Abstract
High levels of infused anti-human immunodeficiency virus type 1 (HIV-1) neutralizing monoclonal antibodies (MAbs) can completely protect macaque monkeys against mucosal chimeric simian-human immunodeficiency virus (SHIV) infection. Antibody levels below the protective threshold do not prevent infection but can substantially reduce plasma viremia. To assess if HIV-1/SIV-specific cellular immunity could combine with antibodies to produce sterile protection, we studied the effect of a suboptimal infusion of anti-HIV-1 neutralizing antibodies in macaques with active cellular immunity induced by interleukin-2 (IL-2)-adjuvanted DNA immunization. Twenty female macaques were divided into four groups: (i) DNA immunization plus irrelevant antibody, (ii) DNA immunization plus infusion of neutralizing MAbs 2F5 and 2G12, (iii) sham DNA plus 2F5 and 2G12, and (iv) sham DNA plus irrelevant antibody. DNA-immunized monkeys developed CD4 and CD8 T-cell responses as measured by epitope-specific tetramer staining and by pooled peptide ELISPOT assays for gamma interferon-secreting cells. After vaginal challenge, DNA-immunized animals that received irrelevant antibody became SHIV infected but displayed lower plasma viremia than control animals. Complete protection against SHIV challenge occurred in three animals that received sham DNA plus MAbs 2F5 and 2G12 and in two animals that received the DNA vaccine plus MAbs 2F5 and 2G12. Thus, although DNA immunization produced robust HIV-specific T-cell responses, we were unable to demonstrate that these responses contributed to the sterile protection mediated by passive infusion of neutralizing antibodies. These data suggest that although effector T cells can limit viral replication, they are not able to assist humoral immunity to prevent the establishment of initial infection.
Existing human immunodeficiency virus type 1 (HIV-1) vaccine candidates elicit reasonably potent cellular immune responses but only low levels of neutralizing antibodies. Such T-cell immunity-based vaccines do not prevent infection but can have a beneficial effect on disease course (1, 7, 13, 15, 22, 30, 33). In contrast, passively infused antibodies that neutralize free virus can provide complete protection in lentiviral animal models, but the serum antibody levels required are higher than can be generated by current HIV-1 immunization strategies (3, 11, 19, 21, 26, 29). To assess if effector T cells could combine with infused antibodies to produce sterile immunity, we studied the protective effect of a suboptimal dose of neutralizing antibodies in association with active cellular immunity induced by an interleukin-2 (IL-2)-adjuvanted DNA vaccine. Based on prior vaginal simian-human immunodeficiency virus (SHIV) challenge studies, the dose of antibodies infused into the monkeys was estimated to be just below the threshold amount needed to provide complete protection.
Our previous passive antibody transfer studies demonstrated that a systemic infusion of anti-HIV-1 neutralizing monoclonal antibodies (MAbs) 2F5 and 2G12 had a dramatic effect on subsequent vaginal SHIV-89.6P challenge. Some macaques were completely protected against infection; in the animals that did become SHIV infected, peak plasma viremia was blunted and the ensuing viremia was controlled to low or undetectable levels (21). While it is not clear how the infused antibody exerted its protective effect, it is known that transudative immunoglobulin G (IgG) MAbs were present at the mucosal surface after passive infusion (21). Thus, it is possible that local antibodies can reduce or eliminate the infectious viral inoculum at the mucosal surface. Similarly, antibodies may limit early virus spread in submucosal and lymphatic tissues and subsequently blunt the initial systemic viremia (12, 18, 24, 27). The observation that a specific dose of passively infused antibody could be close to the threshold amount required to provide complete protection suggested that a preexisting or anamnestic T-cell response might be able to eliminate the initial low level of infection that is established in the presence of neutralizing antibodies. This led to the hypothesis that cellular immunity might act in concert with antibodies and lead to a higher rate of sterile protection.
We addressed this question by combining DNA plasmid immunization with passive infusion of neutralizing MAbs in the rhesus macaque SHIV-89.6P vaginal challenge model. We chose this model because there were prior data on the dose and effect of passively infused antibody and because the mucosal route of infection might also allow effector T cells more opportunity to eradicate SHIV infection in local tissues. However, despite the use of an IL-2-adjuvanted DNA vaccine that induced robust HIV-1/SIV-specific T-cell immune responses, we were unable to demonstrate that cellular immunity improved the level of sterile protection mediated by passive infusion of antibodies.
MATERIALS AND METHODS
Animal immunizations.
Twenty adult female rhesus macaques were housed in a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care in accordance with standards outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animal study protocol and all procedures were approved by the institutional animal care and use committee. Monkeys were divided into four groups of five, based on age and weight. Eight animals expressed the Mamu-A*01 major histocompatibility complex class I allele; two such animals were included in each experimental group.
The codon-optimized SIVmac239 gag DNA (30), the IL-2/Ig plasmid (5), and the sham pV1J plasmid (5) were constructed as previously described. Plasmids were produced by Vical Inc. (San Diego, Calif.). The HIV-1 env plasmid was constructed at Merck Research Laboratories using the env gp140 sequence from primary isolate JR-FL. All DNA plasmids were formulated in sterile saline without adjuvant. Intramuscular DNA immunizations were given at 0, 4, 8, and 24 weeks, using a needle-free Biojector apparatus and a no. 3 syringe (Bioject, Portland, Oreg.). Ten animals received active DNA vaccine and 10 received sham DNA. Five milligrams of DNA gag and 5 mg of DNA env plasmids were delivered at each immunization. For each plasmid, the 5 mg was split into two aliquots of 0.5 ml each, and 2.5 mg was delivered into each quadriceps muscle. The IL-2/Ig plasmid was delivered 2 days later; 2.5 mg was delivered into approximately the same site of each quadriceps muscle (5 mg total). The IL-2/Ig plasmid was given after DNA immunizations at weeks 0 and 4, but not after the week 8 and 24 immunizations. For the 10 sham DNA-vaccinated animals, 5 mg of the sham DNA plasmid was delivered to each quadriceps muscle.
Antibodies and passive antibody infusion.
The human IgG1 MAbs 2F5 and 2G12 are known to neutralize SHIV-89.6P in vitro and to protect against viral challenge in macaque monkeys (3, 21). MAb 2F5 binds to a linear epitope within the extracellular domain of gp41, and 2G12 binds to a discontinuous, glycan-dependent epitope on gp120 that does not overlap the CD4 binding site. The MAbs were produced by recombinant expression in Chinese hamster ovary cells as previously described (2). Control intravenous immunoglobulin (IVIG) from HIV-1-seronegative individuals was purchased from the manufacturer (Gammagard; Baxter Healthcare Corp., Duarte, Calif.). Twenty-four hours prior to vaginal SHIV challenge, antibodies were infused into the saphenous vein by slow intravenous push. The total infused dose of control IVIG was 10 mg/kg of body weight; the dose of MAbs 2G5 and 2G12 was 5 mg of each MAb/kg.
Vaginal SHIV challenge and assessment of SHIV infection.
Vaginal challenge of female macaques was performed as previously described (21). Briefly, 1 ml of rhesus peripheral blood mononuclear cell (PBMC)-grown SHIV-89.6P viral stock (600 50% tissue culture infectious doses) was gently introduced into the vaginal canal using a 1-ml syringe. This inoculum corresponds to about 40 animal infectious doses. To optimize vaginal infection and control for the macaque estrous cycle, animals received 30 mg of medroxyprogesterone acetate (Depo-Provera; Upjohn, Kalamazoo, Mich.) 30 days prior to SHIV challenge. After viral challenge, animals were followed clinically and by routine hematology and lymphocyte subset analysis. SHIV infection was assessed by a plasma RNA assay with a sensitivity of 400 copies/ml (Bayer Diagnostics). PBMC-associated proviral DNA was measured by a quantitative real-time PCR assay for SIV gag using a Perkin-Elmer ABI 7700 instrument. The sensitivity of detection was 20 gag copies per million PBMC. Proviral DNA was also measured in cells derived from inguinal lymph nodes. The assay was performed as previously described (20), using SIV gag primers and probe as described by Lifson et al. (17). A direct enzyme-linked immunosorbent assay (ELISA) was used to measure plasma titers of anti-gp120 (HIV-MN) and anti-p27 (SIVmac239) antibodies (32).
Immune assays.
Flow cytometric enumeration of CD8 T cells specific for the p11C Gag peptide was performed by staining fresh whole-blood samples with anti-CD3 and anti-CD8 MAbs and with soluble tetrameric Mamu-A*01/p11C complexes conjugated to phycoerythrin-labeled streptavidin, as previously described (5). Gated CD3+ CD8+ T cells were examined for staining with the Mamu-A*01 tetramer complexes using a Becton Dickinson FACSCalibur. Enzyme-linked immunospot (ELISPOT) assays were used to assess gamma interferon (IFN-γ) production by PBMC or PBMC depleted of CD4+ T cells or CD8+ T cells, as previously described (8). IFN-γ responses were measured using pools of overlapping 15-mer peptides derived from HIV-1 89.6P Env or SIVmac239 Gag. Assays for plasma-mediated virus neutralization were done using mitogen-stimulated PBMC or MT2 target cells as indicated. The SHIV-89.6 and 89.6P neutralizing antibody assays on MT2 cells were performed by measuring the plasma dilution that protected 50% of MT2 cells from virus-induced cell killing (9). Neutralization of HIV-1 89.6 was evaluated in a single-round flow cytometric assay that measured intracellular expression of HIV-1 Gag (20).
Statistical analysis.
Peak plasma viral load was defined as the maximum viral RNA copies per milliliter, measured between week 1 and week 3 after SHIV challenge. The viral load set point was defined as the average value of measurements taken between week 7 and week 32. Geometric mean values were used for averages. The set point CD4 T-cell count was defined as the mean CD4 T-cell value (CD4+ T cells per microliter of whole blood) between week 7 and week 32. Comparisons among groups were done using a Wilcoxon two-sample rank sum test.
RESULTS
Study design.
The immunization and challenge schedule are shown in Fig. 1. Ten animals received the experimental vaccine (SIVmac239 Gag and HIV JR-FL Env) and 10 received a sham DNA plasmid. Vaginal challenge with SHIV-89.P was performed 14 weeks after the fourth DNA immunization (week 38). Animals received either irrelevant antibody (IVIG; 10 mg/kg) or anti-HIV-1 MAbs 2F5 and 2G12 (5 mg of each MAb/kg). Passive infusion of antibody was performed 24 h prior to vaginal SHIV challenge.
FIG. 1.
Study scheme and experimental groups. Twenty female macaques were divided into the four experimental groups shown. DNA immunizations were administered at 0, 4, 8, and 24 weeks. SHIV-89.6P vaginal challenge occurred at week 38, 1 day after infusion of either IVIG or the 2F5 and 2G12 MAbs.
Immunogenicity of IL-2-adjuvanted DNA vaccination.
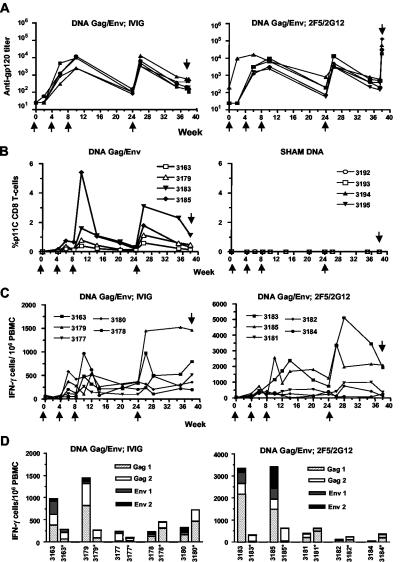
IL-2-adjuvanted DNA Gag/Env immunization generated SHIV-specific cellular and humoral immune responses in all 10 immunized monkeys. Plasma anti-gp120 antibody levels reached peak titers in the range of 1:5,000 to 1:10,000 after the third or fourth DNA immunization (Fig. 2A). Antibody titers in the sham immunized animals were less than 1:50 at all time points measured (data not shown). Plasma from 2 weeks after the fourth immunization had modest neutralizing activity against HIV89.6 but did not neutralize SHIV-89.6P or the homologous JR-FL virus (data not shown). Four of the 10 DNA Gag/Env-immunized monkeys were positive for the Mamu-A*01 allele; p11C tetramer staining of their CD3+ CD8+ lymphocytes is shown in the left panel of Fig. 2B. The tetramer-positive T-cell responses peaked after the third or fourth DNA immunization. For all animals, the frequency of antigen-specific lymphocytes in the peripheral blood was measure by an IFN-γ ELISPOT assay, using peptide pools spanning Gag and Env. Similar to the antibody and tetramer p11C tetramer responses, the number of antigen-specific IFN-γ-secreting cells peaked after the third or fourth immunization. Peak responses ranged from 500 to 5,000 spot-forming cells per million (Fig. 2C). To assess levels of CD4 and CD8 antigen-specific T cells, the ELISPOT assays at week 26 (2 weeks after the fourth DNA immunization) were performed on bulk PBMC and after CD8 T-cell depletion. For all 10 DNA Gag/Env-immunized monkeys, CD8 T-cell depletion decreased the ELISPOT response to one or more of the four peptide pools. However, the magnitude of the effect of CD8 T-cell depletion varied among the animals (Fig. 2D).
FIG. 2.
(A) Plasma anti-gp120 antibody titers were measured during the course of immunizations. In this and subsequent panels, the upward arrows indicate DNA immunizations and the downward arrow indicates the day of SHIV challenge. Note the high antibody titer at week 40 in the second panel, resulting from passive infusion of the MAbs 2F5 and 2G12. (B) Percentage of CD8+ T cells that stained with the Mamu-A01/p11C tetramer. The eight Mamu-A*01 allele-positive animals were evenly distributed into the four groups of animals. Thus, four animals received DNA Env-Gag immunization (left panel) and four received sham DNA (right panel). (C) Pooled peptide ELISPOT assay for IFN-γ-secreting PBMC. All DNA-immunized animals had detectable IFN-γ-secreting cells. In order to best visualize the data, the y axis of the second panel has been expanded to accommodate two animals with particularly high responses. (D) IFN-γ-secreting cells at week 26 (2 weeks after the fourth DNA immunization). All 10 DNA-immunized animals are shown. The response to each peptide pool is shown using a stacked column graph. An asterisk indicates ELISPOT data after CD8 T-cell depletion. Note the expanded y axis used for the right panel.
Passive antibody infusion.
On the day of SHIV challenge, 24 h after IVIG or MAb infusion, plasma neutralizing antibody levels were measured against the challenge virus SHIV-89.6P. Virus neutralizing antibodies were only detected in the 10 animals that had received an infusion of MAbs 2F5 and 2G12. In these animals, 50% virus neutralization was observed at a plasma dilution between 1:35 and 1:90 (Table 1).
TABLE 1.
Plasma neutralizing antibody titers on day of SHIV challengea
| Group | Animal no. | MT2 assayb
|
PBMC IC-p24c assay with HIV-89.6
|
||
|---|---|---|---|---|---|
| SHIV-86.9P IC50 | SHIV-89.6 IC50 | IC50 | IC90 | ||
| Sham DNA, control IVIG | 3189 | <20 | <20 | <5 | <5 |
| 3190 | <20 | <20 | <5 | <5 | |
| 3191 | <20 | <20 | <5 | <5 | |
| 3194 | <20 | <20 | <5 | <5 | |
| 3195 | <20 | <20 | <5 | <5 | |
| DNA Gag-Env, control IVIG | 3163 | <20 | <20 | <5 | <5 |
| 3177 | <20 | <20 | <5 | <5 | |
| 3178 | <20 | <20 | <5 | <5 | |
| 3179 | <20 | <20 | <5 | <5 | |
| 3180 | <20 | <20 | <5 | <5 | |
| Sham DNA, MAb 2F5 and 2G12 | 3186 | 78 | 99 | 164 | 8 |
| 3187 | 90 | 120 | 124 | 9 | |
| 3188 | 69 | 125 | 88 | 5 | |
| 3192 | 73 | 79 | 81 | 6 | |
| 3193 | 40 | 69 | 108 | 7 | |
| DNA Gag-Env, MAb 2F5 and 2G12 | 3181 | 76 | 80 | 134 | 5 |
| 3182 | 68 | 123 | 128 | 7 | |
| 3183 | 41 | 34 | 113 | 6 | |
| 3184 | 61 | 153 | 210 | 16 | |
| 3185 | 35 | 74 | 156 | 11 | |
The plasma samples were obtained just prior to SHIV challenge; i.e., 24 h after IVIG or MAb infusion.
The SHIV-89.6 and SHIV-89.6P neutralizing antibody assays were performed by measuring the reciprocal plasma dilution that protected 50% of MT2 cells from virus-induced killing. IC50, 50% inhibitory concentration.
Reciprocal plasma dilution that neutralized 50% or 90% of HIV-1 89.6. The assay was performed with human PBMC targets, using a single round of replication flow cytometic assay that measures expression of intracellular p24 antigen.
Vaginal SHIV challenge.
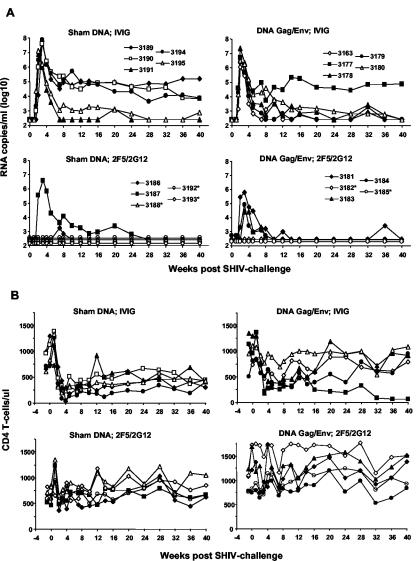
All five control animals displayed high-level plasma viremia that peaked between 2 and 3 weeks after vaginal exposure to virus. Three control animals maintained a viral set point at or above 10,000 RNA copies/ml, while two animals controlled viremia to levels of about 1,000 RNA copies/ml or less (Fig. 3A). Among the five DNA Gag/Env plus IVIG-immunized animals, four controlled plasma viremia to levels of 1,000 copies/ml or less. The geometric mean of peak plasma viremia in control animals was 107.4, compared to 106.5 for the DNA-immunized animals (an eightfold decrease). This difference in peak viremia was statistically significant (P = 0.03) by a nonparametric Wilcoxon rank test. The mean set-point viral load was 103.8 in control animals and 103.1 in DNA-immunized animals, a difference that was not statistically significant. Thus, DNA immunization did not prevent infection after vaginal SHIV challenge but did significantly lower peak viral load. Viral load set point and CD4 T-cell counts (Fig. 3B) also trended toward improvement in the DNA immunization group, compared to the controls group, but the differences were not statistically significant.
FIG. 3.
Postchallenge plasma viral RNA and peripheral CD4 T-cell counts. (A) Plasma viremia was determined using a branched-chain DNA amplification assay with a limit of detection of 500 RNA copies/ml. An asterisk indicates animals with no detectable viremia. (B) CD4 T cells per microliter of whole blood. Animals are identified by the same symbols shown in the plasma viremia graphs above.
Among the 10 animals that received passive infusions of MAbs 2F5 and 2G12, five were completely protected against SHIV infection; i.e., viral RNA was not detected in plasma, proviral DNA was not detected in PBMC or inguinal lymph node cells, and anti-p27 Gag antibodies did not develop (or boost) after SHIV challenge (Table 2). The remaining five animals were SHIV infected but displayed blunted peak viremia and low or undetectable levels of plasma viral RNA at set point. Complete protection was observed in three of five animals that received the sham DNA and MAbs 2F5 and 2G12 and in two of five animals that received active immunization with the DNA gag/env and MAbs 2F5 and 2G12. These results suggest that DNA immunization and the generation of SHIV-specific cellular immune responses did not contribute to the complete protection afforded by MAb infusion. To test for the possibility that the level of PBMC-associated proviral DNA was lower in animals that received DNA immunization plus antibodies, compared to the level in animals that received antibodies alone, a quantitative real-time PCR assay for viral gag was performed on PBMC collected on days 9, 20, 55, 97, 169, and 224 after SHIV challenge. By definition, proviral DNA was not detected in any of the animals that were protected against SHIV challenge. Although limited by the fact that only five of the animals that received passive antibody were infected, we did not observe any suggestion that the levels of viral DNA were lower in those animals that had received prior DNA immunization (Table 2 and data not shown).
TABLE 2.
Evidence for complete protection after SHIV challenge
| Group | Animal no. | Peak plasma RNAa | SIV gag DNA
|
Anti-p27 antibodyd | Complete protectione | |
|---|---|---|---|---|---|---|
| PBMCb | LNc | |||||
| Sham DNA, control IVIG | 3189 | 56,625,000 | 546,551 | 54,628 | 128,000 | No |
| 3190 | 39,403,000 | 207,192 | 72,138 | 48,000 | No | |
| 3191 | 7,609,000 | 18,314 | 43,628 | 21,300 | No | |
| 3194 | 76,300,000 | 118,141 | 45,200 | 42,600 | No | |
| 3195 | 13,440,000 | 30,306 | 49,262 | 12,800 | No | |
| DNA Gag/Env, control IVIG | 3163 | 1,147,000 | 44,867 | ND | 170,600 | No |
| 3177 | 5,214,000 | 32,796 | ND | 614,000 | No | |
| 3178 | 23,345,000 | 97,977 | ND | 25,600 | No | |
| 3179 | 2,306,437 | 85,217 | ND | 682,000 | No | |
| 3180 | 3,165,596 | 77,792 | ND | 51,200 | No | |
| Sham DNA, 2F5/2G12 | 3186 | 1,720 | 53 | 26 | <50 | No |
| 3187 | 3,924,800 | 53,146 | 26,504 | 6,400 | No | |
| 3188 | <500 | <20 | <20 | <50 | Yes | |
| 3192 | <500 | <20 | <20 | <50 | Yes | |
| 3193 | <500 | <20 | <20 | <50 | Yes | |
| DNA Gag/Env, 2F5/2G12 | 3181 | 638,502 | 46,027 | 19,638 | 102,400 | No |
| 3182 | <500 | <20 | <20 | <50 | Yes | |
| 3183 | 26,928 | 861 | 1,460 | 19,200 | No | |
| 3184 | 80,678 | 2,774 | 724 | 16,000 | No | |
| 3185 | <500 | <20 | <20 | <50 | Yes | |
Peak plasma viral RNA (copies/milliliter). Values of <500 indicate that no plasma viral RNA was detected at any time point after SHIV challenge.
Viral gag DNA in PBMC was measured at weeks 1, 3, 8, 14, 24, and 32 after SHIV-challenge; the value listed is the peak number of gag copies per million PBMC.
Viral gag DNA was measured in cells derived from an inguinal lymph node (LN) taken at week 36 or 40; the value listed is the number of gag copies per million cells. ND, not done (i.e., no lymph node biopsy performed).
Indicates peak ELISA level of anti-p27 Gag antibodies measured between 4 and 14 weeks after SHIV challenge.
Complete protection was defined if three criteria were met: (i) no detection of viral RNA in plasma, (ii) no detection of viral DNA in PBMC or from lymph node cells; (iii) no development of anti-p27 Gag antibodies after SHIV challenge. Animals depicted in bold type met all three criteria and were defined as completely protected against SHIV challenge.
Immune responses after viral challenge.
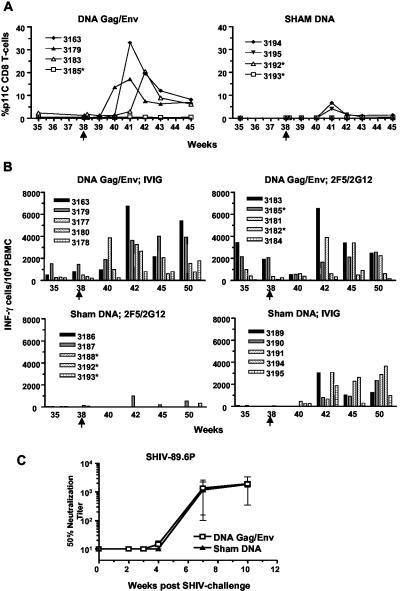
Postchallenge cellular immune responses were measured by p11C tetramer binding (Fig. 4A) and by pooled peptide ELISPOT assays (Fig. 4B). Control monkeys developed primary immune responses at 3 or 4 weeks postchallenge, whereas secondary immune responses of p11C tetramer-positive CD8 T cells and IFN-λ-secreting PBMC could be detected at 2 weeks postchallenge in the DNA-immunized animals. As an example, the primary CD8 T-cell response to the p11C epitope occurred at week 41 (3 weeks after SHIV challenge) and peaked at levels of about 5% of the CD8 T cells (Fig. 4A, right panel, animals 3194 and 3195). In contrast, two DNA-immunized animals (Fig. 4A, left panel, animals 3163 and 3179) developed a secondary CD8 T-cell response to this p11C epitope beginning at 2 weeks after SHIV challenge, peaking at above 15%. These data provide further evidence that DNA immunization produced robust cellular immunity. Neutralizing antibody assays were also done to test if the DNA gag/env immunization primed for a secondary neutralizing antibody response against the SHIV-89.6P challenge virus. As shown in Fig. 4C, neutralizing antibodies developed at about 7 weeks postchallenge. Prior DNA immunization with the JR-FL plasmid did not alter the kinetics or magnitude of this immune response.
FIG. 4.
Postchallenge p11C tetramer-positive CD8 T cells. (A) Four Mamu-A*01 monkeys received DNA gag/env immunization, and four received sham DNA. Closed symbols indicate monkeys that received IVIG infusion; open symbols indicate infusion of the MAbs 2F5 and 2G12. Here and in panel B below, arrows at week 38 indicate time of SHIV challenge. An asterisk indicates animals that were completely protected against SHIV challenge. (B) Postchallenge pooled peptide ELISPOT assays for IFN-γ-secreting PBMC. (C) Development of plasma neutralizing antibodies against the SHIV-89.6P challenge virus is not affected by prior DNA immunization encoding the JR-FL Env glycoprotein. Note that week 0 assays were run prior to passive infusion of antibodies.
DISCUSSION
Neutralizing antibodies are vital for vaccine-mediated protection against many viral diseases. In most cases, they likely act by blunting the initial infection, which is then resolved by cellular immunity. Viral clearance is then mediated by effector T cells such as cytolytic and noncytolytic CD8 T cells (31). However, HIV-1 is a chronic persistent infection that is not cleared by natural immunity, and it is not clear if effector T cells could contribute to the eradication of HIV-1 infection. We hypothesized that cellular immunity might contribute to sterile protection in a setting where SHIV infection was markedly limited by passively infused antibodies. In order to optimize the opportunity for secondary immune responses to affect early events of virus infection, we used a mucosal (vaginal) challenge model of SHIV infection. In this model, passive infusion of HIV-1-specific neutralizing antibodies can confer complete protection against SHIV challenge. In our prior experiments using a combination of MAbs 2F5 and 2G12, a dose of 15 mg of each MAb/kg was shown to completely protect two of five animals; i.e., there was no evidence of plasma viral RNA or cellular proviral DNA in these challenged monkeys. The three remaining animals were chronically SHIV infected, but viremia was controlled to low or undetectable levels. Thus, this dose of antibody appeared to be neutralizing the majority of the infectious viral inoculum, and it seemed possible that an anamnestic response by antigen-specific effector T cells, such as cytotoxic T lymphocytes (CTL), might eradicate the low level of initially infected cells.
The objective of these experiments was to induce robust T-cell immunity with an IL-2-adjuvanted DNA vaccine and infuse a dose of antibody prior to vaginal challenge that would be just below the level required for complete protection. The DNA vaccine encoded the SIV Gag protein and Env gp140 based on the HIV-1 JR-FL strain. As expected, the antibodies elicited by the JR-FL envelope protein did not neutralize the SHIV-89.6P challenge virus. Thus, at the time of challenge, all detectable neutralizing antibodies resulted from passive antibody infusion. We used a 5-mg/kg dose of MAbs, which was threefold less than in our prior passive antibody transfer study. This was an empirical choice based on the data summarized above and from other dose titration experiments (25, 26). Three macaques were completely protected in the antibody-alone group, indicating that the infused antibody was at the threshold of providing complete protection in all animals. Thus, we would expect that effector T cells might have an opportunity to eradicate the remaining infectious virus and tip the balance toward sterile protection in all animals of this group. Since we observed no improvement in sterile immunity in the animals that received DNA immunization prior to MAb infusion, we were unable to demonstrate that cellular immunity could contribute to sterile protection. Our conclusion here is not more definitive, because this study had limited power to detect differences among groups. Ideally, this study would have been performed with a minimum of eight animals per group, but it was not possible to acquire that many female Indian-origin rhesus macaques.
Among the animals that received DNA immunization plus IVIG, we observed a modest amelioration of viral load compared to control animals. This effect on chronic viremia was less dramatic than we reported in our prior experiment using an IL-2-adjuvanted DNA vaccine (7). Two factors may explain this observation. In the prior study, the envelope plasmid immunogen was homologous to the SHIV-89.6P challenge virus, and DNA immunization was shown to prime for an anamnestic neutralizing antibody response to the challenge virus. This antibody response could have contributed to the protection that was observed. In the present experiment, the DNA plasmid encoded the heterologous JR-FL Env, and we did not observe an anamnestic neutralizing antibody response to the challenge virus. Thus, the effect of this DNA immunization was likely limited to the cellular immune responses directed against the Gag and Env proteins encoded by the DNA plasmids. In addition, unlike our prior DNA vaccine study in which all DNA- and IL-2-immunized animals were Mamu-A*01 allele positive, this study included only two Mamu-A*01-positive animals per group.
The primary endpoint of this study was sterile immunity. Our prior experience established that even a suboptimal infusion of neutralizing antibodies could lead to preservation of CD4 T cells and control of plasma viremia in this SHIV-89.6P challenge model. This would have made it difficult to use the CD4 T-cell count or plasma viremia to assess the combined effect of antibody and T-cell immunity. In these experiments, we again observed that the passive infusion of antibody resulted in preserved CD4 T-cell counts and low or undetectable levels of viremia. Thus, the characteristics of this animal challenge model limit our ability to assess the potential benefit of combining humoral and cellular immune responses on the chronic disease course following SHIV infection. It remains possible that, under different experimental conditions, neutralizing antibodies and T-cell immune responses would provide better protection against SHIV infection than either immune response alone. Indeed, Dittmer and colleagues used adoptive transfer experiments to show that multiple lymphocyte subsets were required to provide optimal protection in the retroviral Friend virus mouse model (10). It is possible that CD4 T-helper cells, B lymphocytes, and CTL may all contribute to protective immunity against HIV-1. A lentivirus experimental model using a highly pathogenic SIV, such as Mac251, might be used to demonstrate such an effect.
Our macaque study was also limited to one immunization modality that was delivered intramuscularly. While DNA plasmid immunization can elicit antigen-specific mucosal T cells (4), we did not measure SHIV-specific T-cell immune responses in the vaginal mucosa of these animals. It is possible that a more robust immunization strategy, or one that elicits better mucosal immunity, would produce a different result. Nonetheless, this IL-2-adjuvanted DNA vaccine produced high levels of SHIV-specific cellular immunity, and we documented antigen-specific circulating T cells by staining for Mamu-A*01/p11C CD8 T cells and by a pooled peptide ELISPOT assay for IFN-γ-secreting cells.
Our finding that cellular immunity did not contribute to the eradication of chronic persistent SHIV infection lends support to the concept that cellular immune responses may not be able to eradicate established lentiviral infection. While clearance of acute viral infections occurs as a result of adaptive cellular immune responses, retroviruses and DNA viruses such as herpes simplex virus, cytomegalovirus, and varicella-zoster virus establish a chronic infection that is not eradicated. It is known that cellular immunity plays a key role in controlling SIV and HIV-1 replication in vivo (6, 10, 16, 28), but there is no direct evidence that CTL alone can prevent initial HIV-1 infection. Despite evidence that repeatedly HIV-1-exposed individuals, such as commercial sex workers, can become temporarily immune to infection (14), the mechanism of this effect has not been elucidated. The present data have implications for HIV-1 vaccine development. It has proven difficult to elicit HIV-1-specific neutralizing antibodies similar in potency to the 2F5 and 2G12 MAbs used in our experiments. In contrast, current vaccine candidates generate robust cellular immune responses in nonhuman primates, and these vaccines produce a beneficial effect on the SIV or SHIV disease course. This has led many investigators to focus on such CTL-based vaccines (23). Our data, other published animal model studies, and our knowledge of chronic viral diseases suggest that such vaccines are unlikely to prevent initial HIV-1 infection.
Our experiments confirm both the immunogenicity of IL-2-adjuvanted DNA immunization and the ability of anti-HIV-1 neutralizing antibodies to provide sterile protection against a mucosal SHIV challenge. The data provide no evidence that cellular immunity can tip the balance from near-sterile immunity to sterilizing immunity, even in the setting of high levels of protective neutralizing antibodies. While effector T cells likely contribute to eradication of many acute viral infections, studies of HIV and SIV immunopathogenesis have focused on the effect of CD8 T cells in controlling ongoing viral replication. Our data suggest that neutralizing antibodies, but not CD8 T cells, play a role in preventing the initial establishment of HIV or SIV infection. Thus, humoral and cellular immunity may have distinct effects, particularly during the acute phase of HIV-1 infection. If these animal model data are predictive of protective immunity to HIV-1, vaccine candidates will have to elicit potent neutralizing antibody responses in order to protect against the establishment of chronic persistent HIV-1 infection.
Acknowledgments
D. Burton and N. Letvin contributed equally to this work.
We thank Mark Louder, Jake Yalley-Ogunro, Sherry Rippeon, Tammy Hooper, Jermey Bowling, Ginger Donnlley, Jim Miller, Catherine Griffin, Brenna Hill, Joern Schmitz, Marcelo Kuroda, and Darci Gorgone for technical contributions to this study, Zoe Moodie for statistical analysis, and Rick Koup for helpful advice and discussion. We also thank Chris Butler and Jim Bradac for assistance with DNA plasmid production.
This work was supported in part by grant HL59718 to J.R.M. and T.C.V. and by grants AI 33292 and AI52057 to D.R.B.
REFERENCES
- 1.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 2.Armbruster, C., G. M. Stiegler, B. A. Vcelar, W. Jager, N. L. Michael, N. Vetter, and H. W. Katinger. 2002. A phase I trial with two human monoclonal antibodies (hMAb 2F5, 2G12) against HIV-1. AIDS 16:227-233. [DOI] [PubMed] [Google Scholar]
- 3.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. [DOI] [PubMed] [Google Scholar]
- 4.Baig, J., D. B. Levy, P. F. McKay, J. E. Schmitz, S. Santra, R. A. Subbramanian, M. J. Kuroda, M. A. Lifton, D. A. Gorgone, L. S. Wyatt, B. Moss, Y. Huang, B. K. Chakrabarti, L. Xu, W. P. Kong, Z. Y. Yang, J. R. Mascola, G. J. Nabel, A. Carville, A. A. Lackner, R. S. Veazey, and N. L. Letvin. 2002. Elicitation of simian immunodeficiency virus-specific cytotoxic T lymphocytes in mucosal compartments of rhesus monkeys by systemic vaccination. J. Virol. 76:11484-11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barouch, D. H., A. Craiu, M. J. Kuroda, J. E. Schmitz, X. X. Zheng, S. Santra, J. D. Frost, G. R. Krivulka, M. A. Lifton, C. L. Crabbs, G. Heidecker, H. C. Perry, M. E. Davies, H. Xie, C. E. Nickerson, T. D. Steenbeke, C. I. Lord, D. C. Montefiori, T. B. Strom, J. W. Shiver, M. G. Lewis, and N. L. Letvin. 2000. Augmentation of immune responses to HIV-1 and simian immunodeficiency virus DNA vaccines by IL-2/Ig plasmid administration in rhesus monkeys. Proc. Natl. Acad. Sci. USA 97:4192-4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyerl, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415:335-339. [DOI] [PubMed] [Google Scholar]
- 7.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, and M. G. Lewis. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 8.Barouch, D. H., S. Santra, K. Tenner-Racz, P. Racz, M. J. Kuroda, J. E. Schmitz, S. S. Jackson, M. A. Lifton, D. C. Freed, H. C. Perry, M. E. Davies, J. W. Shiver, and N. L. Letvin. 2002. Potent CD4+ T cell responses elicited by a bicistronic HIV-1 DNA vaccine expressing gp120 and GM-CSF. J. Immunol. 168:562-568. [DOI] [PubMed] [Google Scholar]
- 9.Crawford, J. M., P. L. Earl, B. Moss, K. A. Reimann, M. S. Wyand, K. H. Manson, M. Bilska, J. T. Zhou, C. D. Pauza, P. W. Parren, D. R. Burton, J. G. Sodroski, N. L. Letvin, and D. C. Montefiori. 1999. Characterization of primary isolate-like variants of simian-human immunodeficiency virus. J. Virol. 73:10199-10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dittmer, U., D. M. Brooks, and K. J. Hasenkrug. 1999. Requirement for multiple lymphocyte subsets in protection by a live attenuated vaccine against retroviral infection. Nat. Med. 5:189-193. [DOI] [PubMed] [Google Scholar]
- 11.Foresman, L., F. Jia, Z. Li, C. Wang, E. B. Stephens, M. Sahni, O. Narayan, and S. V. Joag. 1998. Neutralizing antibodies administered before, but not after, virulent SHIV prevent infection in macaques. AIDS Res. Hum. Retrovir. 14:1035-1043. [DOI] [PubMed] [Google Scholar]
- 12.Frankel, S. S., R. M. Steinman, N. L. Michael, S. R. Kim, N. Bhardwaj, M. Pope, M. K. Louder, P. K. Ehrenberg, P. W. Parren, D. R. Burton, H. Katinger, T. C. VanCott, M. L. Robb, D. L. Birx, and J. R. Mascola. 1998. Neutralizing monoclonal antibodies block human immunodeficiency virus type 1 infection of dendritic cells and transmission to T cells. J. Virol. 72:9788-9794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hel, Z., W. P. Tsai, A. Thornton, J. Nacsa, L. Giuliani, E. Tryniszewska, M. Poudyal, D. Venzon, X. Wang, J. Altman, D. I. Watkins, W. Lu, A. von Gegerfelt, B. K. Felber, J. Tartaglia, G. N. Pavlakis, and G. Franchini. 2001. Potentiation of simian immunodeficiency virus (SIV)-specific CD4+ and CD8+ T cell responses by a DNA-SIV and NYVAC-SIV prime/boost regimen. J. Immunol. 167:7180-7191. [DOI] [PubMed] [Google Scholar]
- 14.Kaul, R., S. L. Rowland-Jones, J. Kimani, T. Dong, H. B. Yang, P. Kiama, T. Rostron, E. Njagi, J. J. Bwayo, K. S. MacDonald, A. J. McMichael, and F. A. Plummer. 2001. Late seroconversion in HIV-resistant Nairobi prostitutes despite pre-existing HIV-specific CD8+ responses. J. Clin. Investig. 107:341-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim, J. J., J. S. Yang, L. K. Nottingham, D. J. Lee, M. Lee, K. H. Manson, M. S. Wyand, J. D. Boyer, K. E. Ugen, and D. B. Weiner. 2001. Protection from immunodeficiency virus challenges in rhesus macaques by multicomponent DNA immunization. Virology 285:204-217. [DOI] [PubMed] [Google Scholar]
- 16.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lifson, J. D., J. L. Rossio, M. Piatak, Jr., T. Parks, L. Li, R. Kiser, V. Coalter, B. Fisher, B. M. Flynn, S. Czajak, V. M. Hirsch, K. A. Reimann, J. E. Schmitz, J. Ghrayeb, N. Bischofberger, M. A. Nowak, R. C. Desrosiers, and D. Wodarz. 2001. Role of CD8+ lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J. Virol. 75:10187-10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mascola, J. R., S. S. Frankel, and K. Broliden. 2000. HIV-1 entry at the mucosal surface: role of antibodies in protection. AIDS 14(Suppl. 3):S167-S174. [PubMed] [Google Scholar]
- 19.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mascola, J. R., M. K. Louder, C. Winter, R. Prabhakara, S. C. De Rosa, D. C. Douek, B. J. Hill, D. Gabuzda, and M. Roederer. 2002. Human immunodeficiency virus type 1 neutralization measured by flow cytometric quantitation of single-round infection of primary human T cells. J. Virol. 76:4810-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 22.Matano, T., M. Kano, H. Nakamura, A. Takeda, and Y. Nagai. 2001. Rapid appearance of secondary immune responses and protection from acute CD4 depletion after a highly pathogenic immunodeficiency virus challenge in macaques vaccinated with a DNA prime/Sendai virus vector boost regimen. J. Virol. 75:11891-11896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nabel, G. J. 2001. Challenges and opportunities for development of an AIDS vaccine. Nature 410:1002-1007. [DOI] [PubMed] [Google Scholar]
- 24.Nabel, G. J., and N. J. Sullivan. 2000. Antibodies and resistance to natural HIV infection. N. Engl. J. Med. 343:1263-1265. [DOI] [PubMed] [Google Scholar]
- 25.Nishimura, Y., T. Igarashi, N. Haigwood, R. Sadjadpour, R. J. Plishka, A. Buckler-White, R. Shibata, and M. A. Martin. 2002. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. J. Virol. 76:2123-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parren, P. W., P. A. Marx, A. J. Hessell, A. Luckay, J. Harouse, C. Cheng-Mayer, J. P. Moore, and D. R. Burton. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75:8340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robert-Guroff, M. 2000. IgG surfaces as an important component in mucosal protection. Nat. Med. 6:129-130. [DOI] [PubMed] [Google Scholar]
- 28.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 29.Shibata, R., T. Igarashi, N. Haigwood, A. Buckler-White, R. Ogert, W. Ross, R. Willey, M. W. Cho, and M. A. Martin. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5:204-210. [DOI] [PubMed] [Google Scholar]
- 30.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 31.Thimme, R., S. Wieland, C. Steiger, J. Ghrayeb, K. A. Reimann, R. H. Purcell, and F. V. Chisari. 2003. CD8+ T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J. Virol. 77:68-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VanCott, T. C., J. R. Mascola, L. D. Loomis-Price, F. Sinangil, N. Zitomersky, J. McNeil, M. L. Robb, D. L. Birx, and S. Barnett. 1999. Cross-subtype neutralizing antibodies induced in baboons by a subtype E gp120 immunogen based on an R5 primary human immunodeficiency virus type 1 envelope. J. Virol. 73:4640-4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, S. W., P. A. Kozlowski, G. Schmelz, K. Manson, M. S. Wyand, R. Glickman, D. Montefiori, J. D. Lifson, R. P. Johnson, M. R. Neutra, and A. Aldovini. 2000. Effective induction of simian immunodeficiency virus-specific systemic and mucosal immune responses in primates by vaccination with proviral DNA producing intact but noninfectious virions. J. Virol. 74:10514-10522. [DOI] [PMC free article] [PubMed] [Google Scholar]