Abstract
The human immunodeficiency virus type 1 (HIV-1) accessory protein Nef stimulates viral infectivity by an unknown mechanism. Recent studies have suggested that Nef may act by regulating the efficiency of virus entry into cells. Here we provide evidence to the contrary. Using a quantitative assay of HIV-1 virus-cell fusion, we observed equivalent rates and extents of fusion of wild-type and Nef-defective HIV-1 particles with MT-4 cells and CD4-expressing HeLa cells. In studies using soluble CD4 (sCD4) to inhibit infection, wild-type and Nef-defective HIV-1 escaped the sCD4 block with similar kinetics. We conclude that Nef acts at a postentry step in infection, probably by facilitating intracellular transport of the HIV-1 ribonucleoprotein complex.
Nef is an accessory protein encoded specifically by the primate lentiviruses human immunodeficiency virus type 1 (HIV-1), HIV-2, and simian immunodeficiency virus. Studies with infected macaques and humans indicate that Nef plays a key role in AIDS pathogenesis (15, 17, 23, 25, 31, 39, 40), and the wide array of activities attributed to Nef has led to intensive mechanistic analysis of Nef functions (for a review, see reference 4). Nef regulates the surface expression of numerous T-cell molecules involved in HIV-1 infection and T-cell function, including CD4, MHCI, CD3, DC-SIGN, and Fas ligand (5, 20, 43, 44, 46). In the case of CD4, Nef recruits plasma membrane CD4 into clathrin-coated pits via binding to the intracellular domain of CD4 and to clathrin-associated adaptor proteins (for a review, see reference 16). Nef enhances the infectivity of HIV-1 particles through an additional activity that is distinct from CD4 downregulation (2, 22). Although the mechanism is not well defined, early studies indicated that Nef acts in the virus producer cell to modify the HIV-1 virion (3, 12, 33, 42). Nef does not detectably alter virion protein or RNA composition, nor does it affect the structure or stability of the viral core (3, 34, 42). Indeed, the sole virion modification that has been confirmed is the presence of Nef protein itself within the virion (6, 37), where it is cleaved by the viral protease. Estimates of the quantity of virion-associated Nef range from 10 to 70 molecules per particle (10, 37). Cleavage of Nef is not required for infectivity enhancement (10, 32), and it remains unclear whether Nef must be present in the virion to enhance infectivity. Nef-defective HIV-1 particles are attenuated during an early step in the virus life cycle, as shown by their impaired ability to undergo reverse transcription in target cells (3, 11, 42). However, the virions themselves contain normal levels of reverse transcriptase activity when assayed in vitro. The requirement for Nef is not specific for CD4-mediated HIV-1 entry, since infection of CD4-negative cells by pseudotyped HIV-1 particles bearing the amphotropic murine leukemia virus envelope proteins is also Nef dependent (3, 34, 42). Based on these observations, it was previously concluded that Nef enhances HIV-1 fusion by facilitating an early postfusion step in the virus life cycle. However, a significant limitation of the previous studies was the inability to specifically quantify the efficiency of virus-cell fusion leading to infection.
Results of several recent studies have revived the hypothesis that Nef may stimulate infection by regulating HIV-1 entry into target cells. Nef is associated with lipid rafts (47), and disruption of lipid rafts by extraction of cholesterol from cells resulted in the production of virions that are equally infectious when produced in the presence or absence of Nef (50). Because lipid rafts are implicated in HIV-1 entry into cells, this observation suggested that Nef might act at the level of virus-cell fusion. In another study, Schaeffer et al. fractionated cells following acute HIV-1 infection and quantified the amount of viral capsid protein (CA) in the soluble and membrane fractions (41). More CA was detected in cytosolic fraction of cells infected with wild-type HIV-1 than in the same fraction of cells infected with Nef-defective virus. In addition, we recently reported that when HIV-1 infection was initiated by mixing of receptor-pseudotyped virions with HIV-1 particles containing a defective core, enhancement of infection by Nef was linked to the viral envelope (51). Collectively, these studies suggested that Nef enhances the entry of HIV-1 particles via modification of the viral envelope. In the present study, we employed a recently developed assay of HIV-1 virus-cell fusion to test the hypothesis that Nef enhances HIV-1 infection by facilitating virus entry into target cells.
A quantitative HIV-1 virus-cell fusion assay reveals that Nef does not enhance the extent of HIV-1 fusion with target cells.
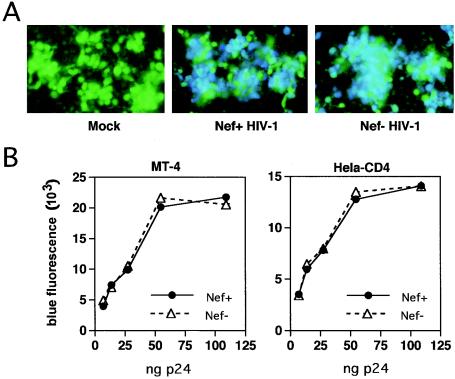
To determine whether Nef enhances HIV-1 entry into cells, we employed a recently developed quantitative assay of HIV-1 fusion with target cells (M. D. Miller et al., submitted for publication). In this system, HIV-1 particles are produced by cotransfection of proviral plasmid DNA with a construct (pMM310) encoding Escherichia coli β-lactamase (BlaM) fused to the amino terminus of HIV-1 Vpr. The BlaM-Vpr fusion protein is specifically incorporated during HIV-1 assembly and is released into target cells upon fusion of virions. Cytosolic BlaM activity is subsequently detected by loading cells with CCF4-AM, a cell-permeant fluorescent BlaM substrate that accumulates within cells due to cleavage of the acetoxymethyl side chain by cytoplasmic esterases (52). Upon excitation of the coumarin ring at 405 nm, this substrate emits at green wavelengths due to fluorescence resonance energy transfer to the attached fluorescein acceptor group; however, upon cleavage by BlaM, the emission spectrum shifts to blue due to release of fluorescein and loss of fluorescence resonance energy transfer. The fusion signal is dependent on the presence of the viral Env proteins and requires expression of CD4 and an appropriate coreceptor on target cells (M. D. Miller et al., unpublished observations). Inhibitors of HIV-1 entry, such as T-20, also block the signal (data not shown), demonstrating that virions that remain attached to the cell without fusing do not score in the assay. Similar HIV-1 fusion assays have recently been reported (7, 36). Using this system, we quantified the ability of wild-type and Nef-defective virions to fuse with target cells. HIV reporter particles containing β-lactamase were generated by cotransfecting 293T cells with pMM310 and the wild-type R8 or Nef-defective R8ΔN HIV-1 proviral plasmids (1) using Fugene6 (60 μl and 5 μg of each plasmid/T75 flask) (Roche, Basel, Switzerland). Importantly, 293T cells do not express CD4, thus allowing specific analysis of the CD4-independent effect of Nef on HIV-1 infectivity. HIV-1 stocks were assayed for p24 by enzyme-linked immunosorbent assay, as previously described (48). Viruses (90 μl) were mixed with cells (0.5 × 105 to 1 × 105 cells/well) in Costar (Corning, N.Y.) 3603 plates, incubated at 37°C for various times to allow virus entry, loaded with CCF4-AM (1 μM; Aurora Biosciences, San Diego, Calif.), and incubated at room temperature for 16 to 18 h. Viruses containing BlaM-Vpr were added to MT-4 cells and to CD4-expressing HeLa (HeLa-CD4) cells, both of which are highly permissive for HIV-1 infection. Following incubation at 37°C for 4 h, CCF4-AM was added, and the cultures were incubated at 23°C to prevent further fusion and to allow substrate cleavage by BlaM. To acquire images of green and blue fluorescence in the cells, we used an Olympus IX-70 inverted microscope with mercury excitation and CCF2 filter set 41031 from Chroma Technology, as previously described (28). Digital images were captured with a SpotRT digital charge-coupled device camera linked to a personal computer. Both wild-type and Nef-defective viruses resulted in cleavage of CCF4-AM, as revealed by conversion of the green emission to blue by fluorescence microscopy of the cultures (Fig. 1A). To quantify the extent of fusion, the blue emission of the cell cultures was measured using a microplate fluorometer (BMG Fluostar) using 405 ± 20 nm excitation and 460 ± 40 nm emission filters. The results revealed that wild-type and Nef-defective virions exhibited identical extents of fusion across the range of input viruses (Fig. 1B).
FIG. 1.
Wild-type and Nef-defective HIV-1 particles fuse with target cells with equal efficiencies. HIV-1 particles containing a BlaM-Vpr fusion protein were produced by transfection of 293T cells, dilutions of viruses were added to MT-4 and HeLa-CD4 cells, and cultures were incubated at 37°C for 4 h. Following addition of the cell-permeant BlaM substrate CCF4-AM, cultures were incubated overnight at 22°C to allow cellular uptake and cleavage of substrate by BlaM. (A) Representative fluorescent images of MT-4 cells inoculated with each virus (25 ng of p24 in each virus inoculum). Images were captured with an inverted fluorescence microscope using green and blue filters, and composite fluorescence images are shown. (B) The extent of fusion was determined by quantifying the fluorescence at 450 nm following excitation at 410 nm using a fluorescence microplate reader. Shown is the blue fluorescence signal as a function of the p24 input in the cultures.
BlaM-Vpr reporter virions retain the requirement for Nef for optimal infectivity.
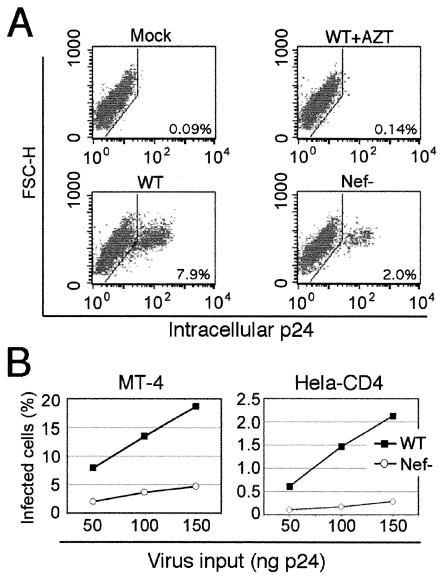
To ensure that incorporation of the BlaM-Vpr fusion protein did not alter the effect of Nef on HIV-1 infectivity, the BlaM-Vpr reporter virus stocks was titrated on MT-4 and HeLa-CD4 cells. Infected cells were quantified by flow cytometry after staining for intracellular Gag expression. HeLa-CD4 and MT4 cells (200,000) were seeded in 24-well tissue culture plates and inoculated with various quantities of viruses. Twelve hours after inoculation, zidovudine (Sigma) was added to a final concentration of 10 μM to prevent viral spread. Following culture for an additional 36 h, cells were collected and fixed in Cytofix solution (Pharmingen). After being washed with Cytostain solution, cells were stained with anti-p24gag mouse monoclonal antibody 183-H12-5C (48) for 30 min at 4°C. Cells were subsequently washed three times with staining buffer and stained with phycoerythrin-conjugated goat anti-mouse polyclonal antibody. Cellular fluorescence was quantified with a FACSCalibur and analyzed with CellQuest software (Becton Dickinson). Infection of MT-4 cells by wild-type HIV-1 was approximately four times as efficient as infection by the Nef-defective virus (Fig. 2). When HeLa-CD4 cells were used as targets, the wild-type virus was approximately eight times more infectious than the Nef-defective virus. Thus, HIV-1 particles produced from cells expressing BlaM-Vpr require Nef for optimal infection. Interestingly, the fusion assay appeared to saturate at lower levels of virus than the infection assay (compare Fig. 1B to Fig. 2B). This observation suggests that a significant fraction of HIV-1 particles may be defective for postfusion steps in infection, or that postentry restriction factors may limit the ability of the fused virions to productively infect the cell. As an additional control, immunoblot analysis of viral lysates revealed that the wild-type and Nef-defective virions were found to incorporate similar quantities of BlaM-Vpr (data not shown). We conclude that Nef does not enhance the overall extent of fusion of HIV-1 particles with target cells.
FIG. 2.
Nef enhances HIV-1 infection of MT-4 and HeLa-CD4 cells. Cell cultures were inoculated with the indicated quantities of viruses. Infection was quantified by flow cytometry following intracellular staining of cells with a p24-specific monoclonal antibody. (A) Representative flow-cytometric data for intracellular staining following inoculation of MT-4 cells with medium (Mock), wild-type (WT) HIV-1, wild-type HIV-1 with 10 μM zidovudine, and Nef-defective HIV-1. Quantities of HIV-1 corresponding to 50 ng of p24 antigen were used in these infections. (B) Percentage of infected cells (p24+) as a function of input virus quantity for MT-4 and HeLa-CD4 cells.
Nef does not affect the kinetics of HIV-1 fusion with target cells.
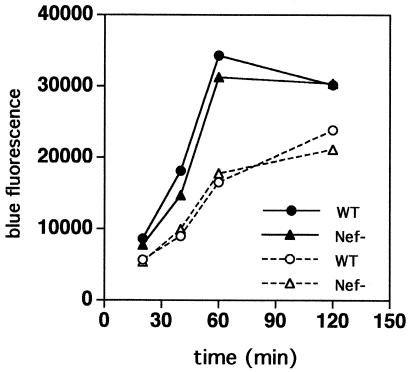
We quantified the fusion of wild-type and Nef-defective HIV-1 following incubation of MT-4 cells with equal quantities of viruses at 37°C for various periods. A time-dependent increase in the fusion signal was observed for both viruses for up to 2 h after inoculation, and wild-type and Nef-defective HIV-1 exhibited virtually identical fusion kinetics (Fig. 3). These data indicate that Nef does not affect the rate or extent of HIV-1 fusion with target cells.
FIG. 3.
Wild-type and Nef-defective viruses exhibit similar kinetics of fusion. MT-4 cells were inoculated with identical quantities (109 ng of p24 [closed symbols]; 54.5 ng of p24 [open symbols]) of wild-type and Nef-defective BlaM-Vpr reporter viruses and incubated at 37°C for the indicated times, after which fusion was quantified by CCF4-AM addition.
Wild-type and Nef-defective HIV-1 particles escape a block to entry at equivalent rates.
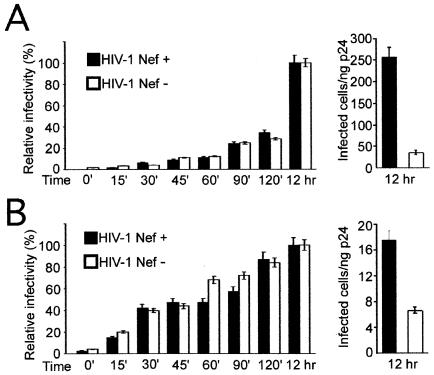
The fusion assay measures the overall virus-cell fusion efficiency, and the signal was correlated with HIV-1 infection when entry inhibitors were tested (J. E. Lineberger and M. D. Miller, unpublished observations). However, the assay does not discriminate between fusion leading to infection and fusion that may be nonproductive for infection. To determine whether wild-type and Nef-defective HIV-1 virions infect cells by fusing at different rates, we quantified the rate of acquisition of resistance to soluble CD4 (sCD4) during infection by each virus, as previously reported (45). The P4 cell line, a HeLa cell clone engineered to express CD4 and a Tat-inducible β-galactosidase gene, was used to quantify virus infectivity as previously described (8). P4 cells were inoculated with fixed quantities of wild-type and Nef-defective HIV-1. Cultures were inoculated with viruses at low multiplicities of infection and allowed to infect the cells for 2 days. An inhibitory concentration of sCD4 (5 μg/ml) was added at intervals, and the cells were cultured for 2 days prior to staining with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) to identify productively infected cells. Infected cells were enumerated by capturing images with a charge-coupled device camera and counting the stained cells by using NIH Image. The results revealed that infection by wild-type and Nef-defective HIV-1 particles escaped a sCD4 block at similar rates (Fig. 4A). In order to synchronize the fusion of the virions in the cultures, we repeated the procedure using cultures of P4 cells to which viruses were prebound at 4°C, washed, and then shifted to 37°C, followed by the addition of sCD4 at timed intervals. Initial attachment of HIV-1 particles to the HeLa-derived P4 cells occurs through interactions of gp120 with cell-surface heparan sulfate proteoglycans, thus permitting subsequent neutralization by sCD4 (35). Addition of sCD4 at the time of the temperature shift resulted in complete inhibition of infection, demonstrating that sCD4 acted rapidly to inhibit HIV-1 entry (Fig. 4B). Prebinding of virions to cells significantly altered the kinetics of sCD4 escape; however, even when fusion was synchronized in this manner, wild-type and Nef-defective HIV-1 particles exhibited identical kinetics of entry leading to infection. Interestingly, prebinding of virions to cells at 4°C resulted in an apparent reduction in the requirement of Nef for infection (Fig. 4B, right panel). We conclude that wild-type and Nef-defective HIV-1 particles exhibit identical kinetics of fusion and that the infectious fractions of both viruses acquire resistance to sCD4 at the same rate.
FIG. 4.
Infection by wild-type and Nef-defective HIV-1 particles acquires resistance to sCD4 at equivalent rates. (A) Viruses were added to P4 cells at 37°C, and sCD4 was added at the indicated times. Cultures were maintained at 37°C for a total of 48 h, and infection was quantified by counting the number of blue cells following staining with X-Gal. Values are expressed as percentages of the infectivity of each virus relative to cultures to which sCD4 was added at 12 h postinoculation. Shown on the right is the relative infectivity of each virus when sCD4 was added at 12 h following inoculation. (B) Analysis of the kinetics of productive virus entry following prebinding of viruses to cells. Viruses were incubated with cells at 4°C, and cells were washed to remove unbound virus and subsequent cultured at 37°C. sCD4 was added at the indicated times following temperature shift.
Nef stimulates HIV-1 infectivity four- to tenfold when assayed in a single round of infection, but the mechanism of infectivity enhancement remains enigmatic. Based on early observations that enhancement by Nef is not specific for the HIV-1 envelope proteins and on the lack of an effect of Nef on the cellular uptake of HIV-1, it has generally been concluded that Nef facilitates a postentry step in infection. However, recent studies have suggested that Nef modifies the viral envelope and enhances the cytoplasmic delivery of the core (41, 50, 51). The results reported here indicate that Nef does not enhance the entry of HIV-1 particles into target cells. Using a novel quantitative assay of HIV-1 virus-cell fusion, we observed identical rates and extents of fusion of wild-type and Nef-defective HIV-1 particles with cells. In addition, Nef did not affect the kinetics of HIV-1 entry leading to infection, since wild-type and Nef-defective virions escaped an entry block with similar kinetics. Collectively, these results support the conclusion that Nef does not enhance HIV-1 infection by facilitating virus entry but rather enhances an early postfusion step in infection.
If Nef does not enhance the efficiency of HIV-1 fusion, then how can the results of Zheng et al. (50) and Schaeffer et al. (41) be accounted for? In the former study, virions produced from cholesterol-depleted cells were found to be independent of Nef for infection, implicating the lipid raft association of Nef in infectivity enhancement. Incorporation of Nef into HIV-1 particles may be required for infectivity enhancement, and because lipid rafts are incorporated into budding HIV-1 particles, it is plausible that depletion of cholesterol from HIV-1 producer cells results in impaired packaging of Nef. Alternatively, cholesterol may be required for an effect of Nef that is manifested following virus-cell fusion. Thus, the effect of cholesterol depletion on Nef function does not necessarily exclude the possibility that Nef enhances a postentry step in infection.
The results of Schaeffer et al. (41) can also be reconciled with our data. Based on the observation that Nef is required for optimal infectivity of HIV-1 particles bearing the amphotropic murine leukemia virus Env proteins (3, 34, 42), we previously concluded that Nef enhances an early postentry step in the virus life cycle. Because Nef is incorporated into HIV-1 virions and localizes to the viral core (26, 49), it is possible that Nef enhances a function of the core, such as uncoating. In the study by Schaeffer et al., cytosolic fractions were recovered as supernatants following ultracentrifugation using conditions under which HIV-1 cores are known to pellet (19, 26, 49). Thus, the greater fraction of cytosolic p24 observed for wild-type virions could result not only from enhanced virus-cell fusion but also from more efficient release of CA from the core following fusion. However, in a recent study of HIV-1 cores isolated from virions, we observed no detectable effects of Nef on HIV-1 core structure or stability under a variety of conditions in vitro (18). Furthermore, assays of permeabilized virions failed to reveal any effect of Nef on endogenous reverse transcription (3, 42). Nonetheless, cell-free assays may not reveal virus-host cell interactions that occur following HIV-1 fusion, and it remains possible that Nef regulates the disassembly of the core in target cells.
If Nef does not enhance the entry of HIV-1 particles, then by what mechanism does Nef render HIV-1 particles more infectious? We and others reported previously that pseudotyping HIV-1 virions by the glycoprotein of vesicular stomatitis virus suppresses the requirement for Nef and renders HIV-1 particles sensitive to inhibitors of endosomal acidification, consistent with an endocytic route of entry for the pseudotyped virions (1, 9, 30). Based on these observations and the lack of an observable effect of Nef on virus-cell fusion, we propose an intracellular trafficking role of Nef in HIV-1 infection. In this model, fusion of virions with target cells releases the viral core into the cytoplasm, where it undergoes partial disassembly. Nef, by virtue of its association with the ribonucleoprotein complex (18), tethers the viral ribonucleoprotein complex to clathrin-coated pits or vesicles by engaging components of the endocytic apparatus (14, 21, 27, 29, 38). Transport of the endocytic vesicle to a specific intracellular compartment may facilitate initiation of reverse transcription, thereby resulting in completion of the remaining steps in infection. This model can account for several additional observations related to infectivity enhancement by Nef. First, mutations in a dileucine motif present in a surface region of the Nef protein impair HIV-1 infectivity enhancement; these mutations also disrupt engagement of adaptor protein complexes by Nef (13, 14). Second, by targeting HIV-1 fusion to an endosomal compartment, vesicular stomatitis virus G bypasses the putative requirement for Nef in intracellular transport. Third, the Nef phenotype is not absolute—i.e., viruses that lack Nef due to nonsense mutations still retain a significant ability to infect cells. How do Nef-defective virions infect cells? HIV-1 fusion is likely a stochastic process, with a small percentage of virions being internalized by endocytosis prior to fusing from within an endosomal membrane, and Nef-defective virions that enter cells by this route may bypass a physical barrier to intracellular transport. Fourth, our observation reported herein, that the requirement for Nef is reduced by prebinding virions to cells prior to allowing fusion, is also consistent with the model. Binding of virions at reduced temperatures may facilitate initial attachment in the absence of productive receptor engagement, thereby promoting endocytosis of the virions prior to fusion. Finally, the model is consistent with a recent study in which initiation of intravirion reverse transcription resulted in complete rescue of the impaired infectivity of Nef-defective virions (24). By “jumpstarting” reverse transcription, the putative requirement for intracellular transport mediated by Nef may be relieved. A crucial test of the model will be to determine whether incorporation of Nef into HIV-1 particles is required for HIV-1 infectivity enhancement.
Acknowledgments
We thank Jing Zhou for excellent technical assistance. The following reagents were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: human sCD4 from Norbert Schuelke, the MT-4 cell line from Douglas Richman, and p24 monoclonal antibody from Bruce Chesebro.
This work was supported by NIH grant AI40364.
REFERENCES
- 1.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 71:5871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiken, C., L. Krause, Y.-L. Chen, and D. Trono. 1996. Mutational analysis of HIV-1 Nef: identification of two mutants that are temperature-sensitive for CD4 downregulation. Virology 217:293-300. [DOI] [PubMed] [Google Scholar]
- 3.Aiken, C., and D. Trono. 1995. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J. Virol. 69:5048-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arora, V. K., B. L. Fredericksen, and J. V. Garcia. 2002. Nef: agent of cell subversion. Microbes Infect. 4:189-199. [DOI] [PubMed] [Google Scholar]
- 5.Bell, I., C. Ashman, J. Maughan, E. Hooker, F. Cook, and T. A. Reinhart. 1998. Association of simian immunodeficiency virus Nef with the T-cell receptor (TCR) zeta chain leads to TCR down-modulation. J. Gen. Virol. 79:2717-2727. [DOI] [PubMed] [Google Scholar]
- 6.Bukovsky, A., T. Dorfman, A. Weimann, and H. G. Gottlinger. 1997. Nef association with human immunodeficiency virus type 1 virions and cleavage by the viral protease. J. Virol. 71:1013-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavrois, M., C. De Noronha, and W. C. Greene. 2002. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat. Biotechnol. 20:1151-1154. [DOI] [PubMed] [Google Scholar]
- 8.Charneau, P., M. Alizon, and F. Clavel. 1992. A second origin of DNA plus-strand synthesis is required for optimal human immunodeficiency virus replication. J. Virol. 66:2814-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chazal, N., G. Singer, C. Aiken, M.-L. Hammarskjold, and D. Rekosh. 2001. Human immunodeficiency virus type 1 particles pseudotyped by envelope proteins that fuse at low pH no longer require Nef for optimal infectivity. J. Virol. 75:4014-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, Y.-L., D. Trono, and D. Camaur. 1998. The proteolytic cleavage of human immunodeficiency virus type 1 Nef does not correlate with its ability to stimulate virion infectivity. J. Virol. 72:3178-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chowers, M. Y., M. W. Pandori, C. A. Spina, D. D. Richman, and J. C. Guatelli. 1995. The growth advantage conferred by HIV-1 nef is determined at the level of viral DNA formation and is independent of CD4 downregulation. Virology 212:451-457. [DOI] [PubMed] [Google Scholar]
- 12.Chowers, M. Y., C. A. Spina, T. J. Kwoh, N. J. S. Fitch, D. D. Richman, and J. C. Guatelli. 1994. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J. Virol. 68:2906-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Craig, H. M., M. W. Pandori, and J. C. Guatelli. 1998. Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc. Natl. Acad. Sci. USA 95:11229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craig, H. M., T. R. Reddy, N. L. Riggs, P. P. Dao, and J. C. Guatelli. 2000. Interactions of HIV-1 nef with the mu subunits of adaptor protein complexes 1, 2, and 3: role of the dileucine-based sorting motif. Virology 271:9-17. [DOI] [PubMed] [Google Scholar]
- 15.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, V. A. Lawson, S. Crowe, A. Maerz, S. Sonza, J. Learmont, J. S. Sullivan, A. Cunningham, D. Dwyer, D. Dowton, and J. Mills. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988-991. [DOI] [PubMed] [Google Scholar]
- 16.Doms, R. W., and D. Trono. 2000. The plasma membrane as a combat zone in the HIV battlefield. Genes Dev. 14:2677-2688. [DOI] [PubMed] [Google Scholar]
- 17.Du, Z., S. M. Lang, V. G. Sasseville, A. A. Lackner, P. O. Ilyinskii, M. D. Daniel, J. U. Jung, and R. C. Desrosiers. 1995. Identification of a nef allele that causes lymphocyte activation and acute disease in macaque monkeys. Cell 82:665-674. [DOI] [PubMed] [Google Scholar]
- 18.Forshey, B. M., and C. Aiken. 2003. Disassembly of human immunodeficiency virus type 1 cores in vitro reveals association of Nef with the subviral ribonucleoprotein complex. J. Virol. 77:4409-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forshey, B. M., U. von Schwedler, W. I. Sundquist, and C. Aiken. 2002. Formation of a human immunodeficiency virus type 1 core of optimal stability is crucial for viral replication. J. Virol. 76:5667-5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia, J. V., and A. D. Miller. 1991. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 350:508-511. [DOI] [PubMed] [Google Scholar]
- 21.Geyer, M., H. Yu, R. Mandic, T. Linnemann, Y.-H. Zheng, O. T. Fackler, and B. M. Peterlin. 2002. Subunit H of the V-ATPase binds to the medium chain of adaptor protein complex 2 and connects Nef to the endocytic machinery. J. Biol. Chem. 277:28521-28529. [DOI] [PubMed] [Google Scholar]
- 22.Goldsmith, M. A., M. T. Warmerdam, R. E. Atchison, M. D. Miller, and W. C. Greene. 1995. Dissociation of the CD4 downregulation and viral infectivity enhancement functions of human immunodeficiency virus type 1 Nef. J. Virol. 69:4112-4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 24.Khan, M., M. Garcia-Barrio, and M. D. Powell. 2001. Restoration of wild-type infectivity to human immunodeficiency virus type 1 strains lacking Nef by intravirion reverse transcription. J. Virol. 75:12081-12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332:228-232. [DOI] [PubMed] [Google Scholar]
- 26.Kotov, A., J. Zhou, P. Flicker, and C. Aiken. 1999. Association of Nef with the human immunodeficiency virus type 1 core. J. Virol. 73:8824-8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Gall, S., L. Erdtmann, S. Benichou, C. Berlioz-Torrent, L. Liu, R. Benarous, J. M. Heard, and O. Schwartz. 1998. Nef interacts with the mu subunit of clathrin adaptor complexes and reveals a cryptic sorting signal in MHC I molecules. Immunity 8:483-495. [DOI] [PubMed] [Google Scholar]
- 28.Lineberger, J. E., R. Danzeisen, D. J. Hazuda, A. J. Simon, and M. D. Miller. 2002. Altering expression levels of human immunodeficiency virus type 1 gp120-gp41 affects efficiency but not kinetics of cell-cell fusion. J. Virol. 76:3522-3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu, X., H. Yu, S.-H. Liu, F. M. Brodsky, and B. M. Peterlin. 1998. Interactions between HIV1 Nef and vacuolar ATPase facilitate the internalization of CD4. Immunity 8:647-656. [DOI] [PubMed] [Google Scholar]
- 30.Luo, T., J. L. Douglas, R. L. Livingston, and J. V. Garcia. 1998. Infectivity enhancement by HIV-1 Nef is dependent on the pathway of virus entry: implications for HIV-based gene transfer systems. Virology 241:224-233. [DOI] [PubMed] [Google Scholar]
- 31.Mariani, R., F. Kirchhoff, T. C. Greenough, J. L. Sullivan, R. C. Desrosiers, and J. Skowronski. 1996. High frequency of defective nef alleles in a long-term survivor with nonprogressive human immunodeficiency virus type 1 infection. J. Virol. 70:7752-7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller, M. D., M. T. Warmerdam, S. S. Ferrell, R. Benitez, and W. C. Greene. 1997. Intravirion generation of the C-terminal core domain of HIV-1 Nef by the HIV-1 protease is insufficient to enhance viral infectivity. Virology 234:215-225. [DOI] [PubMed] [Google Scholar]
- 33.Miller, M. D., M. T. Warmerdam, I. Gaston, W. C. Greene, and M. B. Feinberg. 1994. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J. Exp. Med. 179:101-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller, M. D., M. T. Warmerdam, K. A. Page, M. B. Feinberg, and W. C. Greene. 1995. Expression of the human immunodeficiency virus type 1 (HIV-1) nef gene during HIV-1 production increases progeny particle infectivity independently of gp160 or viral entry. J. Virol. 69:570-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mondor, I., S. Ugolini, and Q. J. Sattentau. 1998. Human immunodeficiency virus type 1 attachment to HeLa CD4 cells is CD4 independent and gp120 dependent and requires cell surface heparans. J. Virol. 72:3623-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munk, C., S. M. Brandt, G. Lucero, and N. R. Landau. 2002. A dominant block to HIV-1 replication at reverse transcription in simian cells. Proc. Natl. Acad. Sci. USA 99:13843-13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandori, M. W., N. J. S. Fitch, H. M. Craig, D. D. Richman, C. A. Spina, and J. C. Guatelli. 1996. Producer-cell modification of human immunodeficiency virus type 1: Nef is a virion protein. J. Virol. 70:4283-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piguet, V., Y. L. Chen, A. Mangasarian, M. Foti, J. L. Carpentier, and D. Trono. 1998. Mechanism of Nef-induced CD4 endocytosis: Nef connects CD4 with the mu chain of adaptor complexes. EMBO J. 17:2472-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Premkumar, D. R., X. Z. Ma, R. K. Maitra, B. K. Chakrabarti, J. Salkowitz, B. Yen-Lieberman, M. S. Hirsch, and H. W. Kestler. 1996. The nef gene from a long-term HIV type 1 nonprogressor. AIDS Res. Hum Retrovir. 12:337-345. [DOI] [PubMed] [Google Scholar]
- 40.Sawai, E. T., I. H. Khan, P. M. Montbriand, B. M. Peterlin, C. Cheng-Mayer, and P. A. Luciw. 1996. Activation of PAK by HIV and SIV Nef: importance of AIDS in rhesus macaques. Curr. Biol. 6:1519-1527. [DOI] [PubMed] [Google Scholar]
- 41.Schaeffer, E., R. Geleziunas, and W. C. Greene. 2001. Human immunodeficiency virus type 1 Nef functions at the level of virus entry by enhancing cytoplasmic delivery of virions. J. Virol. 75:2993-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwartz, O., V. Marechal, O. Danos, and J.-M. Heard. 1995. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J. Virol. 69:4053-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J.-M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 44.Sol-Foulon, N., A. Moris, C. Nobile, C. Boccaccio, A. Engering, J. P. Abastado, J. M. Heard, Y. van Kooyk, and O. Schwartz. 2002. HIV-1 Nef-induced upregulation of DC-SIGN in dendritic cells promotes lymphocyte clustering and viral spread. Immunity 16:145-155. [DOI] [PubMed] [Google Scholar]
- 45.Srivastava, K. K., R. Fernandez-Larsson, D. M. Zinkus, and H. L. Robinson. 1991. Human immunodeficiency virus type 1 NL4-3 replication in four T-cell lines: rate and efficiency of entry, a major determinant of permissiveness. J. Virol. 65:3900-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swigut, T., N. Shohdy, and J. Skowronski. 2001. Mechanism for down-regulation of CD28 by Nef. EMBO J. 20:1593-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, J. K., E. Kiyokawa, E. Verdin, and D. Trono. 2000. The Nef protein of HIV-1 associates with rafts and primes T cells for activation. Proc. Natl. Acad. Sci. USA 97:394-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wehrly, K., and B. Chesebro. 1997. p24 antigen capture assay for quantification of human immunodeficiency virus using readily available inexpensive reagents. Methods 12:288-293. [DOI] [PubMed] [Google Scholar]
- 49.Welker, R., H. Hohenberg, U. Tessmer, C. Huckhagel, and H. G. Krausslich. 2000. Biochemical and structural analysis of isolated mature cores of human immunodeficiency virus type 1. J. Virol. 74:1168-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng, Y. H., A. Plemenitas, T. Linnemann, O. T. Fackler, and B. M. Peterlin. 2001. Nef increases infectivity of HIV via lipid rafts. Curr. Biol. 11:875-879. [DOI] [PubMed] [Google Scholar]
- 51.Zhou, J., and C. Aiken. 2001. Nef enhances human immunodeficiency virus type 1 infectivity resulting from intervirion fusion: evidence supporting a role for Nef at the virion envelope. J. Virol. 75:5851-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zlokarnik, G., P. A. Negulescu, T. E. Knapp, L. Mere, N. Burres, L. Feng, M. Whitney, K. Roemer, and R. Y. Tsien. 1998. Quantitation of transcription and clonal selection of single living cells with beta-lactamase as reporter. Science 279:84-88. [DOI] [PubMed] [Google Scholar]