Abstract
Transmissible spongiform encephalopathies (TSEs) are fatal, untreatable neurodegenerative diseases associated with the accumulation of a disease-specific form of prion protein (PrP) in the brain. One approach to TSE therapeutics is the inhibition of PrP accumulation. Indeed, many inhibitors of the accumulation of PrP associated with scrapie (PrPSc) in scrapie-infected mouse neuroblastoma cells (ScN2a) also have antiscrapie activity in rodents. To expedite the search for potential TSE therapeutic agents, we have developed a high-throughput screening assay for PrPSc inhibitors using ScN2a cells in a 96-well format. A library of 2,000 drugs and natural products was screened in ScN2a cells infected with scrapie strain RML (Chandler) or 22L. Forty compounds were found to have concentrations causing 50% inhibition (IC50s) of PrPSc accumulation of ≤10 μM against both strains. Seventeen had IC50s of ≤1 μM against both strains. Several classes of compounds were represented in the 17 most potent inhibitors, including naturally occurring polyphenols (e.g., tannic acid and tea extracts), phenothiazines, antihistamines, statins, and antimalarial compounds. These 17 compounds were also evaluated in a solid-phase cell-free hamster PrP conversion assay. Only the polyphenols inhibited the cell-free reaction, and their IC50s were near 100 nM. Several of the new PrPSc inhibitors cross the blood-brain barrier and thus have potential to be effective after TSE infection reaches the brain. The fact that many are either approved human drugs or edible natural products should facilitate their use in animal testing and clinical trials.
Transmissible spongiform encephalopathies (TSEs) are neurodegenerative diseases that include Creutzfeldt-Jakob disease, chronic wasting disease, scrapie, and bovine spongiform encephalopathy. These diseases are characterized by the accumulation of a form of prion protein (PrP) that is partially resistant to degradation by proteases (23). The infectious TSE agent is not fully understood but is surmised to contain the proteinase K (PK)-resistant aggregate of PrP (8). The occurrence of TSEs is associated with specific mutations in PrP, inoculation with infectious material, or apparently spontaneous onset (23). Currently, there are no therapies for TSEs, and the diseases are invariably fatal. Thus, it is important to identify compounds with therapeutic or prophylactic activity against these diseases.
The conversion of PrP from the normal, protease-sensitive, and nonaggregated form (PrPC) to the aggregated and protease-resistant form associated with scrapie (PrPSc) or other TSEs (PrPTSE) is a hallmark of the diseases. While the mechanism of neurodegeneration in TSEs is not clear, interactions between PrPC and PrPTSE seem to be important in the pathology of TSEs. Thus, the prevention of PrPTSE formation and/or the elimination of existing PrPTSE may be therapeutic (14, 22, 29).
Chronically scrapie-infected neuroblastoma cells (ScN2a) have been used extensively as a model for studying TSEs (1). The cells produce PrPSc, permitting cellular processes associated with PrPSc production to be examined. ScN2a cells have been used to study the effect of PrP mutations (16, 30), barriers to interspecies transmission (21, 25), PrP metabolism (5), and inhibitors of PrPSc formation (11). To expedite the screening of compounds for anti-PrPSc activity in cell cultures, slot blot and dot blot assays have been developed (24, 31). Many different types of compounds, such as sulfonated dyes (9), sulfated glycans (4), cyclic tetrapyrroles (7), polyene antibiotics (18), curcumin (6), lysosomotropic antimalarial compounds (11), phenothiazines (17), and polyamines (27), can inhibit PrPSc formation when added to the medium of these cells. In addition, several of these classes of inhibitors have prolonged the survival time of scrapie-infected animals when administered near the time of infection (3, 10, 12, 15, 22). Thus, ScN2a cells provide a useful in vitro model for screening compounds for anti-TSE activity.
In the present study, we screened a commercially available library of drugs and natural products to find new candidates for therapeutic intervention against TSEs. The inhibition of PrPSc production was monitored in ScN2a cells infected with scrapie strain RML (Chandler) (4) or 22L. PrPSc from cells plated in a 96-well format was assayed with a modification of the dot blot method of Rudyk et al. (24). Of the 2,000 compounds screened, 17 had concentrations causing 50% inhibition (IC50s) of PrPSc accumulation of ≤1 μM against the RML and 22L strains. A number of these candidates are used for other indications in humans and would therefore be available for immediate clinical trials.
MATERIALS AND METHODS
Compound library.
The library tested was The Spectrum Collection (MicroSource Discovery Inc., Groton, Conn.). The 2,000 compounds in the library are primarily Food and Drug Administration (FDA)-approved compounds or natural products. An alphabetical list of the compounds is available at the MicroSource Discovery website at www.msdiscovery.com/spect.html. The compounds are supplied as 10 mM solutions in dimethyl sulfoxide (DMSO).
Testing for PrPSc inhibitory activity in cell cultures.
Approximately 20,000 RML (4)-infected or 22L-infected mouse neuroblastoma cells in 100 μl of medium were added to each well of a Costar 3595 flat-bottom 96-well plate with a low-evaporation lid (Corning Inc., Corning, N.Y.) prior to the addition of test compounds. 22L-infected cells were developed by reinfection of RML-infected mouse neuroblastoma cells cured by seven passages in 1 μg of pentosan polysulfate/ml of medium (2). The cured cells were reinfected by incubation with PrPSc purified from mouse brains infected with scrapie strain 22L. Others have reported the susceptibility of mouse neuroblastoma cells to 22L infection (20). Neuroblastoma cells reinfected with 22L have stably expressed PrPSc for over 100 passages. The cells were allowed to settle for 4 h before test compounds were added.
The 10 mM solutions of test compounds were diluted in DMSO and then in phosphate-buffered saline (PBS) prior to being introduced to the cell medium. Five-microliter solutions were added to the cell medium. DMSO concentrations in the cell medium were never higher than 0.5% (vol/vol). After a compound was added, the cells were incubated for 5 days at 37°C in a CO2 incubator before being lysed.
Prior to cell lysis, the cells were inspected by light microscopy for toxicity, bacterial contamination, and density compared to controls. After removal of the cell medium, 50 μl of lysis buffer was added to each well. Lysis buffer was composed of 0.5% (wt/vol) Triton X-100, 0.5% (wt/vol) sodium deoxycholate, 5 mM Tris-HCl (pH 7.4 at 4°C), 5 mM EDTA, and 150 mM NaCl. At 5 min after the addition of lysis buffer, 25 μl of PK (0.1 mg/ml; Calbiochem) in Tris-buffered saline (TBS) was added to each well and incubated at 37°C for 50 min. A total of 225 μl of 1 mM Pefabloc (Boehringer Mannheim) was added to each well to inhibit PK activity. A total of 250 μl of 1 mM Pefabloc was added to samples that were not PK treated.
High-throughput measurement of PrPSc by a dot blot procedure.
The dot blot procedure used is a streamlined version of that developed by Rudyk et al. (24). A 96-well dot blot apparatus (Schleicher & Schuell) was set up with a 0.45-μm-pore-size polyvinylidene difluoride (PVDF) membrane (Immobilon-P; Millipore), and each dot was rinsed with 500 μl of TBS. Under vacuum, the lysed and PK-treated samples were added to the apparatus over the PVDF membrane and rinsed with 500 μl of TBS. The PVDF membrane was removed and covered with 3 M GdnSCN (Fluka) for 10 min at ambient temperature. GdnSCN was removed by five PBS rinses, and the membrane was blocked with 5% (wt/vol) milk-0.05% (vol/vol) Tween 20 (Sigma) in TBS (TBST-milk) for 30 min. An appropriate dilution of monoclonal antibody 6B10, an immunoglobulin G2a antibody reactive against mouse, hamster, elk, and sheep PrP in immunoblotting assays and enzyme-linked immunosorbent assays (data not shown), or 8 μg of purified anti-PrP mouse monoclonal antibody 6H4 (Prionics) in 15 ml of TBST-milk was incubated with the membrane for 60 min. After TBST rinsing, a solution of ∼500 ng of an alkaline phosphatase-conjugated goat anti-mouse antibody (Zymed) in 15 ml of TBST-milk was added and incubated for 45 min. After additional TBST rinsing, the membrane was treated with an enhanced chemifluorescence agent (Amersham) for 10 min, allowed to dry, and then scanned with a Storm Scanner (Molecular Dynamics). The intensity of the PrPSc signal from each well was quantitated by using ImageQuant software (Molecular Dynamics). Each 96-well plate had six untreated control wells and six wells treated with curcumin, a known PrPSc inhibitor in RML-infected ScN2a cells (6).
Solid-phase PrP conversion assay.
In brief, for the solid-phase PrP conversion assay (18), a 100-ng suspension of hamster scrapie strain 263K PrPSc in 40 μl of PBS was added to wells of a 96-well plate and air dried to promote adherence of the protein to the surface. The wells were then blocked with 2% bovine serum albumin in PBS. This solution was removed, and another solution, containing ∼20,000 cpm of hamster 35S-labeled PrPC with or without potential inhibitors, was added and incubated at 37°C for 48 h. The 35S-labeled PrPC solution was removed, and the wells were washed. PK (20 μg/ml) was added to the wells and then removed after 1 h to digest unconverted but bound 35S-labeled PrPC. The protein in the wells was eluted by boiling in sodium dodecyl sulfate sample buffer and scintillation counted. To obtain the relative percent conversion, the measured counts in PrPSc wells less the counts in bovine serum albumin-blocked wells lacking PrPSc were compared to the total 35S-labeled PrPC counts added to the wells.
RESULTS
High-throughput screen for PrPSc inhibitors.
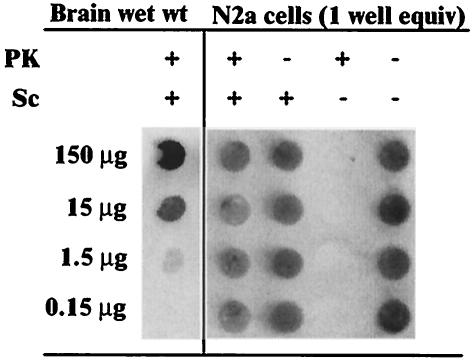
To facilitate the screening of large numbers of compounds for the inhibition of PrPSc accumulation, we developed a high-throughput test using ScN2a cell cultures in combination with a rapid dot blot assay for PrPSc (scrapie cell dot blot [SCDB] assay). PrPSc from one well of ScN2a cells in a 96-well plate was readily detectable by the SCDB assay (Fig. 1). Without PK treatment, PrPC from uninfected N2a cells was also readily detectable, but PK treatment eliminated this signal. Dilutions of scrapie-infected brain homogenates indicated that the PrPSc signal intensity from one well of cells fell between that from samples with 1.5 and 15 μg of brain wet-weight equivalents (Fig. 1) in a linear response range of the PrPSc SCDB assay (data not shown). Similar results were obtained with anti-PrP monoclonal antibodies 6H4 (Fig. 1) and 6B10 (data not shown). A typical dot blot from the SCDB assay with antibody 6B10 is shown in Fig. 2.
FIG. 1.
Dot blot of brain-derived PrPSc and ScN2a cell-derived PrPC and PrPSc. The wells shown are from a single membrane visualized with primary antibody 6H4. The samples in the first lane contain the indicated brain wet-weight (wet wt) equivalents in a lysate from a hamster clinically ill from infection with scrapie strain 263K. The second and third lanes from the left contain lysates from RML-infected ScN2a cells (one well equivalent). The fourth and fifth lanes contain lysates from uninfected N2a cells. PrPC from uninfected cells was detected without any PK treatment.
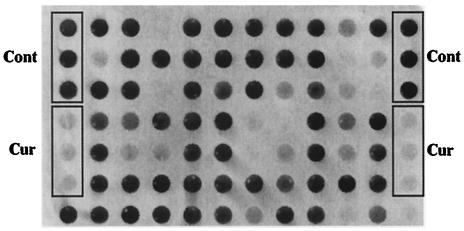
FIG. 2.
Partial 96-well dot blot showing the PK-resistant PrP signal visualized with primary antibody 6B10. Signals from untreated control (Cont) cells and curcumin-inhibited (Cur) cells are indicated. The latter were incubated in the presence of 10 μM curcumin, a known inhibitor of PrPSc in RML-infected cells (6). Other dots represent signals from ScN2a cells after incubation with 10 μM concentrations of various compounds. Some of these spots have an intensity comparable to that of controls, indicating no inhibition of PrPSc formation. Others that are less intense were due to compounds with various inhibitory strengths or toxicities.
Screening of a 2,000-compound library.
The Spectrum Collection, a library of 2,000 drugs and natural products, was screened for PrPSc inhibitory activity with the SCDB assay. The identities of the compounds were not revealed to the investigator until screening was completed. A flowchart of the screening sequence is shown in Fig. 3. The compounds were screened initially at 10 μM against RML-infected cells. Approximately 70% of the compounds showed less than 50% inhibition of PrPSc formation at 10 μM in these cells and were not evaluated further. Approximately 20% (398) of the compounds were cytotoxic at 10 μM and were tested again at 1 μM. A smaller group of 246 compounds inhibited RML PrPSc accumulation by more than 50% at 10 μM without observed toxicity. These 246 compounds were tested further at 10 μM in ScN2a cells infected with scrapie strain 22L, and 40 of them were found to reduce PrPSc accumulation by ≥50% (35 compounds with IC50s of between 1 and 10 μM are shown in Fig. 4). These 40 compounds were then tested at 1 μM against both RML- and 22L-infected cells, revealing 5 compounds with IC50s of ≤1 μM against both strains. Twelve additional inhibitors fitting these criteria were discovered when the 398 compounds cytotoxic at 10 μM were tested at 1 μM against both RML- and 22L-infected cells. Thus, of the 2,000 compounds screened, 17 had an IC50 of ≤1 μM against both scrapie strains without observed toxicity (Fig. 5).
FIG. 3.
Flowchart of the screening of The Spectrum Collection compound library.
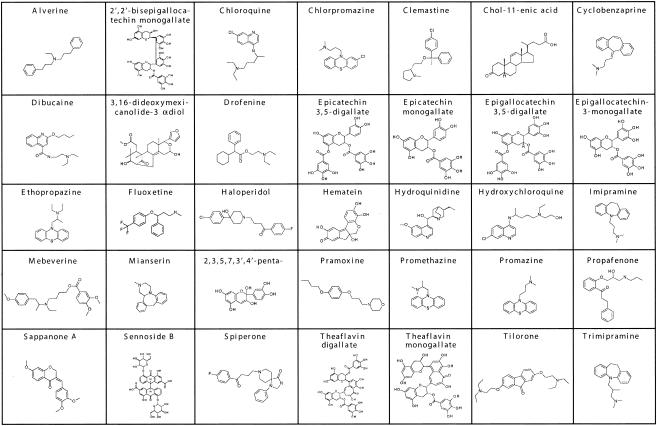
FIG. 4.
Structures of compounds in The Spectrum Collection with IC50s of >1 and ≤10 μM against both the RML and 22L scrapie strains, listed in approximate alphabetical order. 2,3,5,7,3′,4′-penta-, 2,3,5,7,3′,4′-pentahydroxyflavan.
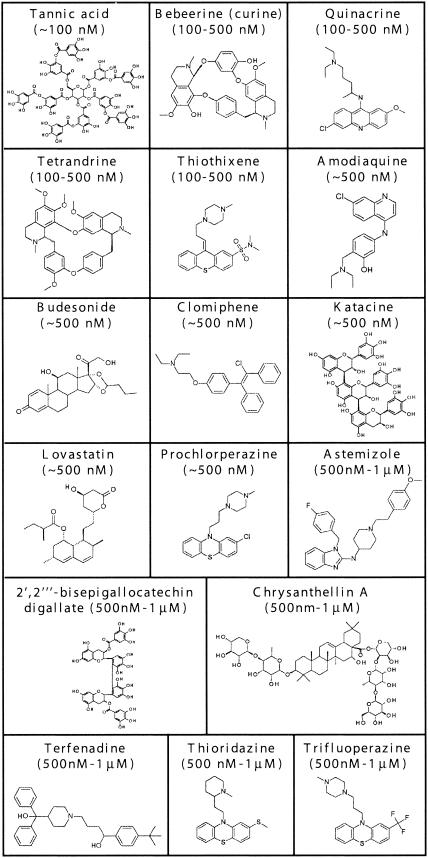
FIG. 5.
Structures of compounds in The Spectrum Collection with IC50s of ≤1 μM against both the RML and 22L strains of scrapie. Compounds are arranged from low to high approximate IC50s.
For compounds to pass through the screen described in Fig. 3, the cells had to grow from low density to confluence and thrive in the presence of test compounds. However, as an additional test for potential cytotoxicity, a 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) cell viability assay was performed with the 17 most potent inhibitors (19). The assay was done in duplicate after 3 and 4 days of incubation with the 17 compounds at 1 μM. The percentage of cell viability with all compounds tested in the MTT assay was in the range of 90 to 129% of controls on both days. In contrast, with aklavine and celastrol, which had visually obvious cytotoxicity, 2 to 9% cell viability was obtained on both days. The inhibition of PrPSc accumulation by these 17 compounds in the SCDB assay was confirmed in six separate experiments. Subsequent testing of these 17 compounds at 500, 100, and 10 nM revealed that tannic acid had an IC50 of ∼100 nM, whereas the other 16 compounds had IC50s of between 100 nM and 1 μM (Fig. 5).
Of the 17 most potent inhibitors in The Spectrum Collection that were active against both scrapie strains, two, quinacrine and lovastatin, were identified previously as PrPSc inhibitors (11, 28). The remaining 15 compounds are novel inhibitors representing multiple classes of drugs or natural products, including polyphenols (e.g., tea and tree gall extracts), antimalarial compounds, antihistamines, phenothiazine analogs (e.g., antipsychotics), statins (hepatic hydroxymethyl glutaryl coenzyme A reductase inhibitors), and others, as indicated in Fig. 5.
Test of inhibition of cell-free PrP conversion.
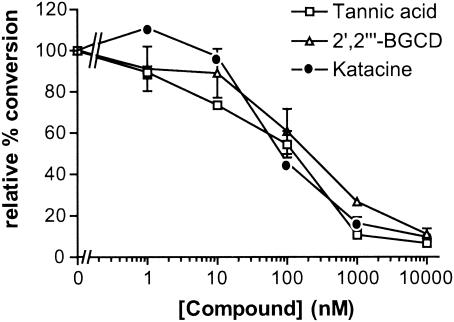
To test for direct effects on PrP conversion, the 17 most potent inhibitors were added to a solid-phase cell-free conversion (SP-CFC) reaction in which hamster PrPSc is used to induce the conversion of radiolabeled hamster PrPC to a PrPSc-like PK-resistant state (18a). Three polyphenols, tannic acid, katacine, and 2′,2′"-bisepigallocatechin digallate, inhibited the SP-CFC reaction, with an IC50 of approximately 100 nM (Fig. 6). The other 14 compounds were not inhibitory in the SP-CFC reaction at concentrations up to 100 μM (data not shown).
FIG. 6.
Inhibition of solid-phase cell-free PrP conversion by polyphenols. The conversion relative to that in control reactions is plotted against the concentration of polyphenol added to the reaction. 2′,2′"-BGCD, 2′,2′"-bisepigallocatechin digallate.
Test for destabilization of preexisting PrPSc.
To search for compounds that can destabilize preexisting PrPSc, compounds with IC50s of ≤10 μM were incubated at 250 μM with ScN2a cell lysates for 24 h at 37°C to determine whether they could increase the PK sensitivity of PrPSc. However, even at a concentration at least 25 times its IC50, no compound was able to increase the PK sensitivity of PrPSc (data not shown).
DISCUSSION
The high-throughput SCDB assay has greatly expedited our search for new, potentially therapeutic inhibitors of PrPSc accumulation. Clearly, one cannot expect that all compounds selected as potent inhibitors with this in vitro screen will prove to be effective anti-TSE drugs in vivo. However, given that a majority of the different classes of compounds that were previously identified as potent inhibitors by use of RML-infected ScN2a cells have prophylactic efficacy against scrapie in vivo, we expect the SCDB assay will be valuable in the initial screening of potential drugs from large compound libraries. The additional use of ScN2a cells infected with the 22L strain of scrapie in the SCDB assay helps to identify compounds with broader, less strain-dependent inhibitory activity. Adaptations of the SCDB assay for use with TSE-infected cell cultures from other species should increase the value of the assay for predicting efficacy against TSE diseases of humans and livestock.
In our screening of the 2,000 compounds of The Spectrum Collection, both new and old inhibitors were identified. Of the 17 most potent inhibitors in the library with activity against the RML and 22L mouse scrapie strains (Fig. 5), 15 were new, whereas quinacrine and lovastatin were already known as PrPSc inhibitors in scrapie-infected cell cultures (11, 28). Other previously identified inhibitors, such as chloroquine (11) and promazine, promethazine, and chlorpromazine (17), also inhibited PrPSc accumulation in the SCDB assay screening (Fig. 4) but were not among the 17 most potent and strain-independent compounds in the library. The fact that several previously known inhibitors were selected by our blind screening of a large compound library inspires confidence in the utility of the SCDB assay.
Polyphenol inhibitors.
Numerous polyphenols were selected as PrPSc inhibitors against both strains of mouse scrapie in the SCDB assay. Tannin (tannic acid), the most potent inhibitor found, is a relatively nontoxic constituent of foods such as tea, red wine, beer, and nuts. 2′,2′"-Bisepigallocatechin digallate is also a component of tea, and katacine is another naturally occurring polyphenol antioxidant. Relatively few studies have been done on the bioavailability of the polyphenols from tea extracts, but significant oral absorption has been shown in humans (32). While at first glance these water-soluble compounds might not be considered likely to cross the blood-brain barrier, radiolabeled epigallocatechin gallate, another tea extract polyphenol, has been detected in mouse brains after oral administration (26). A number of other polyphenols, including epigallocatechin 3,5-digallate and epicatechin monogallate, were included in the group with IC50s of between 1 and 10 μM (Fig. 4). The naturally occurring polyphenols represent a part of the normal human diet and are relatively nontoxic. Even if the ability of these compounds to cross the blood-brain barrier is questionable, they may be useful as prophylactic agents against peripheral infections or as TSE decontaminants.
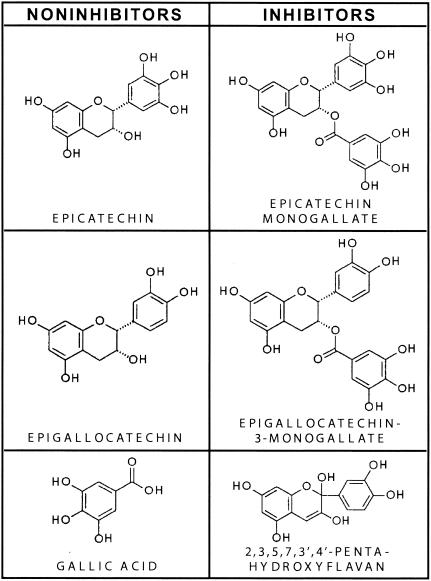
Not all polyphenols tested were PrPSc inhibitors. Epicatechin and epigallocatechin, with molecular weights of about 300, were ineffective, although they represent portions of larger polyphenol molecules that were effective, such as epigallocatechin 3,5-digallate. While most of the polyphenol inhibitors were larger than 350 Da, a similar polyphenol with a molecular weight of 304, 2,3,5,7,3′,4′-pentahydroxyflavan, was an inhibitor. This molecule is more conjugated and planar than epicatechin (Fig. 7). Although these results indicate that minor structural differences can have dramatic effects on polyphenol efficacy, further study is needed to clarify the structure-activity relationships.
FIG. 7.
Structural comparisons of inhibitory and noninhibitory polyphenols. Epicatechin and epigallocatechin were not inhibitors until the addition of a gallate, which was not an inhibitor on its own. Compared to epicatechin, the inhibitor 2,3,5,7,3′,4′-pentahydroxyflavan has one additional conjugated double bond and an additional hydroxyl group. The double-ring system in the flavan should be more planar than the corresponding rings in epicatechin.
Malaria drugs.
Quinacrine and other antimalarial compounds have been reported to inhibit PrPSc formation in cell-based assays (11, 17). Quinacrine was reported to have an IC50 of 400 nM, in good agreement with our present results. However, quinacrine has not shown any long-term benefit against Creutzfeldt-Jakob disease in preliminary clinical trials in humans (13). Since drug bioavailability, transport, and metabolism can depend markedly on structural details, it is possible that the other antimalarial compounds that were identified in this study are more effective than quinacrine in vivo. For instance, amodiaquine and bebeerine were among the 17 most potent inhibitors. Amodiaquine is a 4-aminoquinoline analog of chloroquine that is currently used as an antimalarial drug, but it was a stronger inhibitor of PrPSc formation in the SCDB assay. Chloroquine had an IC50 between 1 and 10 μM, in good agreement with a previous study (11). The closely related compounds hydroxychloroquine and hydroquinidine had similar IC50s. Bebeerine (curine) is a bisbenzylisoquinoline alkaloid naturally produced from the root bark of Chondrondendron platyphyllum. Another naturally produced bisbenzylisoquinoline alkaloid, tetrandrine, was also a potent inhibitor. Although not known to be antimalarial, this compound is a nonselective Ca2+ channel blocker derived from a Chinese medicinal herb, Stephenia tetrandra S. Moore, and has been used to treat hypertension and autoimmune disorders in traditional Chinese medicine.
Antihistamines.
The antihistamines astemizole and terfenadine were both among the most potent PrPSc inhibitors. These compounds are known to be poor at crossing the blood-brain barrier, a fact which may limit their therapeutic usefulness against TSEs. These antihistamines have been used extensively in humans but are currently not marketed in the United States because of a concern for serious, but rare, cardiovascular toxicity and the availability of safer alternatives.
Phenothiazine derivatives and analogs.
The phenothiazine derivatives chlorpromazine, promazine, and promethazine inhibited PrPSc accumulation, in agreement with another study (17). However, our screen identified several more potent phenothiazine inhibitors, including the FDA-approved antipsychotics thioridazine, trifluoperazine, and prochlorperazine. The most potent group of 17 inhibitors identified in this study also included the FDA-approved antipsychotic thiothixene, which is a phenothiazine structural analog. These phenothiazine derivatives and analogs penetrate the blood-brain barrier, a feature that should be beneficial in treating TSEs.
Other inhibitors.
Lovastatin is an FDA-approved hepatic hydroxymethyl glutaryl coenzyme A reductase inhibitor that reduces blood cholesterol levels and is known to cross the blood-brain barrier. Its inhibition of PrPSc accumulation at 500 nM agrees with a previous study (28) and places it among the best inhibitors. Budesonide is a steroid derivative approved by the FDA to treat asthma, chrysanthellin A is a naturally produced steroidal glycoside, and clomiphene is the FDA-approved treatment of choice for anovulatory infertile women with polycystic ovary syndrome.
Inhibition of cell-free PrP conversion.
The SP-CFC reaction monitors direct hamster PrP interactions. Because there presumably are therapeutic targets besides PrP conversion for the TSEs in vivo, a compound could be effective in scrapie-infected cells and animals without being effective in the SP-CFC assay. For example, quinacrine was an effective PrPSc inhibitor in the SCDB assay but was not effective at inhibiting the SP-CFC reaction. Quinacrine is a lysosomotropic amine and may function by altering endosomal or lysosomal microenvironments (11). Another example is lovastatin, which is thought to inhibit PrPSc formation indirectly by depleting cellular cholesterol (28), consistent with its inability to block the SP-CFC reaction. Indeed, a majority of the 17 most potent inhibitors in the SCDB assay were unable to block the SP-CFC reaction. Another possible explanation for the discordance between the SCDB and SP-CFC assays is the species specificity of interactions with PrP isoforms. The SCDB and SP-CFC assays involve mouse and hamster PrP molecules, respectively. Regardless, the three polyphenols were potent inhibitors in both types of assays and thus appear to be direct inhibitors of PrP conversion. Although we do not anticipate that the screening of compound libraries with the SP-CFC assay alone would be as predictive of in vivo efficacy as the SCDB assay, we have shown that the cell-free assay can be used to obtain mechanistic insights into whether inhibitors identified in the SCDB assay act via direct or indirect mechanisms.
Conclusion.
This screening has identified new compounds and classes of compounds that are effective PrPSc inhibitors against two scrapie strains in cell cultures. The naturally occurring polyphenols were also effective inhibitors of cell-free PrP conversion. Barring hamster and mouse PrP species differences, these results suggest that the polyphenols inhibit PrPSc formation through direct PrP interactions, whereas the other inhibitors may work indirectly. Among the list of the 17 best inhibitors are FDA-approved compounds and dietary constituents that should be acceptable for testing in infected animals and humans. The fact that a number of the new inhibitors are known to cross the blood-brain barrier makes them attractive as potential anti-TSE therapeutic agents and distinguishes them from many previously identified PrPTSE inhibitors.
Acknowledgments
We are grateful to Lynne D. Raymond for technical assistance and Gary Hettrick for assistance developing the figures.
REFERENCES
- 1.Beranger, F., A. Mange, J. Solassol, and S. Lehmann. 2001. Cell culture models of transmissible spongiform encephalopathies. Biochem. Biophys. Res. Commun. 289:311-316. [DOI] [PubMed] [Google Scholar]
- 2.Birkett, C. R., R. M. Hennion, D. A. Bembridge, M. C. Clarke, A. Chree, M. E. Bruce, and C. J. Bostock. 2001. Scrapie strains maintain biological phenotypes on propagation in a cell line in culture. EMBO J. 20:3351-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caughey, B., and B. Chesebro. 2001. Transmissible spongiform encephalopathies and prion protein interconversions. Adv. Virus Res. 56:277-311. [DOI] [PubMed] [Google Scholar]
- 4.Caughey, B., and G. J. Raymond. 1993. Sulfated polyanion inhibition of scrapie-associated PrP accumulation in cultured cells. J. Virol. 67:643-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caughey, B., G. J. Raymond, D. Ernst, and R. E. Race. 1991. N-terminal truncation of the scrapie-associated form of PrP by lysosomal protease(s): implications regarding the site of conversion of PrP to the protease-resistant state. J. Virol. 65:6597-6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caughey, B., L. D. Raymond, G. J. Raymond, L. Maxson, J. Silveira, and G. S. Baron. 2003. Inhibition of protease-resistant prion protein accumulation in vitro by curcumin. J. Virol. 77:5499-5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caughey, W. S., L. D. Raymond, M. Horiuchi, and B. Caughey. 1998. Inhibition of protease-resistant prion protein formation by porphyrins and phthalocyanines. Proc. Natl. Acad. Sci. USA 95:12117-12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chesebro, B. 1999. Prion protein and the transmissible spongiform encephalopathy diseases. Neuron 24:503-506. [DOI] [PubMed] [Google Scholar]
- 9.Demaimay, R., B. Chesebro, and B. Caughey. 2000. Inhibition of formation of protease-resistant prion protein by Trypan Blue, Sirius Red and other Congo Red analogs. Arch. Virol. Suppl. 16:277-283. [DOI] [PubMed] [Google Scholar]
- 10.Demaimay, R., R. Race, and B. Chesebro. 1999. Effectiveness of polyene antibiotics in treatment of transmissible spongiform encephalopathy in transgenic mice expressing Syrian hamster PrP only in neurons. J. Virol. 73:3511-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doh-Ura, K., T. Iwaki, and B. Caughey. 2000. Lysosomotropic agents and cysteine protease inhibitors inhibit scrapie-associated prion protein accumulation. J. Virol. 74:4894-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forloni, G., S. Iussich, T. Awan, L. Colombo, N. Angeretti, L. Girola, I. Bertani, G. Poli, M. Caramelli, M. Grazia Bruzzone, L. Farina, L. Limido, G. Rossi, G. Giaccone, J. W. Ironside, O. Bugiani, M. Salmona, and F. Tagliavini. 2002. Tetracyclines affect prion infectivity. Proc. Natl. Acad. Sci. USA 99:10849-10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furukawa, H., M. Takahashi, M. Nakajima, and T. Yamada. 2002. Prospects of the therapeutic approaches to Creutzfeldt-Jakob disease: a clinical trial of the antimalarial, quinacrine. Nippon Rinsho 60:1649-1657. [PubMed] [Google Scholar]
- 14.Heppner, F. L., C. Musahl, I. Arrighi, M. A. Klein, T. Rulicke, B. Oesch, R. M. Zinkernagel, U. Kalinke, and A. Aguzzi. 2001. Prevention of scrapie pathogenesis by transgenic expression of anti-prion protein antibodies. Science 294:178-182. [DOI] [PubMed] [Google Scholar]
- 15.Ingrosso, L., A. Ladogana, and M. Pocchiari. 1995. Congo red prolongs the incubation period in scrapie-infected hamsters. J. Virol. 69:506-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Korth, C., K. Kaneko, and S. B. Prusiner. 2000. Expression of unglycosylated mutated prion protein facilitates PrP(Sc) formation in neuroblastoma cells infected with different prion strains. J. Gen. Virol. 81:2555-2563. [DOI] [PubMed] [Google Scholar]
- 17.Korth, C., B. C. May, F. E. Cohen, and S. B. Prusiner. 2001. Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proc. Natl. Acad. Sci. USA 98:9836-9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mange, A., N. Nishida, O. Milhavet, H. E. McMahon, D. Casanova, and S. Lehmann. 2000. Amphotericin B inhibits the generation of the scrapie isoform of the prion protein in infected cultures. J. Virol. 74:3135-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Maxson, L., C. Wong, L. M. Herrmann, B. Caughey, and G. S. Baron. A solid-phase assay for identification of modulators of prion protein interactions. Anal. Biochem., in press. [DOI] [PubMed]
- 19.May, B. C., A. T. Fafarman, S. B. Hong, M. Rogers, L. W. Deady, S. B. Prusiner, and F. E. Cohen. 2003. Potent inhibition of scrapie prion replication in cultured cells by bis-acridines. Proc. Natl. Acad. Sci. USA 100:3416-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishida, N., D. A. Harris, D. Vilette, H. Laude, Y. Frobert, J. Grassi, D. Casanova, O. Milhavet, and S. Lehmann. 2000. Successful transmission of three mouse-adapted scrapie strains to murine neuroblastoma cell lines overexpressing wild-type mouse prion protein. J. Virol. 74:320-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Priola, S. A., and B. Chesebro. 1995. A single hamster PrP amino acid blocks conversion to protease-resistant PrP in scrapie-infected mouse neuroblastoma cells. J. Virol. 69:7754-7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Priola, S. A., A. Raines, and W. S. Caughey. 2000. Porphyrin and phthalocyanine antiscrapie compounds. Science 287:1503-1506. [DOI] [PubMed] [Google Scholar]
- 23.Prusiner, S. B. 1998. Prions. Proc. Natl. Acad. Sci. USA 95:13363-13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudyk, H., S. Vasiljevic, R. M. Hennion, C. R. Birkett, J. Hope, and I. H. Gilbert. 2000. Screening Congo Red and its analogues for their ability to prevent the formation of PrP-res in scrapie-infected cells. J. Gen. Virol. 81:1155-1164. [DOI] [PubMed] [Google Scholar]
- 25.Scott, M. R., R. Kohler, D. Foster, and S. B. Prusiner. 1992. Chimeric prion protein expression in cultured cells and transgenic mice. Protein Sci. 1:986-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suganuma, M., S. Okabe, M. Oniyama, Y. Tada, H. Ito, and H. Fujiki. 1998. Wide distribution of [3H](−)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis 19:1771-1776. [DOI] [PubMed] [Google Scholar]
- 27.Supattapone, S., H. Wille, L. Uyechi, J. Safar, P. Tremblay, F. C. Szoka, F. E. Cohen, S. B. Prusiner, and M. R. Scott. 2001. Branched polyamines cure prion-infected neuroblastoma cells. J. Virol. 75:3453-3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taraboulos, A., M. Scott, A. Semenov, D. Avrahami, L. Laszlo, S. B. Prusiner, and D. Avrahami. 1995. Cholesterol depletion and modification of COOH-terminal targeting sequence of the prion protein inhibit formation of the scrapie isoform. J. Cell Biol. 129:121-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tremblay, P., Z. Meiner, M. Galou, C. Heinrich, C. Petromilli, T. Lisse, J. Cayetano, M. Torchia, W. Mobley, H. Bujard, S. J. DeArmond, and S. B. Prusiner. 1998. Doxycycline control of prion protein transgene expression modulates prion disease in mice. Proc. Natl. Acad. Sci. USA 95:12580-12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vorberg, I., K. Chan, and S. A. Priola. 2001. Deletion of beta-strand and alpha-helix secondary structure in normal prion protein inhibits formation of its protease-resistant isoform. J. Virol. 75:10024-10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winklhofer, K. F., F. U. Hartl, and J. Tatzelt. 2001. A sensitive filter retention assay for the detection of PrP(Sc) and the screening of anti-prion compounds. FEBS Lett. 503:41-45. [DOI] [PubMed] [Google Scholar]
- 32.Yang, C. S., S. Prabhu, and J. Landau. 2001. Prevention of carcinogenesis by tea polyphenols. Drug Metab. Rev. 33:237-253. [DOI] [PubMed] [Google Scholar]