Abstract
Drugs that induce human immunodeficiency virus type 1 (HIV-1) replication could be used in combination with highly active antiretroviral therapy (HAART) to reduce the size of the latent reservoir that is in part responsible for viral persistence. Protein kinase C (PKC) is a logical target for such drugs because it activates HIV-1 transcription through multiple mechanisms. Here we show that HIV-1 gene expression can be induced by potent synthetic analogues of the lipid second messenger diacylglycerol (DAG) synthesized on a five-member ring platform that reduces the entropy of binding relative to that of the more flexible DAG template. By varying the alkyl side chains of these synthetic DAG lactones, it was possible to maximize their potency and ability to render latently infected T cells sensitive to killing by an anti-HIV-1 immunotoxin while minimizing the side effects of CD4 and CXCR4 downregulation and tumor necrosis factor alpha upregulation. The two lead compounds, LMC03 and LMC07, regulated a series of PKC-sensitive genes involved in T-cell activation and induced viral gene expression in peripheral blood mononuclear cells from HIV-1-infected individuals. These studies demonstrate the potential for the rational design of agents that, in conjunction with HAART and HIV-specific toxins, can be used to decrease or eliminate the pool of latently infected reservoirs by forcing viral expression.
Until recently, pharmacological studies of human immunodeficiency virus type 1 (HIV-1) transcription were dominated by the search for agents that would inhibit viral replication. With the availability of highly active antiretroviral therapies (HAART) directed against viral reverse transcriptase and protease, however, interest has switched to the identification of agents that activate rather than repress viral gene expression. Such agents, in combination with HAART, could be used to reduce the size of the reservoir of latently infected cells by causing them to be directly killed by the cytopathic action of the virus, to be recognized and destroyed by the immune system, or to express proteins that render them susceptible to targeted therapeutics such as immunotoxins (6, 25).
The expression of the HIV-1 genome is regulated by two viral factors, Rev and Tat, as well as by multiple cellular factors whose concentrations and activities are linked to the differentiation and replication status of the host cell. HIV-1-infected CD4+ T cells that have been activated through the T-cell receptor by an antigen or mitogen transcribe the viral genome at a high rate, turn over with a short half-life, and are responsible for most of the virus production in infected individuals. By contrast, HIV-1-infected quiescent memory CD4+ T cells produce little or no viral mRNA or proteins, can survive for prolonged periods, and appear to be responsible for the long-term persistence of HIV-1 even in individuals in whom circulating virus has been reduced to clinically undetectable levels by HAART (2, 8, 16, 33, 37).
Protein kinase C (PKC) is a logical target for HIV-1-inducing drugs because it regulates HIV-1 transcription through multiple mechanisms. First, PKC activates the transcription factor NF-κB, which binds to multiple sites in the enhancer region of the HIV-1 long terminal repeat (LTR), through a phosphorylation cascade that involves the IκB inhibitory factor and IKK kinase (29). Second, PKC activates the AP-1 (c-Jun) transcription factor, which binds both to the HIV-1 enhancer and to downstream sequence elements, through JNK and mitogen-activated protein kinase signaling pathways (18, 34, 41). Finally, PKC has been reported to phosphorylate the virally encoded Tat transcription factor and cellular TAR-binding factors (14, 17).
Physiologically, PKC is activated by antigens, mitogens, and other extracellular ligands through the production of the lipid second messenger 1,2-diacylglycerol (DAG), which binds to the C1 regulatory domain of PKC, thereby exposing the catalytic domain by displacing a negative regulatory pseudosubstrate region and inducing translocation to cellular membranes. PKC comprises at least 12 different serine/threonine kinase isozymes with different cellular distributions, responses to inducers, and substrate preferences. DAG activates the calcium-dependent isozymes α, βI, βII, and γ and the novel calcium-independent isozymes δ, ε, η, and θ. The θ isotype is selectively expressed in T cells and plays a critical role in T-cell activation, proliferation, and cytokine production through NF-κB- and AP-1-mediated signaling pathways (1, 19, 22).
Consistent with its role as a second messenger involved in cellular homeostasis, DAG binds weakly to PKC and is metabolically unstable. More potent and long-lasting activation of PKC can be achieved by a number of naturally occurring compounds such as phorbol esters, teleocidins, and macrocyclic lactones such as the bryostatins and aplysiatoxins. Unfortunately, however, most of these natural products have undesirable side effects such as local irritation, tumor promotion, platelet aggregation, and release of inflammatory cytokines that make them problematic as potential human therapeutics. In addition, they are not easily accessed synthetically because of their complex structures.
The use of pharmacophore- and receptor-guided approaches to designing and synthesizing DAG analogues that function as high-affinity ligands for the C1 domain of PKC has resulted in a number of compounds that are structurally simpler and synthetically more readily accessible than the phorbol esters (27). DAG lactones, in which the entropic penalty for ligand binding is reduced by constraining the glycerol backbone into a five-member lactone ring, have proven to be especially potent and selective as antitumor agents (27, 30, 36). Recent studies have correlated various cellular effects of these DAG lactones, such as induction of apoptosis, to the specific activation of PKC isotypes and their translocation to different cellular compartments (13).
Although the chemical properties of synthetic DAG analogues have been studied extensively, their biological activities are just beginning to be understood. In the present work, we tested the activity of a series of DAG lactones on HIV-1 expression both in a latently infected cell line in vitro and in peripheral blood mononuclear cells (PBMC) from HIV-1-infected humans ex vivo. We also explored the effects of these compounds on cell surface receptor expression, inflammatory cytokine secretion, and gene expression, as well as their ability to render latently infected cells sensitive to killing by an immunotoxin. Our results provide proof in principle for the rational design of HIV-1-inducing agents with desirable pharmacological characteristics.
MATERIALS AND METHODS
Compounds and cell lines.
DAG lactones were synthesized and purified as described previously (30, 36). 12-Deoxyphorbol 13-phenylacetate (DPP) was purchased from LC Laboratories (Woburn, Mass.). The anti-HIV-1 envelope immunotoxin 3B3:N31H/Q100eY(dsFv)-PE was produced in and purified from bacteria (28). The latently HIV-1 infected T-cell line ACH-2 and the parental line A3.01 were obtained from the National Institutes of Health (NIH) AIDS Research and Reagent Reference Program and maintained in RPMI 1640 medium containing 2 mM glutamine, 10% fetal bovine serum, and antibiotics.
Assays.
Antigen capture enzyme-linked immunosorbent assay (ELISA) kits were used to measure HIV-1 p24 antigen (ZeptoMetrix) and tumor necrosis factor alpha (TNF-α) (R&D Systems). Flow cytometry (FACScalibur; Becton Dickinson) was used to assay cell surface CD4 expression with fluorescein isothiocyanate-conjugated antibody SK3 (BD Biosciences), CXCR4 expression with allophycocyanin-conjugated antibody 12G5 (BD Biosciences), and annexin V binding with the Annexin V Apoptosis Detection kit (Oncogene Research Products). Cell viability was measured by the 3-(4,5-dimethylthiazole-2-yl)-2,4-diphenyltetrazolium bromide (MTT) oxidation procedure with Cell Proliferation Kit I (Roche Diagnostics). HIV-1 RNA was measured with the ultrasensitive Amplicor HIV-1 Monitor Test kit (Roche Diagnostics).
Microarray analysis.
Total RNA was extracted from A3.01 cells grown for 24 h either with no inducer or with 100 nM DPP, LMC01, LMC03, or LMC07 by using TriZOL (Gibco/BRL). The RNA was reverse transcribed by using SuperScript II (Gibco/BRL) in the presence of Cy3- or Cy5-labeled dUTP and was hybridized to microarray slides (Advanced Technology Center, National Cancer Institute [NCI]) spotted with 9,984 clones from the UniGEM2 collection (Incyte Genomics) as described previously (10). Slides were scanned in a GenePix 4000A scanner (Axon Instruments), and the Cy3 and Cy5 signal intensities were normalized for each sample by using NCI mAdb database software (http://madb.nci.nih.gov). Data filters based on signal intensity and spot quality were used to exclude less reliable spots or insufficiently expressed genes. Repeated measurements showed that the data collected in this fashion were highly reproducible; for three experiments using DPP as the inducer, the reliability coefficient was 0.94 and the standardized item α was 0.96 (chi-square = 12.5; df = 2; P = 0.0019).
Patients and PBMC culture.
PBMC were obtained from three HIV-1-positive individuals according to protocols approved by NIH institutional review boards. Patient 1 was treated with lamivudine, abacavir, and lopinavir-ritonavir for 18 months and had a viral load of <50 copies/ml and a CD4 count of 780 cells/μl. Patient 2 was a treatment-naïve long-term nonprogressor with a viral load of 530 copies/ml and a CD4 count of 409 cells/μl. Patient 3 was treatment naïve and had a viral load of 30,000 copies/ml and a CD4 count of 530 cells/μl. The PBMC from patients 1 and 2 were depleted of CD8+ T cells by using anti-CD8-coated magnetic beads (Dynal Biotech, Oslo, Norway). Resting CD4+ T cells were purified from patient 3 by incubation with antibodies against CD8, CD14, CD16, CD19, CD41, CD25, HLA-DR, and glycoporin, followed by magnetic column separation (Stem Cell Technologies, Vancouver, Canada) (6, 7). The cells were cultured in RPMI 1640 medium containing 2 mM glutamine, 10% fetal bovine serum, antibiotics, and 5 U of interleukin-2/ml, with changes of the medium twice per week. HIV-1 production was determined by p24 ELISA or RNA measurements on the cell supernatant.
RESULTS
Design and synthesis of DAG lactones.
Structure-activity studies have shown that the potency of DAG analogues can be improved by two types of chemical modification: cyclization of the glycerol backbone to restrict conformational flexibility, thereby decreasing the entropy of ligand binding, and addition of lipophilic side chains, which optimizes membrane binding and interactions with conserved hydrophobic amino acids along the rim of the C1 domain of PKC (27). As diagramed in Fig. 1, the DAG lactones used in this study were generated by intramolecular folding of DAG at sn-2, which maintains a single asymmetric center, and attachment of various carbon side chains to the carbonyl group for acyl branching (R1) or to the lactone via a methylene group for E or Z α-alkylidene branching (R2). Table 1 shows the structures, calculated partition coefficients (log P), and PKCα binding affinities for the different DAG analogues. PKCα binding affinities are expressed as Ki values, which reflect the abilities of the compounds to displace bound [20-3H]phorbol-12,13-dibutyrate from the receptor (30, 36). For comparison, a noncyclic DAG analogue (LMC01) was included to highlight the importance of restricting the conformation of the glycerol backbone in improving potency (31, 36). DPP, a potent phorbol ester originally isolated from Euphorbia poissonii (11, 15), was used as a positive control for PKC activation and HIV-1 induction (4).
FIG. 1.
Synthesis scheme for DAG lactones. For details, see references 27, 30, and 36.
TABLE 1.
Structures, biochemical properties, and biological activities of DAG lactones
Calc., calculated.
Expressed as the mean Ki (nanomolar concentrations), with the standard error in parentheses, as determined by the ability to displace bound [20-3H]phorbol-12,13-dibutyrate from PKCα.
Mean (standard error) from two to four determinations similar to those for which results are shown in Fig. 2.
See reference 32.
See reference 36.
See reference 26.
See reference 31.
See reference 21.
Induction of viral expression in a latently HIV-1 infected T-cell line.
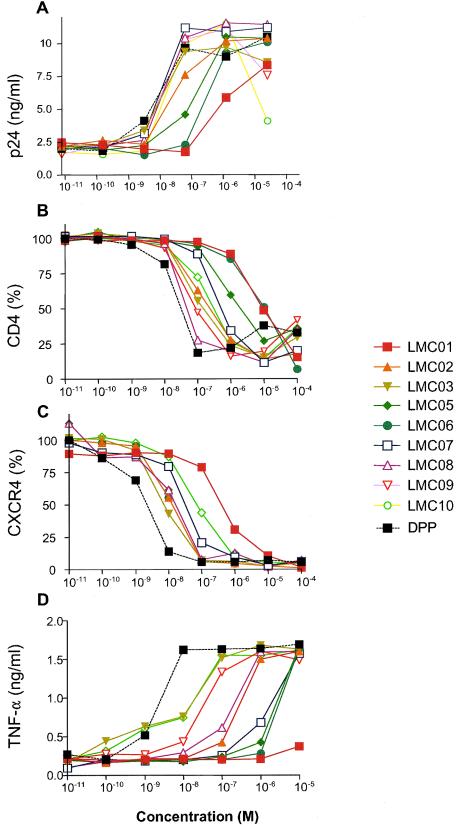
The ability of the DAG analogues to induce HIV-1 expression was initially examined in ACH-2 cells, a latently infected T-cell line that contains a single integrated copy of the viral genome (9, 12). The cells were incubated for 48 h with various concentrations of the synthetic compounds or DPP, then tested for production of p24 antigen in the culture supernatant. The dose-response curves are shown in Fig. 2A, and the IC50 (the drug concentrations required for half-maximal induction) are summarized in Table 1.
FIG. 2.
Biological activities of DAG compounds compared to that of DPP. (A) ACH-2 cells were grown in the presence of various concentrations of the indicated compounds for 48 h, and p24 antigen production was measured by ELISA. (B) ACH-2 cells were grown in the presence of various concentrations of the indicated compounds for 16 h, and cell surface CD4 expression was measured by antibody staining and fluorescence-activated cell sorting; results were normalized to 100% for no added compound. (C) ACH-2 cells were grown in the presence of various concentrations of the indicated compounds for 16 h, and cell surface CXCR4 expression was measured by antibody staining and fluorescence-activated cell sorting; results were normalized to 100% for no added compound. (D) PBMC were grown in the presence of various concentrations of the indicated compounds for 18 h, and TNF-α secretion was measured by ELISA.
All of the DAG lactones induced viral gene expression to final levels similar to those induced by DPP, but their potencies varied by more than 2 orders of magnitude. The order of efficacy, from lowest to highest, was as follows: LMC01, LMC06, LMC05, LMC02, LMC08, LMC10, LMC07, DPP, LMC09, LMC03. With the exception of the weakest DAG analogue (LMC01), there is not a strict correlation between the potencies displayed by the DAG lactones (LMC02 to LMC10) in this assay and their in vitro PKCα binding affinities. However, within each E/Z isomeric pair of DAG lactones, the Z isomer was found to be more potent than its corresponding E isomer (LMC05 was more potent than LMC06, LMC07 was more potent than LMC08, and LMC09 was more potent than LMC10).
Effects on cellular receptor and cytokine expression.
Phosphorylation by PKC results in internalization and decreased cell surface expression of various receptor molecules including CD4, the primary receptor for HIV-1, and CXCR4, a T-cell coreceptor. To determine the activities of the DAG analogues in the downregulation of these proteins, A3.01 T cells (the parental line for ACH-2) were incubated with various concentrations of the synthetic compounds or DPP for 16 h, then analyzed for cell surface CD4 and CXCR4 expression by flow cytometry. As shown in Fig. 2B and C and summarized in Table 1, the DAG lactones downregulated CD4 and CXCR4 to the same extent as DPP, but again with strikingly varied potencies. The order of efficacy for CD4 downregulation, from lowest to highest, was as follows: LMC06, LMC01, LMC05, LMC07, LMC10, LMC02, LMC03, LMC09, LMC08, DPP. For CXCR4 downregulation, the order of efficacy, from lowest to highest, was as follows: LMC01, LMC06, LMC05, LMC07, LMC08, LMC02, LMC10, LMC03, LMC09, DPP. CD4 and CXCR4 downregulation was also observed in PBMC cultures (data not shown).
PKC also plays an important role in the secretion of inflammatory cytokines such as TNF-α from T cells. Because the A3.01 cell line does not synthesize appreciable quantities of TNF-α, the abilities of the DAG lactones to stimulate this activity were assessed in PBMC cultures. As shown in Fig. 2D and summarized in Table 1, all of the cyclic DAGs induced TNF-α production to the same final extent as DPP, whereas the linear compound LMC01 was relatively ineffective in this assay. The order of potency for the DAG lactones, from lowest to highest, was as follows: LMC05, LMC06, LMC07, LMC02, LMC08, LMC09, LMC03, LMC10, DPP.
DAG lactones render cells sensitive to an immunotoxin.
For clinical use, it would be advantageous to combine agents that induce HIV-1 gene expression with those that specifically kill cells expressing HIV-1 proteins. Recently we described an immunotoxin termed 3B3:N31H/Q100eY(dsFv)-PE in which the translocation and cytotoxic domains of Pseudomonas exotoxin A are fused to the variable region of a genetically engineered antibody that tightly binds to the conserved CD4-binding site of the HIV-1 envelope glycoprotein gp120 (28). This molecule specifically recognizes and kills cells that express gp120 at the cell surface.
To test the ability of combined inducer and immunotoxin treatment to eliminate latently infected cells, ACH-2 cells were cultured with 100 nM each DAG compound or DPP in the presence or absence of 3B3:N31H/Q100eY(dsFv)-PE. After 4 days, cell viability was measured by the MTT oxidation method, and apoptosis was estimated by annexin V binding. Figure 3 shows that the immunotoxin by itself was not toxic but that it caused high levels of apoptosis and cell death when combined with LMC02, LMC03, LMC07, LMC08, LMC09, LMC10, or DPP. There was also some cell killing by LMC08, LMC09, and LMC10 by themselves, indicating a certain level of toxicity for these compounds at a 100 nM concentration. LMC01, LMC05, and LMC06 failed to promote immunotoxin-dependent cell death, presumably because these compounds cause little HIV-1 induction at a concentration of 100 nM (Fig. 1A). The best ratios of specific to nonspecific cell killing were observed for LMC03 (30-fold) and LMC07 (45-fold). Therefore, we focused on these compounds for the microarray analysis and ex vivo experiments described below.
FIG. 3.
Induction of immunotoxin sensitivity. ACH-2 cells were grown with no inducer or with 100 nM concentrations of the indicated compounds in the presence or absence of 5 nM 3B3:N31H/Q100eY(dsFv)-PE for 5 days. (A) Cell viability was assayed by the MTT oxidation procedure, and the percentage of cell killing was calculated relative to that with untreated cells. (B) Annexin V binding was assayed by fluorescence-activated cell sorting.
Gene expression analysis.
Microarray analysis was used to determine the spectrum of genes regulated by the lead DAG lactones LMC03 and LMC07 compared to the relatively inactive linear compound LMC01 and the potent PKC activator DPP. RNA was prepared from A3.01 cells incubated for 24 h with 100 nM each compound, or with no inducer as a control, copied into cDNA, and hybridized to a cDNA microarray. The expression ratio for each induced sample compared to the untreated sample was calculated for each of the approximately 7,400 clones that were clearly expressed in the A3.01 cells.
Correlation analysis of the resulting gene expression matrices showed that LMC03 and LMC07 induce and repress very similar sets of genes to one another and to DPP; the Pearson correlations were 0.92 for LMC03 versus LMC07, 0.88 for LMC03 versus DPP, and 0.92 for LMC07 versus DPP. By contrast, LMC01 had little effect on gene regulation, generating almost the same pattern as untreated cells; the Pearson correlations were <0.2 for LMC01 versus LMC03, LMC07, and DPP.
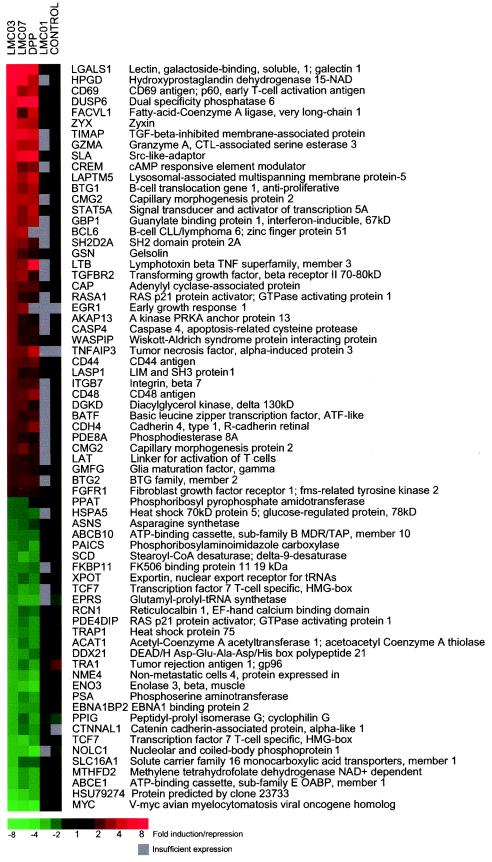
Figure 4 shows the expression data for genes that were induced or repressed at least twofold by both LMC03 and LMC07. The functions of these genes are described in the Discussion, and the complete locus information can be found at http://source.stanford.edu and http://www.ncbi.nlm.nih.gov/omim/.
FIG. 4.
Microarray analysis. cDNA prepared from A3.01 cells grown for 24 h with 100 nM LMC01, DPP, LMC03, or LMC07 was labeled with Cy3 (red). Each sample was mixed with Cy5 (green)-labeled cDNA from uninduced cells and hybridized to a microarray. The control lane contains Cy3-labeled cDNA from uninduced cells. Shown are those genes that were induced or repressed at least twofold by LMC03 and LMC07.
Induction of viral replication in PBMC from HIV-1-infected individuals.
The ability of the DAG lactones to activate expression of the primary virus present in HIV-1-infected individuals was tested by ex vivo culture experiments. Total CD8-depleted PBMC or highly purified resting CD4+ T cells were isolated from infected individuals with undetectable, intermediate, or high viral loads and grown in the presence of no inducer as a negative control, an anti-CD3 antibody or DPP as a positive control, or various DAG lactones as the test agents. The extent of viral replication was determined by p24 assays of the cell supernatants and in some cases was confirmed by RNA assays.
Figure 5 shows that for each of the three HIV-1-infected individuals tested, PBMC grown without any inducer produced no detectable p24, indicating functional latency, whereas growth of PBMC with LMC03 or LMC07 clearly activated viral expression. Furthermore, in each case, LMC03 and LMC07 gave yields of p24 similar (within fourfold) to those obtained with anti-CD3 or DPP, indicating a similar efficiency of viral induction. The DAG lactones other than LMC03 and LMC07 failed to activate viral expression in patient 1, probably because the 100 nM concentration used in these experiments was too low for efficient induction (Fig. 2) or caused nonspecific cell death (Fig. 3.) Virus was rescued both from total CD8+-depleted PBMC (patients 1 and 2) and highly purified resting CD4+ T cells (patient 3). The p24 data for patients 1 and 3 were confirmed by viral RNA assays of the culture supernatants. Furthermore, sequencing of the C2-V3 region of the envelope gene from the RNA samples isolated from patient 1 showed that anti-CD3 and LMC03 induced indistinguishable viral quasispecies (data not shown).
FIG. 5.
Induction of HIV-1 ex vivo. CD8-depleted (patients 1 and 2) or highly purified CD4+ T cells from HIV-1-infected individuals were cultured for 3 weeks with no inducer, anti-CD3 (1:4,000), DPP (100 nM), or DAG lactones (100 nM). HIV-1 p24 levels in the cell-free culture fluid were measured by ELISA; the detection limit of the assay was 50 pg/ml.
DISCUSSION
The existence of HAART-persistent reservoirs of HIV-1 has led to increasing interest in agents that can activate the replication of latent provirus, a strategy referred to as stimulation or immune activation therapy (2, 6, 25). The ideal agent would be completely specific for HIV-1 and have no effects on cellular gene expression. Unfortunately, such a molecule is not yet known and will probably be difficult to develop given the extensive dependence of HIV-1 on cellular transcription factors. The alternative is to find drugs that activate latent HIV-1 through cellular factors but have sufficiently few side effects to allow at least periodic clinical use, possibly in combination with agents that eliminate HIV-1-infected cells.
The present study demonstrates the feasibility of rationally designing such agents. Our efforts focused on a series of synthetic analogues of DAG, which activates HIV-1 transcription through PKC, an enzyme increasingly regarded as an important therapeutic target for cancer and other diseases because of its central role in cellular signal transduction. By conformationally restricting the DAG backbone into a lactone ring, it was possible to increase affinity for PKC while reducing some of the typical side effects of potent PKC activators such as the phorbol esters by systematically altering their side-chains.
Of particular interest are DAG-lactones LMC03 and LMC07. Both of these compounds induced HIV-1 replication in a latently infected T-cell line at the same low nanomolar concentration as DPP, a potent deoxyphorbol ester, thereby rendering the cells susceptible to killing by an anti-HIV-1 immunotoxin. In addition, both compounds activated HIV-1 replication in white blood cells isolated from infected patients, demonstrating their ability to act on viral gene expression ex vivo at concentrations that allow cell survival. Preliminary experiments indicate that LMC03 and LMC07, like the deoxyphorbol ester prostratin (23, 24), induce the differentiation of mononuclear phagocytes but do not stimulate resting T cells to proliferate.
DAG lactones have several potentially useful features as therapeutics, including lack of immunogenicity and ease of synthesis. However, an important obstacle to clinical utilization of any PKC agonist is that it may have downstream effects in addition to HIV upregulation, including respiratory distress syndrome, hypotension, and other toxicities due to the release of cytokines following nonspecific T-cell activation (39). In this regard, it was encouraging that LMC03 and LMC07 were 10- to 80-fold less potent than DPP at upregulating TNF-α expression in PBMC. These compounds were also 6- to 30-fold less potent than DPP at downregulating the cell surface expression of CD4 and 3- to 16-fold less potent than DPP at decreasing CXCR4 expression in A3.01 cells. Thus, although it may be impossible to completely eliminate the ancillary toxicities of PKC activators, it may be feasible to minimize them to a clinically acceptable level.
The other DAG compounds we tested had less desirable activity and pharmacological profiles. As expected from its structure and previous biochemical assays, the linear compound LMC01 had low potency in all assays. LMC02, LMC05, LMC06, and LMC08 were also relatively weak for HIV-1 induction, whereas LMC09 and LMC10 were more potent but had the drawback of nonspecific cell toxicity.
Microarray analysis was used to characterize the molecular action profiles of the lead compounds LMC03 and LMC07. As expected from the role of PKC in mediating signals from the T-cell receptor-CD28 complex to the transcriptional apparatus including NF-κB and AP-1, many of the regulated genes are involved in T-cell activation, growth control, signal transduction, and cytoskeleton reorganization.
LAT (linker for activation of T cells) is directly involved in the T-cell antigen receptor signal transduction pathway and is one of the most prominently tyrosine-phosphorylated proteins detected following T-cell receptor ligation. CD69 (p60) is a cell surface molecule involved in lymphocyte proliferation that is often employed as a marker of early T-cell activation. LGALS1 (galectin-1) encodes a conserved beta-galactoside-binding protein that regulates T-cell receptor signaling and apoptosis by ligation of glycoepitopes on T-cell activation markers. GZMA (granzyme 1), a cytotoxic T-cell- and natural killer cell-specific trypsin-like serine protease, is overexpressed during CD8 cell activation. TNFAIP3 (TNF-α-induced protein 3) is a cytoplasmic zinc finger protein that is upregulated in TNF-induced NF-κB responses.
EGR1 (early growth response 1) and DUSP6 (dual-specificity phosphatase 6) modulate cellular proliferation and differentiation through the mitogen-activated protein kinase-ERK pathway. Other genes involved in signal transduction include CREM (cAMP-responsive element modulator), RASA1 (GTPase-activating protein p21), and DGKD (diacylglycerol kinase). LAPTM5 (lysosomal-associated multispanning membrane protein-5), BTG1 (B-cell translocation gene 1), and BTG2 (BTG family member 2) are thought to have antiproliferative functions but have not previously been analyzed in T cells.
A number of the LMC03- and LMC07-activated genes are involved in cytoskeleton reorganization, which plays an important role in T-cell activation by assembling an immunological synapse of antigen receptors, coreceptors, and adhesion molecules and creating a scaffold for signaling molecules. ZYX (zyxin) binds to mitotic spindles and adhesion plaques and participates in a signal transduction pathway that mediates adhesion-stimulated changes in gene expression. LASP1 (LIM and SH3 protein 1) regulates actin-based cytoskeletal activities. CD44 (a component of the Indian blood group system) encodes the hyaluronan receptor, which binds to the cytoskeleton through ankyrin and plays a role in matrix adhesion, lymphocyte activation, and lymph node homing; both its binding activity and its expression level are regulated by PKC. ITGB7 (integrin beta-7) is a receptor for fibronectin that plays a role in adhesive interactions of leukocytes.
Several of the genes repressed by LMC03 and LMC07 play a negative role in T-cell differentiation. PPIG (petidyl-prolyl isomerase G) codes for one of a series of cyclophilins that, in concert with cyclosporins, act as immunosuppressants by inhibiting calcineurin; expression of the genes for peptidyl-prolyl isomerases B and F was also repressed by the DAG lactones, but less strongly. FKB11 (FK506 binding protein 11) is related to a series of immunophilins that inhibit T-cell activation through the calcineurin pathway when complexed with the immunosuppressive drug FK506; the related genes FKBP4 and FKBP5 were also somewhat downregulated by LMC03 and LMC07. Unexpectedly, expression of TCF7 (positive T-cell transcription factor 7) was consistently repressed by the DAG lactones and other activators of PKC including DPP, phorbol myristate acetate, and mezerein (data not shown). Since TCF7 acts on the promoter of the CD3-ɛ gene, this might represent a form of feedback regulation.
The biological reasons for the improved therapeutic ratios of LMC03 and LMC07 compared to the other DAG lactones and DPP are not yet clear, but there is accumulating evidence that different PKC activators can elicit various cellular responses and display unique pharmacological profiles. For example, it has been shown previously that the 12-deoxyphorbol esters DPP and prostratin induce HIV-1 replication and regulate numerous PKC-sensitive genes (4, 23, 24). Although these compounds activate PKC in vitro, they are not tumor promoters but rather are inhibitors of tumor promotion (38). Likewise, bryostatin 1 is a potent PKC activator in vitro but is a functional antagonist of the phorbol esters for many but not all phorbol ester-induced responses (3). Although in neither case is the basis for the distinct mechanism of action understood, it is clear that these different compounds cause distinct patterns of intracellular localization of PKC isoforms, in particular PKCδ (40). Since intracellular localization controls access to substrates, this altered pattern of localization provides one attractive mechanism that may account for the distinct patterns of biological response.
Recent biochemical studies have revealed that PKC isotype activation in cells is rather complex, involving phosphorylation and autophosphorylation events followed by specific translocation mediated by the binding of second messengers, such as DAG, or pharmacologically mediated by binding to phorbol esters. The results obtained with phorbol esters and, by extension, with the potent DAG lactones described here have to be examined carefully, because these compounds can activate many of the cPKC (classical) and nPKC (novel) isotypes. Further complicating this picture is the discovery of at least five alternative types of high-affinity DAG/phorbol ester receptors (5, 20). These receptors have in common at least one cysteine-rich or zinc finger domain of ca. 50 to 51 amino acids, also known as C1 domains, to which phorbol esters and DAGs can bind (22). Although the amino acid sequence of these C1 domains is highly conserved, there is enough variability in the amino acid composition around the rim formed by the two β-sheets surrounding the binding site that, depending on the nature of the side chains attached to either the phorbol esters or the DAG lactones, specific interactions with the lipid environment of the cell or with scaffolding proteins, such as RACKS, can direct the activated isozyme to a specific cellular organelle, thus precipitating different biological events (35). Given this degree of receptor heterogeneity, it is not surprising that the various biological functions studied in this paper are not quantitatively correlated with the in vitro Ki values of the DAG lactones, which reflect only their affinities for the single isotype PKCα.
Interestingly, LMC03 and LMC07, which were selected as the most effective agents in this study, have widely different structures in spite of their common lactone template. In LMC03, the large branched chain is part of an α-alkylidene chain directly attached to the lactone, whereas in LMC07 the large branched chain is part of the acyl group located on the opposite side of the molecule. However, it is possible that both compounds can direct their bulky branched chains into the same area of the receptor by binding in opposite orientations that engage either the ester carbonyl (sn-1 carbonyl) or the lactone carbonyl (sn-2) in forming the requisite hydrogen bond at the binding site. These two alternative binding modes have been described previously (30, 36), and efforts to understand and modulate their preference continue.
The eventual clinical goal of these studies is to reduce or eradicate the reservoir of HAART-resistant HIV-1-infected cells by first inducing HIV-1 gene expression. This will necessitate the development of inducing agents with acceptable pharmacologic profiles. The variability in biological responses elicited by the small set of DAG-lactones studied here indicates that considerable selectivity and specificity can be engineered into this class of molecules by altering the nature and location of the branched side chains on the lactone template. In future studies, the activation of PKC in cells could be monitored by using isotype-specific antibodies or green fluorescent protein-tagged isozymes to visualize the specific translocation to subcellular compartments that correlates with structural features of the agonists. The results obtained in this study give us hope that through the construction of large combinatorial libraries, even more specific and selective HIV-1-inducing agents will be discovered.
Acknowledgments
We thank the NIH AIDS Research and Reference Reagent Program for providing cell lines and Claude Klee and the anonymous referees for critical reading of the manuscript. We are indebted to the research volunteers who participated in this study.
REFERENCES
- 1.Altman, A., and M. Villalba. 2002. Protein kinase C-theta (PKCθ): a key enzyme in T cell life and death. J. Biochem. (Tokyo) 132:841-846. [DOI] [PubMed] [Google Scholar]
- 2.Blankson, J. N., D. Persaud, and R. F. Siliciano. 2002. The challenge of viral reservoirs in HIV-1 infection. Annu. Rev. Med. 53:557-593. [DOI] [PubMed] [Google Scholar]
- 3.Blumberg, P. M., P. Acs, D. K. Bhattacharyya, and P. S. Lorenzo. 2000. Inhibitors of protein kinase C and related receptors for the lipophilic second messenger sn-1,2-diacylglycerol, p. 347-364. In J. S. Gutkind (ed.), Signal transduction and cell cycle inhibitors. Humana Press, Totowa, N.J.
- 4.Bocklandt, S., P. M. Blumberg, and D. H. Hamer. 2003. Activation of latent HIV-1 expression by the potent anti-tumor promoter 12-deoxyphorbol 13-phenylacetate. Antiviral Res. 59:89-98. [DOI] [PubMed]
- 5.Brose, N., and C. Rosenmund. 2002. Move over protein kinase C, you've got company: alternative cellular effectors of diacylglycerol and phorbol esters. J. Cell Sci. 115:4399-4411. [DOI] [PubMed] [Google Scholar]
- 6.Chun, T. W., D. Engel, S. B. Mizell, L. A. Ehler, and A. S. Fauci. 1998. Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. J. Exp. Med. 188:83-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chun, T.-W., D. Engel, M. M. Berrey, T. Shea, L. Corey, and A. S. Fauci. 1998. Early establishment of a pool of latently infected, resting CD4+ T cells during primary HIV-1 infection. Proc. Natl. Acad. Sci. USA 95:8869-8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chun, T. W., and A. S. Fauci. 1999. Latent reservoirs of HIV: obstacles to the eradication of virus. Proc. Natl. Acad. Sci. USA 96:10958-10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clouse, K. A., D. Powell, I. Washington, G. Poli, K. Strebel, W. Farrar, P. Barstad, J. Kovacs, A. S. Fauci, and T. M. Folks. 1989. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J. Immunol. 142:431-438. [PubMed] [Google Scholar]
- 10.Eisen, M. B., and P. O. Brown. 1999. DNA arrays for analysis of gene expression. Methods Enzymol. 303:179-205. [DOI] [PubMed] [Google Scholar]
- 11.Evans, F. J., and R. J. Schmidt. 1979. The succulent euphorbias of Nigeria. III. Structure and potency of the aromatic ester diterpenes of Euphorbia poissonii Pax. Acta Pharmacol. Toxicol. (Copenhagen) 45:181-191. [DOI] [PubMed] [Google Scholar]
- 12.Folks, T. M., K. A. Clouse, J. Justement, A. Rabson, E. Duh, J. H. Kehrl, and A. S. Fauci. 1989. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc. Natl. Acad. Sci. USA 86:2365-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Bermejo, M. L., F. C. Leskow, T. Fujii, Q. Wang, P. M. Blumberg, M. Ohba, T. Kuroki, K. C. Han, J. Lee, V. E. Marquez, and M. G. Kazanietz. 2002. Diacylglycerol (DAG)-lactones, a new class of protein kinase C (PKC) agonists, induce apoptosis in LNCaP prostate cancer cells by selective activation of PKCα. J. Biol. Chem. 277:645-655. [DOI] [PubMed] [Google Scholar]
- 14.Han, X. M., A. Laras, M. P. Rounseville, A. Kumar, and P. R. Shank. 1992. Human immunodeficiency virus type 1 Tat-mediated trans-activation correlates with the phosphorylation state of a cellular TAR RNA stem-binding factor. J. Virol. 66:4065-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hergenhahn, M., S. Kusumoto, and E. Hecker. 1984. On the active principles of the spurge family (Euphorbiaceae). V. Extremely skin-irritant and moderately tumor-promoting diterpene esters from Euphorbia resinifera Berg. J. Cancer Res. Clin. Oncol. 108:98-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho, D. D. 1998. Toward HIV eradication or remission: the tasks ahead. Science 280:1866-1867. [DOI] [PubMed] [Google Scholar]
- 17.Jakobovits, A., A. Rosenthal, and D. J. Capon. 1990. Trans-activation of HIV-1 LTR-directed gene expression by tat requires protein kinase C. EMBO J. 9:1165-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kagnoff, M. F., and K. A. Roebuck. 1999. Human immunodeficiency virus type 1 (HIV-1) infection and expression in intestinal epithelial cells: role of protein kinase A and C pathways in HIV-1 transcription. J. Infect. Dis. 179(Suppl. 3):S444-S447. [DOI] [PubMed] [Google Scholar]
- 19.Kanashiro, C. A., and R. A. Khalil. 1998. Signal transduction by protein kinase C in mammalian cells. Clin. Exp. Pharmacol. Physiol. 25:974-985. [DOI] [PubMed] [Google Scholar]
- 20.Kazanietz, M. G. 2002. Novel “nonkinase” phorbol ester receptors: the C1 domain connection. Mol. Pharmacol. 61:759-767. [DOI] [PubMed] [Google Scholar]
- 21.Kazanietz, M. G., L. B. Areces, A. Bahador, H. Mischak, J. Goodnight, J. F. Mushinski, and P. M. Blumberg. 1993. Characterization of ligand and substrate specificity for the calcium-dependent and calcium-independent protein kinase C isozymes. Mol. Pharmacol. 44:298-307. [PubMed] [Google Scholar]
- 22.Kazanietz, M. G., M. J. Caloca, P. Eroles, T. Fujii, M. L. Garcia-Bermejo, M. Reilly, and H. Wang. 2000. Pharmacology of the receptors for the phorbol ester tumor promoters: multiple receptors with different biochemical properties. Biochem. Pharmacol. 60:1417-1424. [DOI] [PubMed] [Google Scholar]
- 23.Korin, Y. D., D. G. Brooks, S. Brown, A. Korotzer, and J. A. Zack. 2002. Effects of prostratin on T-cell activation and human immunodeficiency virus latency. J. Virol. 76:8118-8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulkosky, J., D. M. Culnan, J. Roman, G. Dornadula, M. Schnell, M. R. Boyd, and R. J. Pomerantz. 2001. Prostratin: activation of latent HIV-1 expression suggests a potential inductive adjuvant therapy for HAART. Blood 98:3006-3015. [DOI] [PubMed] [Google Scholar]
- 25.Kulkosky, J., and R. J. Pomerantz. 2002. Approaching eradication of highly active antiretroviral therapy-persistent human immunodeficiency virus type 1 reservoirs with immune activation therapy. Clin. Infect. Dis. 35:1520-1526. [DOI] [PubMed] [Google Scholar]
- 26.Lee, J., K. C. Han, J. H. Kang, L. L. Pearce, N. E. Lewin, S. Yan, S. Benzaria, M. C. Nicklaus, P. M. Blumberg, and V. E. Marquez. 2001. Conformationally constrained analogues of diacylglycerol. 18. The incorporation of a hydroxamate moiety into diacylglycerol-lactones reduces lipophilicity and helps discriminate between sn-1 and sn-2 binding modes to protein kinase C (PK-C). Implications for isozyme specificity. J. Med. Chem. 44:4309-4312. [DOI] [PubMed] [Google Scholar]
- 27.Marquez, V. E., K. Nacro, S. Benzaria, J. Lee, R. Sharma, K. Teng, G. W. Milne, B. Bienfait, S. Wang, N. E. Lewin, and P. M. Blumberg. 1999. The transition from a pharmacophore-guided approach to a receptor-guided approach in the design of potent protein kinase C ligands. Pharmacol. Ther. 82:251-261. [DOI] [PubMed] [Google Scholar]
- 28.McHugh, L., S. Hu, B. K. Lee, K. Santora, P. E. Kennedy, E. A. Berger, I. Pastan, and D. H. Hamer. 2002. Increased affinity and stability of an anti-HIV-1 envelope immunotoxin by structure based mutagenesis. J. Biol. Chem. 277:34383-34390. [DOI] [PubMed] [Google Scholar]
- 29.Nabel, G., and D. Baltimore. 1987. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature 326:711-713. [DOI] [PubMed] [Google Scholar]
- 30.Nacro, K., B. Bienfait, J. Lee, K. C. Han, J. H. Kang, S. Benzaria, N. E. Lewin, D. K. Bhattacharyya, P. M. Blumberg, and V. E. Marquez. 2000. Conformationally constrained analogues of diacylglycerol (DAG). 16. How much structural complexity is necessary for recognition and high binding affinity to protein kinase C? J. Med. Chem. 43:921-944. [DOI] [PubMed] [Google Scholar]
- 31.Nacro, K., B. Bienfait, N. E. Lewin, P. M. Blumberg, and V. E. Marquez. 2000. Diacylglycerols with lipophilically equivalent branched acyl chains display high affinity for protein kinase C (PK-C). A direct measure of the effect of constraining the glycerol backbone in DAG lactones. Bioorg. Med. Chem. Lett. 10:653-655. [DOI] [PubMed] [Google Scholar]
- 32.Nacro, K., D. M. Sigano, S. Yan, M. C. Nicklaus, L. L. Pearce, N. E. Lewin, S. H. Garfield, P. M. Blumberg, and V. E. Marquez. 2001. An optimized protein kinase C activating diacylglycerol combining high binding affinity (Ki) with reduced lipophilicity (log P). J. Med. Chem. 44:1892-1904. [DOI] [PubMed] [Google Scholar]
- 33.Pomerantz, R. J. 2002. Reservoirs of human immunodeficiency virus type 1: the main obstacles to viral eradication. Clin. Infect. Dis. 34:91-97. [DOI] [PubMed] [Google Scholar]
- 34.Roebuck, K. A., D. S. Gu, and M. F. Kagnoff. 1996. Activating protein-1 cooperates with phorbol ester activation signals to increase HIV-1 expression. AIDS 10:819-826. [DOI] [PubMed] [Google Scholar]
- 35.Schechtman, D., and D. Mochly-Rosen. 2001. Adaptor proteins in protein kinase C-mediated signal transduction. Oncogene 20:6339-6347. [DOI] [PubMed] [Google Scholar]
- 36.Sigano, D., M. Peach, K. Nacro, Y.-S. Choi, N. E. Lewin, M. C. Nicklaus, P. M. Blumberg, and V. E. Marquez. 2003. Differential binding modes of diacylglycerol (DAG) and DAG-lactones to protein kinase C (PK-C). J. Med. Chem. 46:1571-1579. [DOI] [PubMed] [Google Scholar]
- 37.Sonza, S., and S. M. Crowe. 2001. Reservoirs for HIV infection and their persistence in the face of undetectable viral load. AIDS Patient Care STDS 15:511-518. [DOI] [PubMed] [Google Scholar]
- 38.Szallasi, Z., L. Krsmanovic, and P. M. Blumberg. 1993. Nonpromoting 12-deoxyphorbol 13-esters inhibit phorbol 12-myristate 13-acetate induced tumor promotion in CD-1 mouse skin. Cancer Res. 53:2507-2512. [PubMed] [Google Scholar]
- 39.van Praag, R. M., J. M. Prins, M. T. Roos, P. T. Schellekens, I. J. Ten Berge, S. L. Yong, H. Schuitemaker, A. J. Eerenberg, S. Jurriaans, F. de Wolf, C. H. Fox, J. Goudsmit, F. Miedema, and J. M. Lange. 2001. OKT3 and IL-2 treatment for purging of the latent HIV-1 reservoir in vivo results in selective long-lasting CD4+ T cell depletion. J. Clin. Immunol. 21:218-226. [DOI] [PubMed] [Google Scholar]
- 40.Wang, Q. J., D. Bhattacharyya, S. Garfield, K. Nacro, V. E. Marquez, and P. M. Blumberg. 1999. Differential localization of protein kinase C delta by phorbol esters and related compounds using a fusion protein with green fluorescent protein. J. Biol. Chem. 274:37233-37239. [DOI] [PubMed] [Google Scholar]
- 41.Yang, X., Y. Chen, and D. Gabuzda. 1999. ERK MAP kinase links cytokine signals to activation of latent HIV-1 infection by stimulating a cooperative interaction of AP-1 and NF-κB. J. Biol. Chem. 274:27981-27988. [DOI] [PubMed] [Google Scholar]