Abstract
Naturally arising CD4+ regulatory T cells, which engage in the maintenance of immunologic self-tolerance, specifically express FOXP3, which encodes a transcription-repressor protein. Genetic defects in FOXP3 cause IPEX, an X-linked autoimmune/inflammatory syndrome. With FOXP3 as a specific marker for regulatory CD4+ T cells in humans, it is now possible to determine their origin and developmental pathway .
The immune system discriminates between self and non-self, maintaining immunologic self-tolerance (i.e., unresponsiveness to self-constituents). It is known that potentially hazardous self-reactive T and B cells are clonally deleted at immature stages of their development or inactivated upon encounter with self-antigens in the periphery. There is now accumulating evidence that, in addition to these passive mechanisms of self-tolerance, a population of CD4+ T cells, called regulatory T cells (TR cells), engage in the maintenance of peripheral self-tolerance by actively suppressing the activation and expansion of self-reactive T cells (1–3). The majority, if not all, of such naturally occurring CD4+ TR cells constitutively express CD25 (IL-2 receptor α chain) in the physiologic state. Indeed, removal of CD25+CD4+ T cells, which constitute 5–10% of CD4+ T cells in rodents and humans, leads to spontaneous development of various autoimmune diseases in otherwise normal mice (4). The removal of CD25+CD4+ TR cells also triggers excessive or misdirected immune responses to microbial antigens, causing immunopathology, such as inflammatory bowel disease (IBD), due to hyper-reaction of the remaining T cells to commensal bacteria in the intestine (3).
FOXP3: master control gene for the development and function of natural CD4+ TR cells
There is now evidence not only for the presence of CD25+CD4+ TR cells in humans but also for their essential roles in controlling autoimmunity, immunopathology, and allergy in human diseases (5). This is best illustrated by IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome), a rare monogenic disease of male children that is accompanied by autoimmune disease (such as type 1 diabetes), IBD, and severe allergy similar to those produced in mice by depletion of CD25+CD4+ TR cells (6). The causative gene, FOXP3 (Foxp3 in mice), which encodes a transcription repressor (7–10), is specifically expressed in CD25+CD4+ T cells in the thymus and periphery (11–13). Forced expression of the Foxp3 gene can convert murine naive T cells to TR cells that phenotypically and functionally resemble naturally arising CD25+CD4+ TR cells (11, 12). Furthermore, inoculation of CD25+CD4+ T cells prepared from normal mice can prevent autoimmune disease in Foxp3-defective mice (12). These findings collectively indicate that FOXP3 is a master control gene for the development and function of natural CD25+CD4+ TR cells.
The origin and the developmental pathway of FOXP3-expressing TR cells
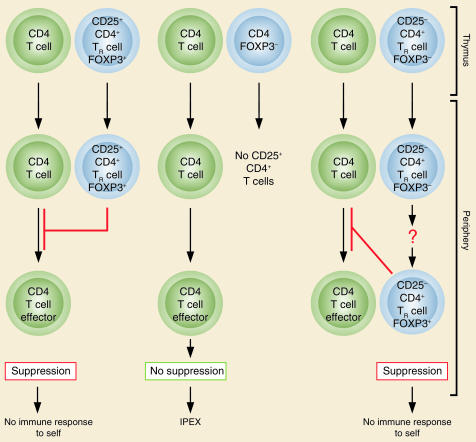
The discovery of FOXP3/Foxp3 as a specific and stable marker for natural TR cells now makes it possible to determine the origin and the developmental pathway of TR cells in humans, as reported by Walker et al. in this issue of the JCI (14). It has been shown, mainly in rodents, that the normal thymus continuously produces CD25+CD4+ TR cells as a functionally mature T cell subpopulation that recognizes a broad repertoire of self- and non-self antigens, and that abrogation of the thymic production of TR cells leads to the development of autoimmune disease (1–3). Walker et al. (14) show that CD25+CD4+ T cells in the peripheral blood lymphocytes express FOXP3 and are capable of suppressing the activation and expansion of other T cells in vitro, as shown in rodents (11–13). Furthermore, they show that, in contrast with murine Foxp3 expression, activation of CD25–CD4+ T cells by T cell receptor (TCR) stimulation induces FOXP3 expression, and that FOXP3-expressing T cells derived from CD25–CD4+ T cells are equally as suppressive as natural CD25+CD4+ TR cells (Figure 1) (14). This interesting finding suggests two possibilities regarding the origin of CD25+CD4+ TR cells. One is that naive T cells can differentiate to CD25+CD4+ TR cells upon TCR stimulation, in a manner similar to that in which the expression of the transcription factors T-bet and GATA-3 instruct naive T cells to differentiate to Th1 and Th2 cells, respectively (15, 16). Another possibility is that some of the functionally mature TR cells produced by the thymus are CD25– or lose CD25 expression with retention of their suppressive function, as has been shown in rodents (17–19). Such CD25– TR cells may become CD25+ upon activation, especially when other T cells respond to antigen stimulation, and IL-2 secreted by them may trigger the expansion of TR cells. Given the specific expression of FOXP3 in TR cells whether they are of thymic or peripheral origin, it remains to be determined whether other T cells with regulatory functions, such as IL-10–secreting Tr1 or TGF-β–secreting Th3 cells, may also express FOXP3 (20).
Figure 1.
The normal thymus produces FOXP3-expressing CD25+CD4+ TR cells. Some of the naive CD25–CD4+ T cells may also differentiate to FOXP3-expressing CD25+CD4+ TR cells in the periphery. These TR cells suppress the activation and expansion of self-reactive T cells that may cause autoimmune disease. Genetic defects of FOXP3 cause IPEX due to developmental or functional defects of TR cells. Adapted with permission from Nature Immunology (21).
Besides self-tolerance and autoimmunity, evidence is now accumulating that natural CD4+ TR cells actively engage in negative control of a broad spectrum of immune responses to quasi-self or non-self antigens, as in tumor immunity, organ transplantation, allergy, and microbial immunity (1–3). With FOXP3 as a useful tool for investigating TR cells, further characterization of their developmental pathways will facilitate better control of pathologic as well as physiologic immune responses by expansion or reduction of TR cell populations.
Footnotes
See the related article beginning on page 1437.
Conflict of interest: The author has declared that no conflict of interest exists.
Nonstandard abbreviations used: regulatory T (TR); inflammatory bowel disease (IBD); T cell receptor (TCR).
References
- 1.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 2.Shevach EM. Regulatory T cells in autoimmunity. Annu. Rev. Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 3.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat. Immunol. 2001;2:816–822. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25): breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 5.Shevach EM. Certified professionals: CD4+CD25+ suppressor T cells. J. Exp. Med. 2001;193:F41–F46. doi: 10.1084/jem.193.11.f41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr. Opin. Rheumatol. 2003;15:430–435. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Brunkow ME, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 8.Chatila TA, et al. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J. Clin. Invest. 2000;106:R75–R81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wildin RS, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 10.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 11.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 12.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 13.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 14.Walker MR, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25– T cells. J. Clin. Invest. 2003;112:1437–1443. doi:10.1172/JCI200319441. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorstenson KM, Khoruts A. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J. Immunol. 2001;167:188–195. doi: 10.4049/jimmunol.167.1.188. [DOI] [PubMed] [Google Scholar]
- 16.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat. Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 17.Annacker O, Burlen-Defranoux O, Pimenta-Araujo R, Cumano A, Bandeira A. Regulatory CD4 T cells control the size of the peripheral activated/memory CD4 T cell compartment. J. Immunol. 2000;164:3573–3580. doi: 10.4049/jimmunol.164.7.3573. [DOI] [PubMed] [Google Scholar]
- 18.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4+CD25+ suppressor T cells in vivo. Nat. Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 19.Stephens LA, Mason D. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and CD25– subpopulations. J. Immunol. 2000;165:3105–3110. doi: 10.4049/jimmunol.165.6.3105. [DOI] [PubMed] [Google Scholar]
- 20.Levings MK, et al. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor beta, but not interleukin 10, and are distinct from type 1 T regulatory cells. J. Exp. Med. 2002;196:1335–1346. doi: 10.1084/jem.20021139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Garra A, Vieira P, et al. Twenty-first century Foxp3. Nat. Immunol. 2003;4:304–306. doi: 10.1038/ni0403-304. [DOI] [PubMed] [Google Scholar]