself-recognition during vegetative growth is unclear. During vegetative growth, filamentous fungi possess the remarkable feature of being able to undergo hyphal fusion between different individuals to form vegetative heterokaryons that contain genetically distinct nuclei within a common cytoplasm. The viability of such heterokaryons is determined by the genetic constitution at het (heterokaryon) or vic (vegetative incompatibility) loci (8, 9, 10). The phenomenon of heterokaryon (vegetative, heterogenic, or somatic) incompatibility has been described in numerous filamentous ascomycetes and basidiomycetes (9, 10, 11). Nuclear components of a heterokaryon that contain different specificities at any of several het loci can give rise to unstable nuclear ratios or can result in growth inhibition of heterokaryotic cells, which are frequently destroyed by a lytic process (8, 9, 12, 13).
Heterokaryon incompatibility in filamentous fungi may confer selective advantages by preventing hyphal fusions that could spread mycoviruses, debilitated organelles, and deleterious plasmids throughout a fungal population (14). It also has been proposed that het genes may play a role in limiting outbreeding in certain fungal species (11). However, it is unclear whether heterokaryon incompatibility loci are bona fide self/nonself-recognition systems in filamentous fungi or whether their existence is a simple consequence of variation in genes with critical cellular functions (15). If a primary function of het loci is to mediate self/nonself-recognition during vegetative growth, selection may have favored polymorphisms at het loci. Alleles at het loci would then exhibit evolutionary features similar to those found in other loci that regulate self/nonself-recognition, such as trans-species polymorphism. Alternatively, het loci could be polymorphic in populations as a result of genetic variation and encode products for which heteroallelism becomes lethal or detrimental. In this case, the number and constitution of het loci might vary in different fungal species, including the types and frequency of polymorphism at individual het loci.
Genes involved in heterokaryon incompatibility have been cloned and characterized from two haploid filamentous ascomycetes, Podospora anserina (for review, see ref. 15) and Neurospora crassa (8, 16). In N. crassa, the het-c locus is one of 11 genetically identified het loci (17). Individuals that are nearly isogenic, but carry different alleles at het-c, do not form viable heterokaryons (18). The het-c locus encodes a glycine-rich polypeptide that contains a coiled-coil domain (16). Three distinct and mutually incompatible alleles termed het-cOR, het-cPA, and het-cGR have been characterized (19, 20), and a region of 34–48 amino acids that is polymorphic among HET-COR, HET-CPA, and HET-CGR (Fig. 1) determines het-c allelic specificity. Mutants in het-c are unaffected in vegetative or sexual phenotype but have lost the capacity to mediate incompatibility via differences at het-c (16). The identification of polymorphisms within a domain that confers het-c specificity has allowed us to examine the evolutionary pattern of alleles at this locus in species within the Sordariaceae.
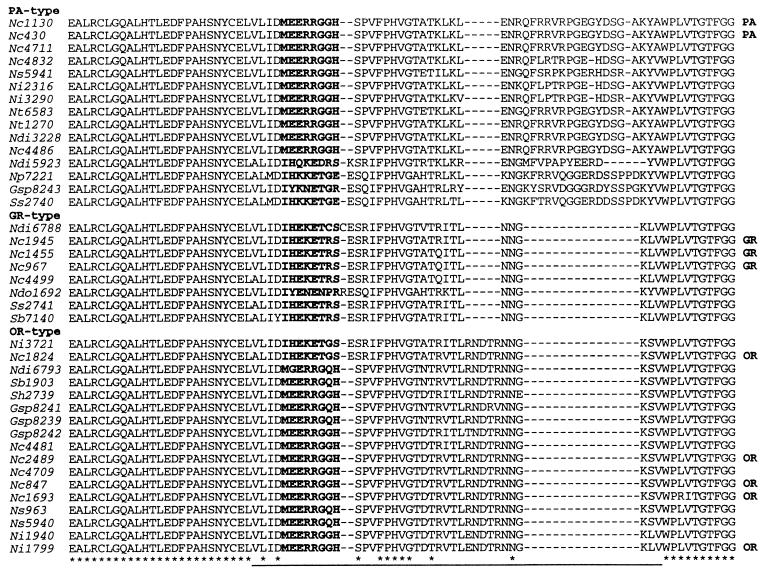
Figure 1.
Inferred amino acid sequences of PCR amplified het-c allelic specificity motif from 40 isolates. The alignment was obtained by clustalw 6.1 and modified manually. Nc, Neurospora crassa; Ni, N. intermedia; Ndi, N. discreta; Ns, N. sitophila; Nt, N. tetrasperma; Np, N. pannonica; Ndo, N. dodgei; Ss, Sordaria sclerogenia; Sh, S. heterothallis; Sb, S. brevicollis; Gsp, Gelasinospora sp. Numbers following indicate the Fungal Genetics Stock Center (FGSC) number or P number (4481, 4486, and 4499 strains). PA (Panama), GR (Groveland), and OR (Oakridge) refer to the three molecularly characterized alleles that confer different het-c specificities (20). The het-c specificity of alleles designated in the figure was determined by partial diploid analysis or heterokaryon tests (refs. 19 and 20 and unpublished results). Asterisk and dashes indicate identical and deletion residue sites, respectively. Underlined section is het-c specificity variable region. Bold region is polymorphic block.
In this study, we find that het-c shares evolutionary features with loci in the MHC and the S systems, in which allelic polymorphisms are believed to be maintained by balancing selection. We find that the specificity region of het-c displays trans-species polymorphism, that het-c allele frequencies are approximately equal within a N. crassa population sample, and that high frequency of nonsynonymous substitutions are present within the het-c specificity region. These findings support our assertion that het-c functions as a self/nonself-recognition locus and that this function is critical during the vegetative growth phase of filamentous fungi.
MATERIALS AND METHODS
Strains and Cultural Conditions.
For het-c phylogenetic analysis, we used 40 strains representing 11 species and three different genera within the Sordariaceae. All strains were obtained from the Fungal Genetics Stock Center (FGSC; Department of Microbiology, University of Kansas Medical Center, Kansas City, KS). N. crassa strains were: FGSC 1130 (Panama), FGSC 4711 (Haiti), FGSC 4832 (Ivory Coast), FGSC 2489 (Louisiana), FGSC 1455 (unknown), FGSC 4709 (Haiti), FGSC 430 (Ivory Coast), FGSC 847 (Louisiana), FGSC 1693 (Louisiana), FGSC 1824 (Pakistan), FGSC 1945 (Florida), and FGSC 967 (Liberia). N. intermedia strains were: FGSC 2316 (Florida), FGSC 1940 (Florida), FGSC 3290 (Oahu), FGSC 3721 (Kauai), and FGSC 1799 (Malaya). N. discreta strains were: FGSC 3228 (Texas), FGSC 5923 (Florida), FGSC 6788 (New Guinea), and FGSC 6793 (Brazil). N. sitophila strains were: FGSC 963 (France), FGSC 5940 (Tahiti), and FGSC 5941 (Tahiti). Gelasinospora sp. strains were: FGSC 8241, FGSC 8239, FGSC 8242, and FGSC 8243 (all from the Yucatan Peninsula.). Sordaria brevicollis strains were: FGSC 7140 (New York) and FGSC 1904 (New York). S. heterothallis strain was: FGSC 2739 (Texas). S. sclerogenia strains were: FGSC 2741 (Ceylon) and FGSC 2740 (Ceylon). All of the above strains are heterothallic in mating behavior. The following pseudohomothallic N. tetrasperma strains were used: FGSC 6583 (Tahiti) and FGSC 1270 (unknown). Homothallic species used were: N. pannonica, FGSC 7221 (Hungary), and N. dodgei, FGSC 1692 (Puerto Rico).
For population analysis, an additional 36 N. crassa strains isolated by D. J. Jacobson (Dept. of Biological Sciences, Stanford University) from a 5-hectare sugar cane field in Louisiana (ref. 21; P4448-P4501) were obtained from A. J. F. Griffiths, Botany Department, University of British Columbia. All strains were maintained on vegetative growth medium (22).
PCR and DNA Sequence Analysis.
Genomic DNA from all isolates was prepared as described by Oakley et al. (23). Genomic DNA was digested with restriction enzymes and subjected to Southern blotting as described (16). The region of het-c allelic specificity was amplified by PCR from genomic DNA with two primers designed from the sequence of het-cOR allele (20): 5′-(598)GGAGACATGGCGATATCG(615)-3′ and 5′-(1441)GTGAGGCACAACCCACTC(1424)-3′. Single amplification products corresponding to het-c were obtained for all isolates. The PCR products were cloned into the pCRII vector by using the TA cloning kit (Invitrogen) and subjected to DNA sequence analysis by using the ABI automatic sequencing procedure at the Nucleic Acid and Protein Sequencing Unit (University of British Columbia). For restriction fragment length polymorphism (RFLP) analysis, the PCR products from the het-c specificity domain were subjected to HaeIII digestion and probed with het-cOR.
Phylogenetic and Statistical Analyses.
DNA and deduced amino acid sequences of the specificity region from the 40 isolates described above were aligned by clustalw 6.1 (Institute for Biomedical Computing, Washington University, St. Louis, Mo.) and adjusted by eye. Phylogenetic analyses were performed by the neighbor-joining and maximum-likelihood methods by using phylip (24). Genetic distances were estimated based on Kimura’s two-parameter method (24). Insertions and deletions were excluded from analysis in pairwise comparisons. The number of nonsynonymous substitutions per nonsynonymous site (dN) and synonymous substitutions per synonymous site (dS) were calculated by using mega software (25), with pairwise deletion of gap sites. A standard z test was used to determine whether (i) mean dS = mean dN and (ii) mean dN (of the het-c specificity region) = mean dN (of the het-c nonspecificity region). The expected values of dN:dS and the associated standard errors were calculated by using the delta method (26, 27).
RESULTS
The Three het-c Allelic-Types Are About Equally Frequent Both Within and Between Neurospora crassa Populations.
A variable domain characterized by particular insertion/deletion motifs (20) determines allelic specificity at het-c. Of 15 N. crassa isolates in which the DNA sequence of the het-c specificity region was determined, five alleles were het-cPA-type, four were het-cGR-type, and six were het-cOR-type (Fig. 1). There was no obvious correlation between geographical location and het-c allelic type. If the different het-c allelic types are maintained by selection, the expectation is that a near-equal frequency of each type of allele would be observed within a fungal population, assuming equivalent reactions among alleles and no intrinsic fitness differences. The near-equal frequency of the three het-c allelic types among geographically dispersed N. crassa isolates suggested the possibility that each allelic type also might be present at equal frequencies within a population of N. crassa.
Although a large number of wild-type isolates of N. crassa are available, most had been isolated from scattered regions in the sub-tropics. However, a population sample of 39 individuals had been recovered from a 5-hectare sugarcane field in Louisiana (21). RFLP analysis of the het-c specificity region PCR product from 36 isolates (three isolates did not grow) showed that 14 of the N. crassa isolates had a het-cOR pattern, 14 had a het-cPA pattern, and eight had a het-cGR pattern (data not shown). The DNA sequence of the het-c specificity region was determined for a representative of each polymorphic class, which confirmed the allele designations made by RFLP analysis (Fig. 1; Nc4481, Nc4486, and Nc4499). When digested genomic DNA from the 36 isolates was probed with a 35-kbp cosmid containing het-c, twenty-four different RFLP patterns could be identified, demonstrating that the population was not clonal or, if clonal, was not of recent origin.
The HET-C Sequences from Different Species and Genera in the Sordariaceae Were Either HET-COR, HET-CPA, or HET-CGR-Type.
If het-c polymorphisms are present because of genetic variation in N. crassa and if they are not under selection, the expectation is that het-c polymorphisms would be lost by genetic drift in species distantly related to N. crassa. To determine whether polymorphisms at het-c are restricted to N. crassa, the het-c specificity regions from an additional 25 isolates representing 11 different species and three different genera in the Sordariaceae were analyzed. DNA hybridization analysis was consistent with the presence of only a single het-c allele within the genome of each isolate (data not shown). Analysis was restricted mainly to heterothallic species. Unlike Neurospora, most other heterothallic species in the Sordariaceae are not represented in collections by large numbers of individual strains that could be analyzed.
The DNA sequences of the het-c specificity region were divergent among the 40 isolates, with the two most distantly related members of the 40 isolate data set (N. pannonica 7221 and S. heterothallis 2739) showing only 66% identity. Although the DNA sequences of the het-c specificity region were divergent, the deduced amino acid sequences from the 40 isolates grouped into one of the three HET-C specificity types based on the pattern of insertion/deletion first observed in the N. crassa het-c alleles (Fig. 1).
A comparison of the HET-C region from the 40 isolates showed conserved and variable domains. The regions flanking the HET-C specificity domain showed 100% amino acid identity among the 40 isolates (Fig. 1). A highly polymorphic amino acid block [MEERRGG(Q)H and IH(Q)EKET(D)R(G/C)S] is highlighted in Fig. 1. The 40 isolates have either of these polymorphic blocks, although the HET-CGR-type isolates all contained a similar motif [IHEK(N)ET(N)R(C, P)S]. The amino acid sequence in this polymorphic block apparently does not affect het-c specificity in N. crassa because Nc1824 and Nc2489 have identical het-c specificities based on partial diploid analysis (19, 20). Following this polymorphic region is a five-amino acid block (FPHVG) that is conserved in all 40 isolates.
For all species in which more than one isolate was characterized (with the exception of the pseudohomothallic species, N. tetrasperma), at least two of the three het-c allelic variants were found. In the heterothallic species S. brevicollis, the two interfertile isolates from this species (both isolated from zebra dung in the New York Zoological Park) contained het-c alleles with different HET-C types (HET-CGR and HET-COR) (Fig. 1). The two S. sclerogenia interfertile isolates from Ceylon also have different HET-C allelic types, one being HET-CPA (Ss2740) and the other HET-CGR (Ss2741). Similarly, the HET-C specificity domain from interfertile Gelasinospora sp. isolates from the Yucatan Peninsula fell into either HET-CPA or HET-COR types. All three of the het-c allelic types were identified in all of the heterothallic Neurospora species. From the amino acid sequence alignment shown in Fig. 1, it is evident that HET-C polymorphism (as characterized by insertion/deletion motif and the associated highly polymorphic block within het-c) is shared between species and genera within the Sordariaceae.
The DNA Sequence of the het-c Specificity Region Shows Trans-Species Polymorphism.
Evidence for shared polymorphism at the DNA level between species within the Sordariaceae would indicate that allelic specificities at het-c were generated in an ancestral species for genera within the Sordariaceae and have been maintained through multiple speciation events. Alternatively, each of the species within the Sordariaceae may have independently evolved similar insertion/deletion polymorphisms within the het-c specificity region that are only evident at the amino acid level. To distinguish between these two alternatives, the relationship of the DNA sequence of the het-c region from the 40 alleles was examined by phylogenetic analysis using a neighbor-joining algorithm based on Kimura’s two-parameter distance with pairwise elimination of insertion/deletion sites (24). Nearly identical trees were produced by DNA maximum likelihood and DNA parsimony analyses (data not shown). In addition, a neighbor-joining analysis that excluded the entire deletion/insertion motif [from the beginning of the Oakridge (OR)-type insertion to the end of the Panama (PA)-type insertion] also gave a similar tree, indicating that tree topology was not an artifact of alignment.
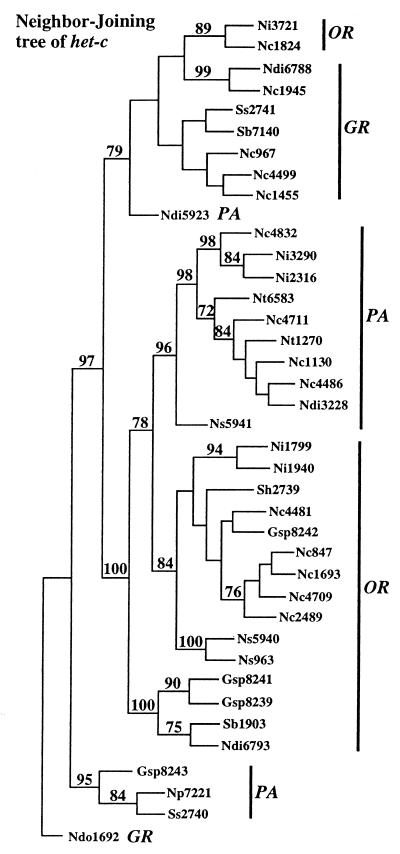
From Fig. 2, it is apparent that the DNA sequences of the 40 alleles fell into groups by het-c allelic type rather than according to species. Thus, the DNA sequences of the specificity region of het-c displayed trans-species polymorphism. Although only three allelic specificities have been identified in N. crassa (het-cOR, het-cPA, and het-cGR-type), phylogenetic analysis of the het-c DNA sequences from the 40 isolates showed additional lineages. In all of the trees, two het-cOR-type strains, Ni3721 and Nc1824, were included in the Groveland (GR)-type lineage. The clustering of these two OR-type strains with the GR-type strains was due to the intragenic combinatorial association of the MEERRGGH/IHEKETRS polymorphic block (bold in Fig. 1) with the het-cOR insertion/deletion type. Similarly, strains Ndi5923, Gsp8243, Np7221, and Ss2740 were separated from the PA-lineage because of the linkage of the IHEKETRS-type polymorphic block with the het-cPA insertion. Because gap sites were eliminated in pairwise comparisons in genetic distance estimation, the polymorphic block in het-c affected placement of these strains in phylogenetic analysis. Combinatorial associations of polymorphic blocks also have been observed in MHC class I DNA polymorphisms (28).
Figure 2.
Neighbor-joining tree using 390-bp nucleotide sequences spanning the het-c specificity motif from 39 isolates (N. crassa FGSC 1130 (Panama) and 430 (Ivory Coast) have identical nucleotide sequences in this region). The het-c sequence from the homothallic species, N. dodgei (Ndo1692), was used as an outgroup. Distance was calculated by Kimura’s two parameter method in the phylogenetic analysis package phylip 3.5 (24). Numbers are bootstrap percentages (out of 1,000 resamplings) in support of each node. Bootstrap percentages below 70% were not included. Each insertion and deletion was treated as a single event in the analysis. Species abbreviations are indicated as in Fig. 1. The designation OR, het-cOR allelic-type; PA, het-cPA allelic-type; and GR, het-cGR allelic-type is based on alignment in Fig. 1.
Although strains from the different species/genera are included in each het-c lineage (OR, PA, or GR-type), the tips of lineages often show the same or closely related species. These data indicate that a number of closely related alleles with similar het-c polymorphisms are present within a single species, which one would expect if these alleles have been retained in populations and if gene flow among species is rare. However, representatives of a single species within a single allelic type do not form discrete groupings, suggesting that selection also may be operating to maintain diversity even within het-c allelic types.
In both the MHC and the S locus, trans-species polymorphisms have been retained during multiple speciation events. The allele polymorphisms at the DRB1 locus have been calculated to have been retained for >30 million years (1), whereas the S locus polymorphisms have been retained for at least 36 million years (3). To determine how long het-c polymorphisms have been retained in species in the Sordariaceae, we calculated the divergence time between Neurospora and Sordaria. The molecular clock for the 18S rRNA sequences has been calibrated by using fossil evidence and ages of fungal hosts and symbionts to estimate the rate of substitution (29). The DNA sequence of the 18S rRNA gene from Sordaria fimicola (X69851) and N. crassa (29) show a 1.46% difference, indicating a 0.73% substitution per lineage. Assuming a reasonably high substitution rate of 2% per 100 million years (29), Sordaria diverged from Neurospora at least 36 million years ago. The persistence of het-c polymorphisms in different genera during such a long time period indicate that the allelic variants have been maintained by selection in populations across multiple speciation events.
HET-C Shows Constrained and Unconstrained Regions.
Pairwise sequence comparisons of different S- or MHC-encoded products show that the level of amino acid divergence is highly constrained in certain regions of the proteins, whereas other regions appear not only to be unconstrained, but under selection for diversity. This aspect is evidenced by an excess of nonsynonymous over synonymous substitutions in the hypervariable regions conferring specificity, consistent with a diversifying selection thought to operate at these loci (4, 30, 31). A frequent occurrence of nonsynonymous substitutions also was observed among alleles of the het-s (32) and among alleles at het-c (33) loci in P. anserina. Among the 40 HET-C sequences in this study, the amino acids in the region that flanked the specificity region were 100% identical, whereas those in the specificity region were variable (Fig. 1). A comparison of synonymous to nonsynonymous substitution ratios in the het-c specificity domain among the 40 isolates also showed variability. The frequency of nonsynonymous substitutions significantly increased both within and around the insertion/deletion motif of the het-c specificity region (Fig. 3).
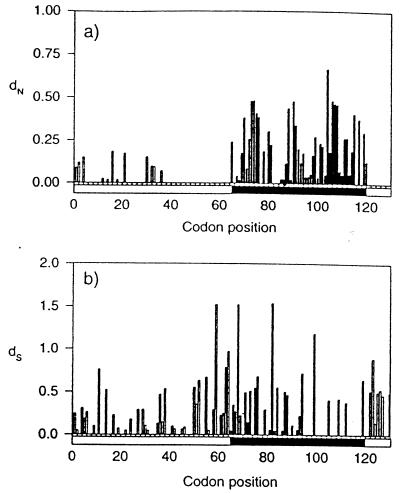
Figure 3.
Histograms of dN and dS substitutions per site mapped onto the codons for both the polymorphic and conserved regions in het-c from the 40 isolates. Sites 40–129 correspond to the sequences aligned in Fig. 1. Filled bar sites (codons 65–119) are polymorphic and correspond to the underlined region in Fig. 1. (a) The average number of nonsynonymous substitutions per nonsynonymous site (dN) grouped by codons, and (b) the average number of synonymous substitutions per synonymous site (dS) grouped by codons. Calculations were done by mega according to the Cantor–Jukes correction (25).
The numbers of nucleotide substitutions per synonymous (dS) site and per nonsynonymous (dN) site (26) were estimated among all 40 het-c alleles (Table 1). For the region flanking the specificity domain (non-specificity region), the mean dS significantly exceeded the mean dN (dN/dS = 0.11 for all alleles; z test P < 0.05). However, the value of dN increased significantly within the 57 codons that make up the het-c specificity region (underlined in Fig. 1; dN/dS = 0.54 for all alleles; z test P < 0.05). The most variable alleles were within the het-cPA-type insertion, where dN/dS ratio was 1.3. The analysis of dN/dS of het-c indicated that the polymorphic alleles are derived from an evolutionarily highly conserved gene and suggest that most amino acid differences between the polypeptides are not tolerated. However, a high occurrence of nonsynonymous substitutions and a significant increase in nonsynonymous substitutions per site near and within the insertion/deletion motif resulted in amino acid diversity of het-c encoded products.
Table 1.
Comparison of dS and dN substitutions per 100 sites in het-c
| Alleles/species | n | Specificity region
|
Non-specificity region
|
||
|---|---|---|---|---|---|
| dS | dN | dS | dN | ||
| OR-type* | 17 | 25.5 (5.6) | 10.3 (1.7) | 19.2 (3.5) | 2.1 (0.6) |
| GR-type* | 8 | 16.2 (5.1) | 6.0 (1.5) | 14.0 (3.3) | 1.4 (0.6) |
| PA-type† | 15 | 16.3 (4.5) | 21.5 (2.9) | 13.8 (3.0) | 1.2 (0.4) |
| All† | 40 | 38.0 (7.1) | 20.4 (2.8) | 24.5 (3.5) | 2.6 (0.6) |
| N. crassa‡ | 15 | 23.9 (7.8) | 17.0 (3.3) | 20.5 (4.3) | 2.1 (0.7) |
| Sordaria† | 5 | 37.0 (8.9) | 20.3 (3.8) | 27.3 (5.5) | 1.5 (0.7) |
Specificity and non-specificity region refer to the codons of filled and clear bar regions in Fig. 3, respectively. n, the number of alleles surveyed. Standard errors are in parentheses. GR, OR, and PA-types refer to het-cGR, het-cOR, and het-cPA allelic specificity types, respectively. Test of hypothesis: dN/dS ratio has no significant difference in the two regions (26, 27). *P < 0.05,
P < 0.001, and
P < 0.01.
DISCUSSION
The S locus and the MHC systems are examples in which balancing selection has caused the retention of ancestrally polymorphic alleles, resulting in several common evolutionary features. First, each has a large number of alleles present within a single species. Second, these alleles differ at a number of nucleotide sites, often displaying large divergences in DNA sequence. Third, trans-species polymorphisms are apparent. Fourth, the ratio of nonsynonymous to synonymous substitutions within the specificity domain exceeds that outside of the specificity domain. The ratio of nonsynonymous to synonymous mutations (dN/dS) within the hypervariable domain (peptide-binding region) of the DRB1 alleles can be as high as 10 for within species (salmon species; ref. 34), whereas a 40-amino acid variable domain of S alleles in the Solanaceae has a dN/dS ratio that is closer to 1 (31). In this study, we examined the evolutionary features of the het-c specificity region in different filamentous fungal species in the Sordariaceae. We show that the het-c region conferring specificity displays large divergences in DNA sequence, that DNA polymorphisms present in the het-c specificity region exhibit trans-species polymorphisms, and that this region has an increased frequency of nonsynonymous substitutions. The het-c locus thus shares with loci in the MHC and the S locus particular evolutionary features that are unexpected under the neutral model of evolution but that are predicted under balancing selection. Although the het-c locus shares these features with loci in the MHC and S locus, only three het-c allelic specificities have been identified in N. crassa, which are characterized by particular insertion/deletion motifs (19, 20). In N. crassa, vegetative incompatibility can be mediated by any of 11 different loci (17). If even a portion of these loci are relevant to self/nonself-recognition in N. crassa, the number of incompatible individuals in a segregating population would be extremely large. In a population analysis of N. crassa, heterokaryon compatible individuals were not identified, indicating that the population was highly heteromorphic for het loci (35). It is possible that the generation of additional alleles at het-c is constrained by requirements for protein function and that multiple alleles at het-c are not necessary because of multiplicity of het loci found within a species.
Retention of ancestral polymorphisms in neutral genes can be expected if species divergence times have been short and the population size is large. As judged by comparison of 18S rRNA sequences, Sordaria and Neurospora diverged over 36 million years ago. Theoretical analyses indicate that alleles at a locus under balancing selection will have long residence times and expected coalescence times far exceeding those of neutral alleles (1). Based on the data in this study, het-c polymorphisms must have originated in a species ancestral to Sordaria and Neurospora over 36 million years ago and have been retained during subsequent multiple speciation events. We believe that the wide occurrence of het-c trans-species polymorphisms within the Sordariaceae and the approximately equal frequency of each allelic type within a N. crassa population sample make it extremely unlikely that het-c diversity has been maintained under neutrality.
An alternative explanation for the occurrence of trans-species polymorphisms in het-c is that horizontal transfer occurs among species in the Sordariaceae. DNA sequences from a number of other genes and RFLP analysis support the hypothesis that strains assigned to N. sitophila, N. tetrasperma, and N. discreta form respective monophyletic groups within the genus Neurospora (36). It also is possible that the maintenance of het-c polymorphism results from “hitchhiking” after selection on a tightly linked locus. This hypothesis is not supported, however, because it predicts that a high ratio of dN/dS would be present throughout het-c and not just within the specificity region.
In P. anserina, an excess of nonsynonymous substitutions was observed between two het-s alleles (involved in allelic incompatibility; ref. 32) and between four het-c alleles (involved in non-allelic incompatibility; ref. 33). In this study, we observed a significant increase in the frequency of nonsynonymous substitutions within the het-c specificity region of the 40 alleles as compared with regions flanking the specificity domain. The fact that an excess of nonsynonymous substitutions has been observed in alleles from three different het loci suggests that selection to maintain diversity is a common evolutionary feature of these loci. The het-c diversity and presence of lineages within the OR, PA, and GR groups in our phylogenetic analysis also suggest that some of the het-c variants observed in other species and genera within the Sordariaceae may confer het-c specificities other than those identified in N. crassa. It will be of interest to determine whether het-c functions as a vegetative incompatibility locus in other species and whether balancing selection operates to maintain diversity of allelic-types at other het loci in filamentous fungi.
Allelic polymorphisms can be maintained through speciation events either by over-dominance in a diploid organism (heterozygote superiority) or by frequency dependent selection (6, 7); controversy surrounds the selection pressure for maintenance of MHC diversity (37, 38). If het-c allelic differences are expressed only in the haploid vegetative phase, then heterozygote superiority cannot be the driving force for maintenance of het-c polymorphisms, and the maintenance of allelic diversity would be attributed to frequency-dependent selection. However, until het-c is shown to be inactive in the sexual diplophase or dikaryophase, overdominance cannot be ruled out. Our analysis of the het-c specificity domain in the genera of the Sordariaceae supports a role for this locus as a self/nonself-recognition system limiting heterokaryon formation between unlike individuals during vegetative growth. These data shed light not only on the biological role of vegetative incompatibility in filamentous fungi but also reinforce the view that balancing selection is an evolutionary mechanism common to different self/nonself-recognition systems.
Acknowledgments
We thank Drs. Daniel Haydon, Sally Otto, and Mary Berbee for assistance with data analysis. We also thank Drs. Sally Otto, Daniel Haydon, Tony Griffiths, Mary Berbee, Don Natvig, and Richard Todd for critical reading of the manuscript. This work is supported by a Natural Science and Engineering Research Council of Canada (NSERC) grant.
ABBREVIATIONS
- MHC
major histocompatibility complex
- RFLP
restriction fragment length polymorphism
- dN
nonsynonymous
- dS
synonymous
- FGSC
Fungal Genetics Stock Center
- PA
Panama
- GR
Groveland
- OR
Oakridge
Footnotes
References
- 1. Ayala F J, Escalante A, O’hUigin C, Klein J. Proc Natl Acad Sci USA. 1994;91:6787–6794. doi: 10.1073/pnas.91.15.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Charlesworth D. BioEssays. 1995;17:31–38. [Google Scholar]
- 3.Ioerger T R, Clark A G, Kao T. Proc Natl Acad Sci USA. 1990;87:9732–9735. doi: 10.1073/pnas.87.24.9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hinata K, Watanabe M, Yamakawa S, Satta Y, Isogai A. Genetics. 1995;140:1099–1104. doi: 10.1093/genetics/140.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein J. Hum Immunol. 1987;19:155–162. doi: 10.1016/0198-8859(87)90066-8. [DOI] [PubMed] [Google Scholar]
- 6.Takahata N, Satta Y, Klein J. Genetics. 1992;130:925–938. doi: 10.1093/genetics/130.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahata N, Nei M. Genetics. 1990;124:967–978. doi: 10.1093/genetics/124.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glass N L, Kuldau G A. Annu Rev Phytopathol. 1992;30:201–224. doi: 10.1146/annurev.py.30.090192.001221. [DOI] [PubMed] [Google Scholar]
- 9.Leslie J F. Annu Rev Phytopathol. 1993;31:127–150. doi: 10.1146/annurev.py.31.090193.001015. [DOI] [PubMed] [Google Scholar]
- 10.Worrall J J. Mycologia. 1997;89:24–36. [Google Scholar]
- 11.Esser K, Blaich R. In: The Mycota I: Growth, Differentiation and Sexuality. Wessels J G H, Meinhardt F, editors. Heidelberg, Germany: Springer; 1994. pp. 211–232. [Google Scholar]
- 12.Garnjobst L, Wilson J F. Proc Natl Acad Sci USA. 1956;42:613–618. doi: 10.1073/pnas.42.9.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Begueret J, Bernet J. Nat New Biol. 1973;243:94–96. [PubMed] [Google Scholar]
- 14.Caten C E. J Gen Microbiol. 1972;72:221–229. doi: 10.1099/00221287-72-2-221. [DOI] [PubMed] [Google Scholar]
- 15.Begueret J, Turcq B, Clave C. Trends Genet. 1994;10:441–446. doi: 10.1016/0168-9525(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 16.Saupe S J, Kuldau G A, Smith M L, Glass N L. Genetics. 1996;143:1589–1600. doi: 10.1093/genetics/143.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perkins D D, Radford A, Newmeyer D, Bjorkman M. Microbiol Rev. 1982;46:426–570. doi: 10.1128/mr.46.4.426-570.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garnjobst L. Am J Bot. 1953;40:607–614. [Google Scholar]
- 19.Howlett B J, Leslie J F, Perkins D D. Fungal Genet Newsletter. 1993;40:40–42. [Google Scholar]
- 20.Saupe S J, Glass N L. Genetics. 1997;146:1299–1309. doi: 10.1093/genetics/146.4.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcinko-Kuehn M, Yang X, Debets F, Jacobson D J, Griffiths A J F. Curr Gen. 1994;26:336–343. doi: 10.1007/BF00310498. [DOI] [PubMed] [Google Scholar]
- 22.Vogel H J. Am Nat. 1964;98:435–446. [Google Scholar]
- 23.Oakley C E, Weil P L, Dretz P L, Oakley B R. Gene. 1987;53:293–298. doi: 10.1016/0378-1119(87)90019-9. [DOI] [PubMed] [Google Scholar]
- 24.Felsenstein J. phylip. University of Washington, WA: Department of Genetics; 1993. , Version 3.5. [Google Scholar]
- 25.Kumar S, Tamura M, Nei M. Molecular Evolutionary Genetics Analysis. University Park, PA: Pennsylvania State Univ. Press; 1993. [Google Scholar]
- 26.Kotz S, Johnson N L. The Encyclopedia of Statistical Sciences. Vol. 8. New York: Wiley; 1989. p. 646. [Google Scholar]
- 27.Haydon D, Lea S, Fry L, Knowles N, Samuel A R, Stuart D, Woolhouse M E J. J Mol Evol. 1998;46:465–475. doi: 10.1007/pl00006327. [DOI] [PubMed] [Google Scholar]
- 28.Yuhki N, O’Brien S J. J Mol Evol. 1994;39:22–33. doi: 10.1007/BF00178246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berbee M L, Taylor J W. Can J Bot. 1993;71:1114–1127. [Google Scholar]
- 30.Hughes A L, Nei M. Proc Natl Acad Sci USA. 1989;86:958–962. doi: 10.1073/pnas.86.3.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark A G, Kao T. Proc Natl Acad Sci USA. 1991;88:9823–9827. doi: 10.1073/pnas.88.21.9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turcq B, Deleu C, Denayrolles M, Begueret J. Mol Gen Genet. 1991;228:265–269. doi: 10.1007/BF00282475. [DOI] [PubMed] [Google Scholar]
- 33.Saupe S, Turcq B, Begueret J. Curr Genet. 1995;27:466–471. doi: 10.1007/BF00311217. [DOI] [PubMed] [Google Scholar]
- 34.Miller K M, Withler R E. Immunogenetics. 1996;43:337–351. doi: 10.1007/BF02199802. [DOI] [PubMed] [Google Scholar]
- 35.Mylyk O M. Genetics. 1976;83:275–284. doi: 10.1093/genetics/83.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skupski M P, Jackson D A, Natvig D O. Fungal Gen Biol. 1997;21:153–162. [PubMed] [Google Scholar]
- 37.Slade R W, McCallum H I. Genetics. 1992;132:861–862. doi: 10.1093/genetics/132.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hughes A L, Nei M. Nature (London) 1992;355:402–403. doi: 10.1038/355402b0. [DOI] [PubMed] [Google Scholar]