Abstract
Proteins of the Bcl-2 family are important regulators of apoptosis in many tissues of the embryo and adult. The recently isolated bcl-w gene encodes a pro-survival member of the Bcl-2 family, which is widely expressed. To explore its physiological role, we have inactivated the bcl-w gene in the mouse by homologous recombination. Mice that lack Bcl-w were viable, healthy, and normal in appearance. Most tissues exhibited typical histology, and hematopoiesis was unaffected, presumably due to redundant function with other pro-survival family members. Although female reproductive function was normal, the males were infertile. The testes developed normally, and the initial, prepubertal wave of spermatogenesis was largely unaffected. The seminiferous tubules of adult males, however, were disorganized, contained numerous apoptotic cells, and produced no mature sperm. Both Sertoli cells and germ cells of all types were reduced in number, the most mature germ cells being the most severely depleted. The bcl-w−/− mouse provides a unique model of failed spermatogenesis in the adult that may be relevant to some cases of human male sterility.
Keywords: Bcl-2 family/gene disruption/testis/germ cells/Sertoli cells
The programmed death of cells, reflected in the stereotypic process denoted apoptosis, has major roles in molding the embryo, in maintenance of tissue homeostasis, and in defense against pathogens. Disrupted regulation of apoptosis is implicated strongly in cancer and in autoimmune and degenerative diseases. Key regulators include proteins of the Bcl-2 family (reviewed in refs. 1–3), some of which (e.g., Bcl-2, Bcl-xL, Mcl-1, and A1) promote cell survival while others (e.g., Bax and Bak) antagonize it. Because members of these opposing factions can associate and seemingly titrate one another’s function, their relative abundance in a particular cell type may determine its threshold for apoptosis (4). The competitive action of the pro- and antisurvival Bcl-2-related proteins regulates the activation of the proteases (caspases) that dismantle the cell, but how they do so remains uncertain (1–3). The pro-survival proteins may, however, associate with caspase-activating adaptors such as Ced-4 and Apaf-1 and prevent their activity (5, 6) and/or prevent the release of pro-apoptotic proteins from mitochondria (7–9).
The pro-survival family members are expressed in diverse tissues, in distinct but overlapping patterns. Although their biochemical actions are difficult to distinguish, gene inactivation studies suggest that each may have a critical role in particular tissues. Mice which lack Bcl-2 develop normally but later display marked lymphocytopenia, polycystic kidney disease, hypopigmented hair, motoneuron degeneration, and disordered growth of intestinal villi and long bones (10–15). In contrast, mice that lack Bcl-xL die in utero due to massive apoptosis of both hematopoietic and neuronal cells (16).
Bcl-w is a pro-survival protein recently discovered by our laboratory (17). Enforced expression of Bcl-w, like Bcl-2, renders myeloid and lymphoid cell lines refractory to apoptosis induced by cytokine deprivation or irradiation but is relatively ineffective against apoptosis induced by engagement of the CD95 (Fas) “death” receptor (17). Transcripts of bcl-w are present at moderate levels in brain, colon, and salivary gland and at low levels in testis, liver, heart, stomach, skeletal muscle, and placenta, as well as in most myeloid cell lines but few lymphoid lines (17).
To identify the tissues in which Bcl-w plays an essential role, we have inactivated the mouse bcl-w gene by homologous recombination in embryonic stem (ES) cells. Bcl-w was apparently dispensable for the normal development and function of most organs but essential for spermatogenesis. We compare these findings with those recently reported for a mouse having an inadvertent insertion within the bcl-w gene (18), and with the different spermatogenic defects elicited by disruption of the bax gene (19), or by expression of bcl-2 or bcl-xL transgenes (20, 21).
MATERIALS AND METHODS
Disruption of bcl-w.
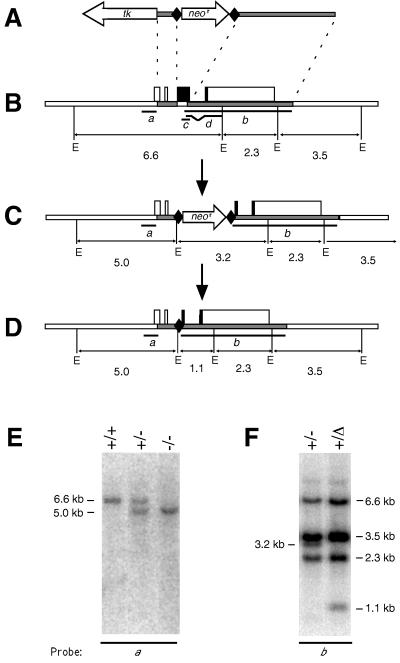
The gene-targeting vector (see Fig. 1A) was assembled in ploxPneo-1 (a kind gift from J. Rossant, University of Toronto), in which a neomycin phosphotransferase gene (neor), driven by a phosphoglycerate kinase promoter (PGK), is flanked by bacteriophage P1 loxP sites. The 129/Sv mouse bcl-w genomic DNA sequences introduced at each end of the loxP-neor-loxP cassette comprised the 876-bp region immediately upstream of the bcl-w start codon and the 4-kb BamHI fragment extending from within exon 3 through the entire 3′-untranslated region. Introduction of a terminal herpes simplex virus thymidine kinase (tk) gene driven by a PGK promoter then completed the vector (Fig. 1A), which was linearized and electroporated into W9.5 ES cells (22). ES cell clones selected for resistance to G418 (i.e., neor gene integration) and gancyclovir (i.e., loss of the tk gene after homologous recombination) (23, 24) were screened for homologous recombination at the bcl-w locus by Southern blot analysis. The bcl-w mutant ES cell clones were injected into the blastocoel cavity of C57BL/6J (B6) blastocysts, which were then implanted into pseudopregnant foster mothers. Male chimeric progeny were crossed to B6 females or, to delete the neor cassette, to B6/FVB F1 females expressing bacteriophage P1 Cre recombinase (Cre), a gift from H. Westphal (25).
Figure 1.
Disruption of the bcl-w gene. (A) The targeting vector pbcl-wlox neor tk. Shaded bars represent regions derived from the bcl-w gene; tk, a thymidine kinase expression cassette; neor, a PGK- neor expression cassette; and diamonds, loxP sequences. (B) The wt bcl-w locus. Boxes represent exons (solid, coding region; open, untranslated region). E, EcoRI sites; sizes of EcoRI fragments are in kb. The bcl-w genomic DNA probes used for Southern blot analyses are labeled a and b, whereas the bcl-w cDNA sequences used as riboprobes are indicated by c and d. (C) Homologous recombination replaces the first 413 bp of the bcl-w coding region with a PGK-neor expression cassette bounded by loxP sites. (D) Cre-mediated recombination deletes the PGK-neor sequence, leaving only 127 bp of exogenous sequence, including a single loxP site. (E) Southern blot of genomic DNA from wt (+/+), heterozygous (+/−), and homozygous mutant (−/−) bcl-w mice (line 228), hybridized with bcl-w cDNA probe a. (F) Southern blot of genomic DNA from heterozygous mice (line 228) before (+/−) and after (+/Δ) the action of Cre recombinase, hybridized with bcl-w probe b.
Analysis of Mouse Weights.
Wild-type (wt) and mutant mice were weighed weekly from birth to 20 wk, and the weights were analyzed by using the split-line model (26). In brief, growth curves before and after puberty were fitted to two straight lines and the slopes of these lines and their point of intersection compared.
Blot Analysis.
Southern blot analysis (24) on cultured ES cells or mouse tail tips used 500-bp StuI–BamHI and 4-kb Pml I genomic DNA fragments (Fig. 1B, probes a and b, respectively). Northern blot analysis was conducted on total RNA (10 μg/lane) prepared (27) from testes of adult mice. For Western blot analysis, tissues or cells were washed in PBS, immediately frozen in isopentane on dry ice, homogenized at 4°C in buffer (50 mM Tris⋅HCl, pH 7.5/2 mM EDTA/1% Nonidet P-40) containing 1 mM phenylmethylsulfonyl fluoride, 2 μg/ml aprotinin, 1 μg/ml pepstatin, and 2 μg/ml leupeptin, and then centrifuged at 10,000 × g at 4°C for 30 min. Proteins (35 μg) in the supernatant were resolved by SDS/PAGE (12% acrylamide gel) and transferred to nitrocellulose membranes (Hybond-C extra, Amersham). As controls for protein loading and integrity, membranes were stained with Ponceau S or with an antibody against the ubiquitous Hsp-70. Bcl-w was detected by incubation of the membranes overnight with a polyclonal rabbit-anti-human Bcl-w antibody (AAP-050, StressGen Biotechnologies, Victoria BC, Canada), followed by horseradish peroxidase-conjugated goat anti-rabbit antibody (Selenius) and chemiluminescent reagents (Amersham).
Histology and BrdUrd Labeling.
Tissues fixed in Bouin’s solution for 5 hr were embedded in paraffin, and 8-μm sections were transferred to silane-coated microscope slides and stained with hematoxylin/eosin. The following tissues were examined: brain, colon, salivary gland, liver, heart, stomach, skeletal muscle, skin, peripheral nerve, pituitary gland, eye, teeth, bone, cartilage, thyroid and parathyroid glands, blood vessels, lung, small intestine, pancreas, kidney, adrenal gland, bladder, uterus, ovary, and testis. To determine mitotic turnover, mice were injected i.p. with BrdUrd (100 μg/g body weight in 7 mM NaOH) 8 hr before killing. Paraffin-embedded sections of testis, small intestine, colon, spleen, thymus, and bone marrow were stained with rat-anti-BrdUrd antibody (Mas 250P, Harlan Ser-Lab, Sussex, United Kingdom). This was detected by biotinylated mouse-anti-rat Igκ antibody (Mar 18.5), avidin-biotinylated horseradish peroxidase (Elite ABC, Vector Laboratories), and diaminobenzidine.
Terminal Transferase-Mediated dUTP Nick End-Labeling (TUNEL).
Paraffin-embedded sections were treated with 20 μg/ml proteinase K in water for 15 min at room temperature, and then DNA free ends were labeled with dUTP-biotin by using terminal deoxynucleotidyl transferase (28) and revealed with avidin-biotinylated horseradish peroxidase. For each testis, TUNEL-labeled (apoptotic) nuclei in approximately twenty-five 0.56 mm2 fields were counted, and the number of apoptotic nuclei per seminiferous tubule were determined.
Hematologic Analyses.
Peripheral blood erythrocytes and leucocytes were enumerated using a Coulter counter and platelets with a Sysmex NE8000 counter (TOA, Kobe, Japan). Leucocytes in peripheral blood, femoral bone marrow, peritoneum, spleen, and thymus were stained with eosin and counted by hemocytometer. Cytocentrifuge preparations were stained with May-Grunwald-Giemsa (Gurr, Poole, United Kingdom). Single cell suspensions prepared from blood, bone marrow, spleen, and thymus were incubated with 2.4G2 anti-Fcγ receptor antibody (29) to reduce background staining, labeled with fluorescent surface marker-specific mAbs, and analyzed by flow cytometry as described elsewhere (30).
To enumerate progenitor cells, bone marrow and spleen cells were cultured in medium containing 0.1% agar (31) and the following cytokines: 10 ng/ml murine granulocyte-macrophage-colony stimulating factor (CSF), 10 ng/ml human granulocyte-CSF, 10 ng/ml murine macrophage-CSF, 10 ng/ml murine interleukin-3, 100 ng/ml murine stem cell factor or 200 ng/ml murine thrombopoietin. To determine the cellular composition of each colony, the agar plates were fixed and stained for acetylcholinesterase and then with Luxol fast blue and hematoxylin (31).
Testis Stereology.
Testes fixed for 5 hr in Bouin’s fixative were embedded in methacrylate; 25-μm sections were transferred to glass slides and stained with hematoxylin and the periodic acid-Schiff reagent. Leydig and Sertoli cells and germ cells were counted by using the “optical disector” approach as described (32).
In Situ Hybridization.
Digoxigenin-labeled riboprobes were generated from linearized plasmid DNA templates (33). Riboprobes c1 (sense) and c2 (anti-sense) (Fig. 1B) were generated from residues 118–410 of the bcl-w cDNA (GenBank accession no. U59746) in the pT7Blue vector (Novagen) and d1 and d2 from residues 330–956 in the pBSIISK vector (Stratagene). Paraffin-embedded tissue sections on microscope slides were treated with 1 μg/ml proteinase K in buffered saline for 30 min at 37°C, hybridized to the riboprobes at 50°C for 16 hr, and washed to 0.1× SSC at 50°C (33). Slides were then exposed to an alkaline phosphatase-conjugated anti-digoxigenin antibody (Boehringer Mannheim), riboprobes detected with the nitroblue tetrazolium chloride/bromo-chloro-indolyl phosphate substrate, and the slides counterstained with hematoxylin.
Serum Gonadotrophin Assay.
The concentration of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) in serum was determined by a double-antibody radioimmunoassay by using reagents for the measurement of rat FSH and LH (34). Their efficacy on the mouse hormones was confirmed. All samples were measured in the same assay with an intra-assay coefficient of variation of 4.8% and 5.9% for the FSH and LH assays, respectively.
RESULTS
Disruption of bcl-w.
The gene-targeting vector was designed to inactivate bcl-w by replacing the first two-thirds of its coding region with a PGK- neor expression cassette bounded by loxP sites (Fig. 1A−C). Any translation of the remainder should be precluded by a preceding stop codon. Homologous recombination was obtained in 8 of 352 selected ES cell clones. The structure of the mutant allele (bcl-w−) was confirmed by Southern blot analysis: bcl-w probe a detected 6.6-kb and 5.0-kb EcoRI fragments diagnostic for the wt and bcl-w− alleles respectively (e.g., Fig. 1E). A neor probe excluded the presence of any copies of the targeting vector integrated elsewhere in the genome. Two independent recombinant ES clones were used to generate chimeric mice, which were bred with B6 females to generate two lines of bcl-w-mutant mice (228 and 229), each of which was subsequently bred to homozygosity.
Regulatory sequences introduced by gene targeting can inadvertently alter the expression of neighboring genes (35). Just 5.5 kb downstream of bcl-w is the gene encoding Poly(A)-binding protein II (mPABII (36), homologue of rox; 17). To avoid altering the expression of this or other neighboring genes, we also generated mice in which the introduced PGK-neor cassette was deleted by crossing both 228 and 229 mice with animals expressing Cre recombinase at the two-cell stage of development (25) (Fig. 1D). Progeny carrying the deleted allele (bcl-wΔ, Fig. 1D) were recognized by a diagnostic 1.1-kb EcoRI fragment (Fig. 1F), and the deletion was confirmed by sequencing a PCR product spanning the recombination site. Crosses with B6 mice then generated lines 228Δ and 229Δ. Northern blot analysis confirmed that expression of the mPABII gene was unaffected in 228Δ mice homozygous for the bcl-wΔ allele (data not shown). Importantly, homozygous mutants of all four lines (228, 229, 228Δ, and 229Δ) proved to be indistinguishable in all analyses presented here.
Bcl-w Is Dispensable for Development.
As expected, the bcl-wΔ/Δ mice expressed neither bcl-w RNA nor protein. No RNA transcript was detected by a bcl-w cDNA probe in Northern blots of RNA extracted from testis (Fig. 2A), and Western blots with an anti-Bcl-w antibody revealed no Bcl-w protein in lysates from brain, testis, or pancreas (Fig. 2B).
Figure 2.
Expression of the bcl-w gene. (A) Northern blot of total RNA (10 μg) extracted from the testes of 4-wk-old wt (+/+) and bcl-wΔ/Δ mice (Δ/Δ), hybridized to a probe containing the first 1.2 kb of the bcl-w cDNA (Upper); glyceraldehyde phosphate dehydrogenase mRNA served as a control (gapdh, Lower). (B) Western blot analysis of protein lysates from the brain, testis, and pancreas of wt and bcl-wΔ/Δ mice, using a polyclonal anti-Bcl-w antibody. The 21-kDa Bcl-w protein is indicated. (C) Western blots of protein lysates from testis cell lines, with the same antibody. GC-1 is a germ cell line derived from type B spermatogonia, TM4 a Sertoli cell line, and TM3 a Leydig cell line; all were obtained from the American Type Culture Collection.
Lack of Bcl-w Did Not Compromise Survival of Fetal or Neonatal Mice. The offspring of bcl-w+/Δ intercrosses were born at normal Mendelian frequency: 25% bcl-w+/+, 47% bcl-w+/Δ, and 28% bcl-wΔ/Δ, and 57% of bcl-wΔ/Δ offspring were male (total n = 210). The bcl-wΔ/Δ mice exhibited no significant abnormality in external appearance or behavior. The growth of bcl-wΔ/Δ pups from birth to 5 wk of age was indistinguishable from that of their wt littermates. Although the average weights of male and female bcl-wΔ/Δ mice at 5, 7, 9, 12, 16, and 20 wk of age were slightly less than that of their bcl-w+/+ and bcl-w+/Δ littermates, the differences were not statistically significant. In addition, the growth curves of wt and bcl-wΔ/Δ mice were indistinguishable when analyzed by using the split-line method (26). Thorough histological examination of numerous tissues (see Materials and Methods) from bcl-wΔ/Δ mice 6 and 52 wk of age revealed no significant abnormalities.
Normal Maintenance of Hematopoiesis.
Because bcl-w RNA is detectable in most myeloid and some lymphoid cell lines (17), the hematopoietic tissues of bcl-wΔ/Δ mice were scrutinized carefully. In mice analyzed at 6 and 52 wk, the weight and histology of the thymus, spleen, lymph node, and bone marrow were normal. Blood cell analysis of three adult mice indicated normal numbers of erythrocytes, platelets, neutrophils, monocytes, eosinophils, and lymphocytes (B and T). The peritoneal leucocyte population also was unaffected. The frequency of apoptotic nuclei in the spleen, thymus, and bone marrow was unaltered, as judged by TUNEL analysis (28). Bcl-2 family members can slow mitotic cycle entry (37, 38), but immunohistochemistry of spleen, thymus, and bone marrow from bcl-wΔ/Δ mice injected with BrdUrd 8 hr before killing (see Materials and Methods) indicated normal numbers of leucocytes in S phase.
Clonogenic assays on bone marrow cells from three adult bcl-wΔ/Δ mice and three wt littermates yielded a comparable frequency of neutrophil, neutrophil-macrophage, macrophage, eosinophil, megakaryocyte, and blast cell colony-forming cells, and the colonies were of similar size and maturation. Moreover, the progenitors were not rendered more sensitive to cytokine deprivation, since a 4-day delay in addition of interleukin-3 to such cultures reduced the number of colonies from wt and mutant marrow to equivalent extents (data not shown).
Bcl-w Is Essential for Spermatogenesis.
Female bcl-wΔ/Δ mice were fertile and competent to feed their pups. Intriguingly, however, all the males were infertile. Although their external genitalia and testicular descent appeared normal, the cauda epididymides of bcl-wΔ/Δ mice of all ages were devoid of sperm (cf. Fig. 3 A and B). In contrast, male heterozygotes exhibited normal fertility and epididymal histology.
Figure 3.
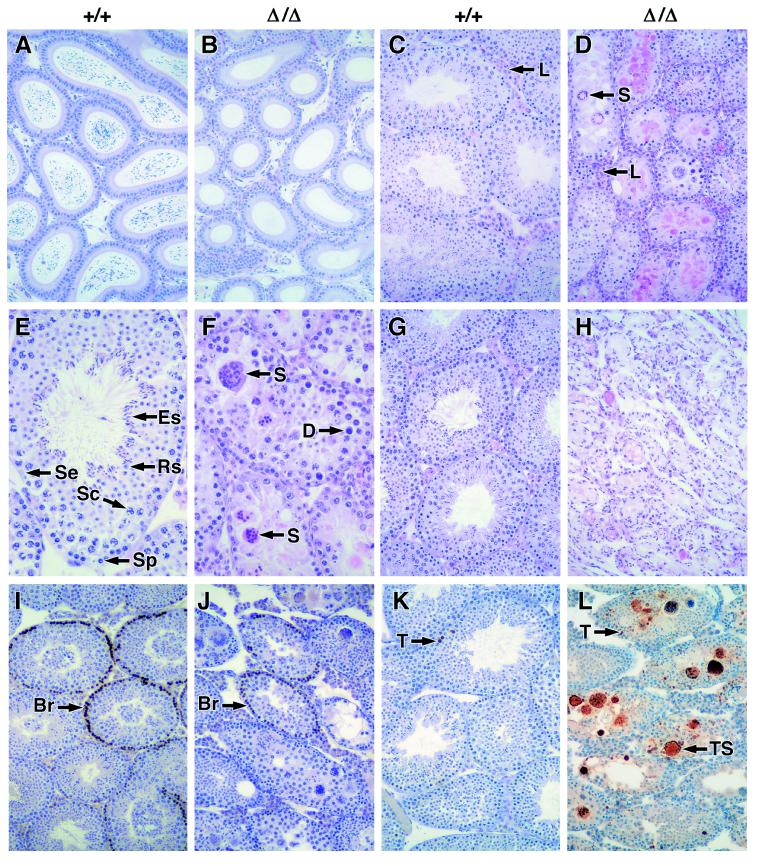
Failed spermatogenesis in mice lacking Bcl-w. Comparison of wt (+/+) and bcl-wΔ/Δ (Δ/Δ) tissues. (A and B) Epididymides of 6-wk-old mice stained with hematoxylin/eosin (85-fold magnification). (C−F) Testis from 8-wk-old mice stained with hematoxylin/eosin (C and D, 85-fold; E and F, 340-fold). S, symplasts; D, degenerating nuclei; L, Leydig cells; Se, Sertoli cells; Sp, spermatogonia; Sc, spermatocytes; Rs, round spermatids; and Es, elongating spermatids. (G and H) Testis from 52-wk-old mice stained with hematoxylin/eosin (85-fold). (I and J) BrdUrd-labeled testis from 6-wk-old mice immunostained with anti-BrdUrd antibody and then counterstained with hematoxylin (85-fold); Br indicates spermatogonia that have incorporated BrdUrd. (K and L) Testis from 6-wk-old mice labeled by TUNEL and counterstained with hematoxylin (85-fold). T, TUNEL-labeled apoptotic nuclei; TS, TUNEL-labeled symplasts.
Spermatogenesis involves an orderly process of germ cell maturation toward the center of the seminiferous tubules: mitotic proliferation of spermatogonia (up to 9 divisions), meiotic division of spermatocytes, differentiation of spermatids, and finally release of spermatozoa into the tubule lumen (reviewed in ref. 39). Histological examination of the testes of adult bcl-wΔ/Δ mice revealed extensive albeit heterogeneous pathology within the seminiferous tubules (Fig. 3 C−F). The tubules were abnormally small in diameter and often lacked a lumen. Numerous degenerating cells appeared throughout the seminiferous epithelium, some in the form of symplasts, giant cells containing several degenerating nuclei (Fig. 3 C−F). There were few elongating spermatids more advanced than stage 13 of the seminiferous cycle and no mature sperm. Indeed, by 52 wk of age, almost no germ cells were discernible, although Sertoli cells remained (Fig. 3 G−H). The defect was not in proliferation, since anti-BrdUrd-immunohistochemistry revealed numerous spermatocytes in S phase (Fig. 3 I−J). Instead there was a striking elevation in the number of TUNEL-labeled apoptotic cells, many of which were contained within symplasts (Fig. 3 K−L).
To determine which cells were affected, we used the well characterized “optical disector” method (see Materials and Methods) to calculate the total number of each cell type within the testes of wt and bcl-wΔ/Δ mice at 6 wk of age. Leydig cells were increased by nearly 50% (see Discussion). For each of the other cell types analyzed however, mutant testes contained significantly fewer cells than wt testes (Student’s t tests, P < 0.05). Sertoli cell numbers had decreased to 16% of their normal level (Fig. 4). Interestingly, germ cell numbers declined progressively with advancing stages of differentiation. Whereas type A spermatogonia were 30% of the normal level, spermatocytes represented only 15–20% of normal numbers, and, during spermatid differentiation, the level fell to 3% of normal (Fig. 4). Cells also were enumerated in the testes of single wt and bcl-wΔ/Δ mice at 12, 14, and 16 wk of age. The deficit of round and elongating spermatids was more severe by 12 wk of age, and by 14 wk, very few cells at or beyond the pachytene spermatocyte stage remained (data not shown). Heterozygotes exhibited none of these alterations (data not shown).
Figure 4.
Reduced numbers of various cell types within the seminiferous tubules of bcl-wΔ/Δ mice. Frequencies of the indicated cell types was determined by the optical disector method for seven 6-wk-old wt mice and eight 6-wk-old bcl-wΔ/Δ mice. The percentage of the wt cell numbers remaining in the testes of bcl-wΔ/Δ mice is indicated. Error bars denote two SEM.
Germ Cell Apoptosis Increases Near Sexual Maturity.
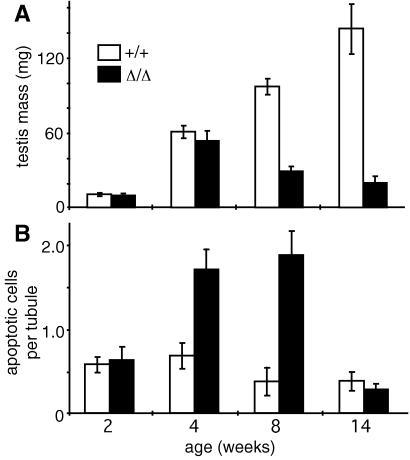
Early testicular development was normal. At 2 wk of age, the testes of bcl-wΔ/Δ mice exhibited normal mass and histology, and the number of TUNEL-labeled apoptotic nuclei per tubule was similar to that of wt littermates (Fig. 5). Even at 4 wk, the testes appeared normal and were of normal weight (Fig. 5A), suggesting that germ cell numbers had not yet fallen substantially, although there were twice as many apoptotic cells as in wt littermates (Fig. 5B). By 8 wk of age, however, the number of apoptotic cells was 5 times the normal level, and the testes had lost 70% of their mass (Fig. 5). Subsequently, the frequency of apoptotic cells declined, probably because so few germ cells remained. Thus, the apoptotic loss commences by 4 wk of age, but severe attrition is evident only at sexual maturity.
Figure 5.
Degeneration of testis in bcl-wΔ/Δ mice. (A) Mean mass of testes (three mice per group). (B) TUNEL-labeled nuclei per tubule, counted at 2, 4, 8, and 14 wk (three mice per group). Error bars denote two SEM.
No Evidence for an Endocrinological Basis.
Germ cell apoptosis is inhibited directly by circulating androgens and FSH and indirectly by LH, which promotes the secretion of androgens by Leydig cells (20, 39, 40). It therefore seemed possible that the spermatogenic defect was caused by reduced levels of these hormones. However, normal androgen levels could be inferred from the unaltered weight and histology of androgen-dependent organs (ventral prostate gland and seminal vesicles). Moreover, the serum FSH and LH concentrations of six wt and six bcl-wΔ/Δ mice were equivalent (Student’s t test, P = 1.0 for FSH and 0.1 for LH). These results, together with the normal histological appearance of the Leydig cells, hypothalamus, and pituitary gland, make it unlikely that altered endocrine levels have a major role in the phenotype.
Expression of bcl-w in the Testis.
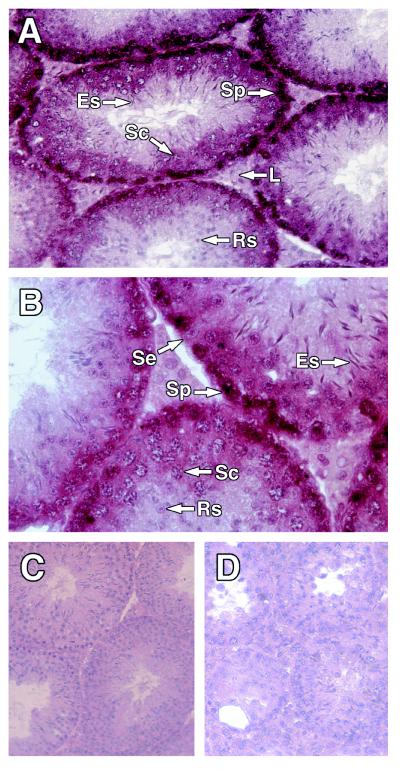
To facilitate interpretation of the phenotype of bcl-wΔ/Δ mice, we explored the expression pattern of bcl-w in wt adult testis. In situ hybridization indicated that bcl-w RNA was very prominent in the basal regions of seminiferous tubules. Antisense bcl-w riboprobes (c1 and d1, Fig. 1B) hybridized strongly to spermatogonia and moderately to spermatocytes, round spermatids, and some Sertoli cells but not detectably to elongating spermatids or mature sperm (Fig. 6 A and B). Corresponding sense riboprobes (c2, d2) did not hybridize to any cell type (Fig. 6C), and the antisense probes failed to detect any cells in the testis of bcl-wΔ/Δ mice (Fig. 6D). Thus, bcl-w expression in adult testis was most conspicuous in premeiotic germ cells and was detectable in Sertoli cells but not in Leydig cells.
Figure 6.
bcl-w expression in testis. (A−D) Paraffin-embedded sections of testis from 14-wk-old mice hybridized to bcl-w riboprobes and counterstained with hematoxylin. (A and B) wt testis hybridized with antisense bcl-w riboprobe c1 (A, 230-fold magnification; B, 460-fold). (C) wt testis hybridized to sense bcl-w riboprobe c2 (165-fold). (D) Testis from a bcl-wΔ/Δ mouse, hybridized to antisense bcl-w riboprobe c1 (165-fold).
The expression profile of Bcl-w in three mouse testicular cell lines was in accord with the in situ hybridization results. Western blot analysis with a polyclonal anti-Bcl-w antibody revealed high levels of Bcl-w protein in the germ cell line GC-1 (derived from type B spermatogonia) and moderate levels in the Sertoli cell line TM4 but none in the Leydig line TM3 (Fig. 2C). Bcl-w also was detected in testes of 10-day-old mice, which contain only Sertoli cells and spermatogonia (data not shown).
DISCUSSION
Bcl-w Is Dispensable in Most Tissues.
Despite its widespread expression (17), this study suggests that bcl-w is dispensable for the development and maintenance of most tissues. Tissues in which bcl-w is known to be expressed, such as brain, colon, salivary gland, liver, heart, stomach, and skeletal muscle, were histologically normal in bcl-wΔ/Δ mice. Although this might mean that Bcl-w does not function in these tissues, it seems more likely that another pro-survival protein suffices for maintenance of their homeostasis. Indeed, Bcl-xL has been observed in some cells of all these tissues (41), Bcl-2 in brain and intestine (42, 43) and Mcl-1 in intestine, heart, stomach, and skeletal muscle (44). Redundancy probably also accounts for the normal hematopoiesis in bcl-w mice. Bcl-2 and Bcl-x are detectable in precursors of several hematopoietic lineages and in certain lymphoid populations (42, 44); Mcl-1 in normoblasts, differentiating myeloid cells, and germinal centers (44); and A1 in spleen, thymus, bone marrow, and macrophages (45). Thus, it appears that most cell types are guarded by more than one pro-survival family member.
Bcl-w Is Required in the Testis.
Spermatogenesis was ablated in adult bcl-wΔ/Δ mice. The incidence of germ cell apoptosis in the testis was elevated, and the numbers of Sertoli cells and germ cells of all types were reduced, with elongating spermatids and spermatozoa the most severely affected (Fig. 7). These testicular defects resemble those of another line of bcl-w-mutant mice (ROSA41), generated by the chance integration of a gene trap retroviral vector 134-nt upstream of bcl-w exon 3 (18). Like the bcl-wΔ/Δ mice, the testes of ROSA41 mice were reduced in size and lacked the most mature generations of elongating spermatids, although the germ cell and Sertoli cell defects in ROSA41 mice were not quantified.
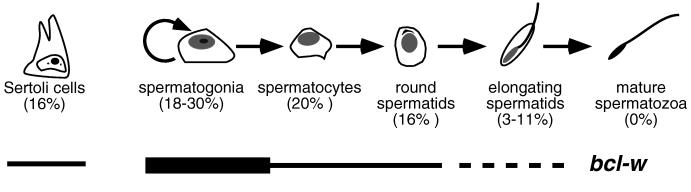
Figure 7.
Consequences of Bcl-w loss in the testis. The percentages of the Sertoli cells and the different types of germ cells remaining in bcl-wΔ/Δ mice are indicated. The expression pattern of the gene is indicated schematically; the broken line indicates that the extent of expression in late stages of germ cell development remains to be clarified (see text).
Our in situ hybridization analysis of wt mice indicated that bcl-w is expressed primarily in immature germ cells (spermatogonia, spermatocytes, and round spermatids) and also in Sertoli cells but not in Leydig cells (Figs. 6, 7). Western blots demonstrating Bcl-w in a pre-meiotic germ cell line and a Sertoli cell line but not in a Leydig cell line (Fig. 2C) corroborated these findings. The ROSA41 study (18) also concluded that bcl-w is expressed in Sertoli cells but reported a different pattern of expression in germ cells. Because ROSA41 mice carry a lacZ sequence integrated downstream of the bcl-w promoter, β-galactosidase activity in heterozygotes was used to identify cells likely to express bcl-w. β-galactosidase was detected in some Sertoli cells and in stage 11–16 elongating spermatids but not consistently in less mature germ cells or in Leydig cells (18). Although staining with a rabbit polyclonal anti-Bcl-w antiserum appeared to support these findings, the antibody crossreacted with several unrelated proteins in Western blots (18), raising questions about its specificity in immunohistochemistry. The discrepancy between the two studies may indicate that the expression of the reporter gene does not entirely reflect that of the native bcl-w gene. We are attempting to produce anti-Bcl-w mAbs for immunohistochemistry to further clarify the pattern of Bcl-w expression.
Basis for the Defect.
The first wave of spermatogenesis, between birth and ≈5 wk of age, is normally accompanied by extensive apoptosis of germ cells. This attrition, which may be mediated by high levels of Bax, is thought to adjust the number of germ cells to that which can be supported by the available Sertoli cells (20). It appears critical for subsequent production of spermatozoa because inhibition of germ cell apoptosis during the first wave, either by inactivation of the bax gene (19) or by expression of bcl-2 or bcl-xL transgenes (20, 21), leads to accumulation of pre-meiotic germ cells and prevents establishment of spermatogenesis in the adult. Importantly, the testes of 2-wk-old bcl-wΔ/Δ mice appeared unaffected, despite abundant expression of bcl-w in Sertoli and germ cells of wt mice at this age (our unpublished in situ hybridization data). Presumably alternative pro-survival proteins such as Bcl-xL, which is highly expressed in the testis from 1 to 3 wk of age (20), can maintain an appropriate balance between cell survival and apoptosis at this early stage.
In marked contrast, adult spermatogenesis is critically dependent on Bcl-w (ref. 18 and this paper). The initial depletion of testicular cells in young adult bcl-wΔ/Δ mice probably occurs by cell-intrinsic mechanisms, due to an excess of pro-apoptotic over pro-survival proteins. Because Sertoli cells contain the pro-death protein Bak (46), absence of Bcl-w may lead to their Bak-induced death. The extensive depletion of germ cells (Fig. 4) also may be primarily cell-intrinsic, possibly via Bax (still present at low levels) or other pro-death proteins such as Bad (20). Compensation would not be expected by the other pro-survival proteins so far surveyed in testis. Bcl-2 has been detected only in mature sperm and not in the seminiferous epithelium (42); Mcl-1 appears to be restricted to Leydig cells (44); and Bcl-xL is present at relatively low levels in adult testis and apparently only in spermatocytes and spermatids (20, 46).
Germ cell depletion in adult bcl-wΔ/Δ mice also may be due to cell-extrinsic effects, in particular to the loss of Sertoli cells. Experimental reduction of Sertoli cell number in the rat has been shown to reduce proportionally the number of round spermatids (47). Death of Sertoli cells may be particularly devastating for elongating spermatids, which reside luminal to the blood-testis barrier and thus rely on Sertoli cells for a variety of metabolic and transport functions. It also is conceivable that dying Sertoli cells actively promote the demise of germ cells because both the expression of Fas ligand on Sertoli cells and the expression of Fas on germ cells are up-regulated by exposure of Sertoli cells to toxins (48). Surprisingly, the number of Leydig cells was increased in adult bcl-wΔ/Δ mice. Since Bcl-w has not been detected in Leydig cells, their increased abundance may reflect the alterations in Leydig cell function known to occur in response to disordered spermatogenesis (49).
Mice that lack Bcl-w provide a unique model of failed spermatogenesis, in which Sertoli cells and germ cells of all types are affected. Further analysis of these animals should clarify the role played by Bcl-w in each cell type in the testis and reveal whether, under stress conditions, Bcl-w is required in other organ systems. The striking spermatogenic defect in these mice raises the possibility that mutations in the bcl-w gene may contribute to human male sterility. Moreover, it seems possible that reagents, which alter cell death in the testis, could be used to modulate fertility.
Acknowledgments
We thank L. Barnett for valuable assistance with targeting the ES cells, J. Rossant for the ploxPneo-1 plasmid, H. Westphal for the EIIa-cre transgenic mice, A. Harris for assistance with interpretation of histology, A. Strasser for the antibodies used in FACS analysis, T. Speed and N. Roberts for assistance with statistics, S. Holmgreen for help with Westerns, S. Mihajlovic and E. Tsui for preparation of tissue sections, L. Di Rago and A. D’Amico for assistance with bone marrow colony assays, A. O’Connor and S. Hayward for LH and FSH assays, E. Rames for technical assistance, and T. Gibbs and J. DeWinter for mouse management. Reagents for LH and FSH assays were kindly provided by the National Hormone and Pituitary Program, USA. C.P. is supported by postdoctoral fellowships from the New Zealand Health Research Council and Australian National Health and Medical Research Council (NHMRC, Reg. Key 977171). This research was supported by the NHMRC (Reg. Keys 973002 and 973218), the U.S. National Cancer Institute (CA43540), and the Howard Hughes Medical Institute (75193-531101).
ABBREVIATIONS
- neor
neomycin phosphotransferase gene
- PGK
phosphoglycerate kinase
- tk
thymidine kinase
- ES
embryonic stem
- B6
C57BL/6J
- Cre
Cre recombinase
- TUNEL
terminal transferase-mediated dUTP nick end-labeling
- CSF
colony-stimulating factor
- FSH
follicle-stimulating hormone
- LH
luteinizing hormone
- wt
wild type
References
- 1. Strasser A, Huang D C S, Vaux D L. Biochim Biophys Acta. 1997;1333:F151–F178. doi: 10.1016/s0304-419x(97)00019-x. [DOI] [PubMed] [Google Scholar]
- 2.Adams, J. M. & Cory, S. (1998) Science, in press.
- 3.Chao D T, Korsmeyer S J. Annu Rev Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 4.Oltvai Z N, Milliman C L, Korsmeyer S J. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 5.Pan G H, O’Rourke K, Dixit V M. J Biol Chem. 1998;273:5841–5845. doi: 10.1074/jbc.273.10.5841. [DOI] [PubMed] [Google Scholar]
- 6.Chinnaiyan A M, O’Rourke K, Lane B R, Dixit V M. Science. 1997;275:1122–1126. doi: 10.1126/science.275.5303.1122. [DOI] [PubMed] [Google Scholar]
- 7.Susin S A, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, Daugas E, Geuskens M, Kroemer G. J Exp Med. 1996;184:1331–1341. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J, Liu X S, Bhalla K, Kim C N, Ibrado A M, Cai J Y, Peng T I, Jones D P, Wang X D. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 9.Kluck R M, Bossy-Wetzel E, Green D R, Newmeyer D D. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 10.Veis D J, Sorenson C M, Shutter J R, Korsmeyer S J. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 11.Kamada S, Shimono A, Shinto Y, Tsujimura T, Takahashi T, Noda T, Kitamura Y, Kondoh H, Tsujimoto Y. Cancer Res. 1995;55:354–359. [PubMed] [Google Scholar]
- 12.Nakayama K-I, Nakayama K, Izumi N, Kulda K, Shinkai Y, Louie M C, Fields L E, Lucas P J, Stewart V, Alt F W, et al. Science. 1993;261:1884–1888. doi: 10.1126/science.8372353. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama K, Nakayama K-I, Negishi I, Kuida K, Sawa H, Loh D Y. Proc Natl Acad Sci USA. 1994;91:3700–3704. doi: 10.1073/pnas.91.9.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amling M, Neff L, Tanaka S, Inoue D, Kuida K, Weir E, Philbrick W M, Broadus A E, Baron R. J Cell Biol. 1997;136:205–213. doi: 10.1083/jcb.136.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michaelidis T M, Sendtner M, Cooper J D, Airaksinen M S, Holtmann B, Meyer M, Thoenen H. Neuron. 1996;17:75–89. doi: 10.1016/s0896-6273(00)80282-2. [DOI] [PubMed] [Google Scholar]
- 16.Motoyama N, Wang F P, Roth K A, Sawa H, Nakayama K, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S, et al. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- 17.Gibson L, Holmgreen S, Huang D C S, Bernard O, Copeland N G, Jenkins N A, Sutherland G R, Baker E, Adams J M, Cory S. Oncogene. 1996;13:665–675. [PubMed] [Google Scholar]
- 18.Ross A J, Waymire K G, Moss J E, Parlow A F, Skinner M K, Russell L D, MacGregor G R. Nat Genet. 1998;18:251–256. doi: 10.1038/ng0398-251. [DOI] [PubMed] [Google Scholar]
- 19.Knudson C M, Tung K S K, Tourtellotte W G, Brown G A J, Korsmeyer S J. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez I, Ody C, Araki K, Garcia I, Vassalli P. EMBO J. 1997;16:2262–2270. doi: 10.1093/emboj/16.9.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furuchi T, Masuko K, Nishimune Y, Obinata M, Matsui Y. Development (Cambridge, UK) 1996;122:1703–1709. doi: 10.1242/dev.122.6.1703. [DOI] [PubMed] [Google Scholar]
- 22.Koentgen F, Grumont R, Strasser A, Metcalf D, Li R, Tarlington D, Gerondakis S. Genes Dev. 1995;9:1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- 23.Mansour S L, Thomas K R, Capecchi M R. Nature (London) 1988;336:348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- 24.Barnett L, Koentgen F. Gene Knockout Protocols. Totowa, NJ: Humana; 1998. , in press. [Google Scholar]
- 25.Lakso M, Pichel J G, Gorman J R, Sauer B, Okamoto Y, Lee E, Alt F W, Westphal H. Proc Natl Acad Sci USA. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hudson D J. J Am Stat Assoc. 1966;61:1097–1129. [Google Scholar]
- 27.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 28.Gorczyca W, Gong J, Darzynkiewicz Z. Cancer Res. 1993;53:1945–1951. [PubMed] [Google Scholar]
- 29.Unkeless J C. J Exp Med. 1979;150:580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strasser A, Harris A W, Cory S. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 31.Metcalf D. The Hemopoietic Colony Stimulating Factors. Amsterdam: Elsevier Science; 1984. [Google Scholar]
- 32.Wreford N. Microsc Res Tech. 1995;32:423–436. doi: 10.1002/jemt.1070320505. [DOI] [PubMed] [Google Scholar]
- 33.Meinhardt A, O’Bryan M K, McFarlane J R, Loveland K L, Mallidis C, Foulds L M, Phillips D J, de Kretser D M. J Reprod Fertil. 1998;112:233–241. doi: 10.1530/jrf.0.1120233. [DOI] [PubMed] [Google Scholar]
- 34.Lee V W K, de Kretser D M, Hudson B, Wang C. J Reprod Fertil. 1975;42:121–126. doi: 10.1530/jrf.0.0420121. [DOI] [PubMed] [Google Scholar]
- 35.Olson E N, Arnold H-H, Rigby P W J, Wold B J. Cell. 1996;85:1–4. doi: 10.1016/s0092-8674(00)81073-9. [DOI] [PubMed] [Google Scholar]
- 36.Lee Y J, Lee J, Yang I C, Hahn Y, Lee Y, Chung J H. Biochim Biophys Acta. 1998;1395:40–46. doi: 10.1016/s0167-4781(97)00147-4. [DOI] [PubMed] [Google Scholar]
- 37.Vairo G, Innes K M, Adams J M. Oncogene. 1996;13:1511–1519. [PubMed] [Google Scholar]
- 38.O’Reilly L, Huang D C S, Strasser A. EMBO J. 1996;15:6979–6990. [PMC free article] [PubMed] [Google Scholar]
- 39.Russell L D, Ettlin R A, Hikim A P S, Clegg E D. Histological and Histopathological Evaluation of the Testes. Clearwater, FL: Cache River Press; 1990. [Google Scholar]
- 40.Billig H, Furuta I, Rivier C, Tapanainen J, Parvinen M, Hsueh J W. Endocrinology. 1995;136:5–12. doi: 10.1210/endo.136.1.7828558. [DOI] [PubMed] [Google Scholar]
- 41.Krajewski S, Krajewska M, Shabaik A, Wang H G, Irie S, Fong L, Reed J C. Cancer Res. 1994;54:5501–5507. [PubMed] [Google Scholar]
- 42.Hockenbery D M, Zutter M, Hickey W, Nahm M, Korsmeyer S. Proc Natl Acad Sci USA. 1991;88:6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merry D E, Veis D J, Hickey W F, Korsmeyer S J. Development (Cambridge) 1994;120:301–311. doi: 10.1242/dev.120.2.301. [DOI] [PubMed] [Google Scholar]
- 44.Krajewski S, Bodrug S, Krajewska M, Shabaik A, Gascoyne R, Berean K, Reed J C. Am J Path. 1995;146:1309–1319. [PMC free article] [PubMed] [Google Scholar]
- 45.Lin E Y, Orlofsky A, Berger M S, Prystowsky M B. J Immunol. 1993;151:1979–1988. [PubMed] [Google Scholar]
- 46.Krajewski S, Krajewska M, Reed J C. Cancer Res. 1996;56:2849–2855. [PubMed] [Google Scholar]
- 47.Orth J M, Gunsalus G L, Lamperti A A. Endocrinology. 1988;122:787–794. doi: 10.1210/endo-122-3-787. [DOI] [PubMed] [Google Scholar]
- 48.Lee J, Richburg L J, Younkin J H, Boekelheide K. Endocrinology. 1997;138:2018–2088. doi: 10.1210/endo.138.5.5110. [DOI] [PubMed] [Google Scholar]
- 49.de Kretser D M. Int Rev Cytol. 1987;109:89–112. doi: 10.1016/s0074-7696(08)61720-9. [DOI] [PubMed] [Google Scholar]