Abstract
Synthesis of Gag-Pol polyproteins of retroviruses requires ribosomes to shift translational reading frame once or twice in a −1 direction to read through the stop codon in the gag reading frame. It is generally believed that a slippery sequence and a downstream RNA structure are required for the programmed −1 ribosomal frameshifting. However, the mechanism regulating the Gag-Pol frameshifting remains poorly understood. In this report, we have defined specific mRNA elements required for sufficient ribosomal frameshifting in equine anemia infectious virus (EIAV) by using full-length provirus replication and Gag/Gag-Pol expression systems. The results of these studies revealed that frameshifting efficiency and viral replication were dependent on a characteristic slippery sequence, a five-base-paired GC stretch, and a pseudoknot structure. Heterologous slippery sequences from human immunodeficiency virus type 1 and visna virus were able to substitute for the EIAV slippery sequence in supporting EIAV replication. Disruption of the GC-paired stretch abolished the frameshifting required for viral replication, and disruption of the pseudoknot reduced the frameshifting efficiency by 60%. Our data indicated that maintenance of the essential RNA signals (slippery sequences and structural elements) in this region of the genomic mRNA was critical for sufficient ribosomal frameshifting and EIAV replication, while concomitant alterations in the amino acids translated from the same region of the mRNA could be tolerated during replication. The data further indicated that proviral mutations that reduced frameshifting efficiency by as much as 50% continued to sustain viral replication and that greater reductions in frameshifting efficiency lead to replication defects. These studies define for the first time the RNA sequence and structural determinants of Gag-Pol frameshifting necessary for EIAV replication, reveal novel aspects relative to frameshifting elements described for other retroviruses, and provide new genetic determinants that can be evaluated as potential antiviral targets.
Ribosomal frameshifting is a translational decoding mechanism that allows expression of a single protein from two or more overlapping genes. All retroviruses contain gag, pro, and/or pol genes, and the expression of Gag-Pol polyprotein is achieved by a −1 ribosomal frameshifting to allow translational readthrough (5). It has been demonstrated that when ribosomes translate the unspliced genomic RNA of retroviruses, 95% of translation yields Gag proteins while only about 5% of translation produces Gag-Pol proteins through −1 ribosomal frameshifting (29). However, the precise mechanisms of the programmed frameshifting remain largely undefined. Therefore, characterization of functional elements involved in ribosomal frameshifting from various retroviruses can provide new insights on genetic determinants that control frameshifting during viral replication. Furthermore, because translational frameshifting is a vital process for retroviruses (13), this function offers a potential target for antiviral drug development (1). Elucidation of frameshifting mechanisms may provide critical information that can be used to design new therapeutics targeting Gag-Pol translational frameshifting to block retroviral replication.
In general, it is believed that the occurrence and frequency of translational frameshifting are determined predominantly by two elements of mRNA: a slippery sequence and a downstream RNA structure. Various slippery sequences have been identified from different retroviruses and from other viruses (5, 18). A typical slippery sequence is a heptanucleotide X XXY YYZ (where X ≠ C, Y = A or U, and Z ≠ G) (14, 19). The slippery sequence itself induces a basal level (∼1%) of frameshifting; downstream stimulatory RNA structures substantially regulate this process, in some cases up to 50% (12, 14). There are to date two kinds of RNA structures associated with frameshifting, stem-loop (3, 14, 15, 19, 21) and pseudoknot (4, 11, 16, 27, 33), and the stability of these structures has been correlated with translational frameshifting efficiency (3, 16). However, not all stem-loop or pseudoknot structures can induce ribosomal frameshifting (16, 17, 32). Thus, the definitive characterization of specific RNA structures and how these RNA structures mediate ribosomal frameshifting remain to be defined.
Equine anemia infectious virus (EIAV) is a member of the lentivirus subfamily of retroviruses that also includes human immunodeficiency virus type 1 (HIV-1). In contrast to HIV-1, study on the genetic elements mediating EIAV translational frameshifting has been very limited. Sequence comparisons to other viruses indicate a putative slippery sequence located within the C terminus of the EIAV NC coding sequence (28). Both stem-loop and pseudoknot structures can be predicted with equal confidence based on thermodynamic stability and the statistical significance of the predicted structures (18, 31) (Fig. 1). In this study, we sought to distinguish between these predicted models and to define RNA elements for EIAV frameshifting and their roles in viral replication.
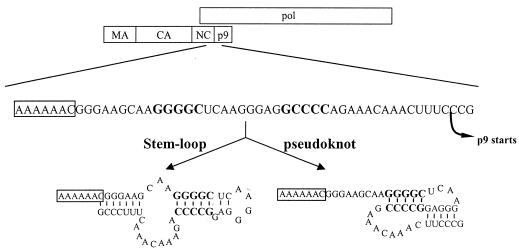
FIG. 1.
Schematic diagram showing the RNA determinants for EIAV Gag-Pol frameshifting. The 58 nucleotides correspond to the coding sequence of C-terminal NC spanning from nucleotide 1707 to 1764 in EIAVuk proviral DNA (AFO16316 [reference 9]). The slippery sequence is boxed, nucleotides involved in the GC-paired stretch are bolded, and the starting point of the p9 protein is indicated. The stem-loop structure was predicted by the stability of the RNA structure (18), and the pseudoknot structure was predicted based on computer-aided comparisons of a number of viral mRNAs (31).
For most studies on ribosomal frameshifting, slippery sequence segments and the downstream sequences are cloned into a reporter gene in order to quantify frameshifting efficiency in vitro (3, 15, 19, 30). However, a limitation of these studies is that it is impossible to evaluate the biological relevance of observed alterations in frameshifting efficiency. Here, we studied EIAV frameshifting in the context of the full-length EIAV provirus and assayed the effects of frameshifting mutations both at the levels of viral replication and expressed viral proteins. The results of our studies revealed for the first time novel sequence and structural elements essential for EIAV Gag-Pol frameshifting.
MATERIALS AND METHODS
DNA mutagenesis.
Two versions of proviral DNA, EIAVuk and CMVuk pro−, were used in the present studies. EIAVuk is an in vivo pathogenic proviral DNA clone described previously (8, 9). CMVuk pro− is derived from the EIAVuk by insertion of a cytomegalovirus (CMV) promoter into the 5′ long terminal repeat region to obtain higher levels of protein expression (8). Additionally, the active-site aspartic acid of the protease was mutated to alanine, resulting in a null protease that is unable to process Gag and Gag-Pol. Consequently, full-length Gag and Gag-Pol polyproteins are accumulated and can be detected in the transfected cells. All the mutants were constructed using overlapping PCR methods as described previously (8) and were sequenced to confirm the specified mutations.
Cell culture and transfection.
Equine dermal (ED) and Cos-1 cells were cultured as described previously (8). All plasmid DNA preparations used for transfection were purified using a Qiagen Midiprep kit (Qiagen, Valencia, Calif.). Transfections were carried out using GenePorter 2 reagents (Gene Therapy Systems, San Diego, Calif.) according to the manufacturer's recommendations. ED cells transfected with the frameshifting mutants in the context of the EIAVuk backbone were assayed to examine replication properties of the mutants (8). Cos-1 cells transfected with the CMVuk pro− proviral constructs were examined to quantify the frameshifting efficiency of specific frameshifting mutants, because the level of protein expression is much higher in transfected Cos-1 cells than in ED cells. Our unpublished data showed that viral particles produced from transfected Cos-1 cells by EIAVuk proviral DNA are infectious to ED cells, indicating adequate ribosomal frameshifting in either cell type.
RT assays.
To characterize replication properties of the specific frameshifting mutants, the reverse transcriptase (RT) activity of culture medium from transfected ED cells was assayed as a measure of viral production. Culture medium was collected at the indicated days posttransfection, and the RT activity of 15 μl of cell culture supernatant was assayed as described previously (20).
Protein assays.
To examine levels of expressed viral proteins, transfected Cos-1 cells grown on six-well plates were lysed with 250 μl of lysis buffer (25 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1% deoxycholic acid, 1% Triton X-100, protease inhibitor cocktail, 0.1% sodium dodecyl sulfate) at 48 h posttransfection. The postnuclear cellular lysates were then resolved by electrophoresis through a 3 to 8% gradient Tris-acetate gel (NuPage; Invitrogen, Carlsbad, Calif.) and immunoblotted using a reference immune serum from a naturally infected horse (24). Horseradish peroxidase-conjugated goat anti-horse immunoglobulin G [F(ab′)2; Jackson ImmunoResearch, West Grove, Pa.] was used as the secondary antibody. The immunoblots were developed by incubation with SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, Ill.). The amounts of viral Gag and Gag-Pol proteins were digitally quantified using a Kodak imaging station model 1000. Each construct, including the wild-type control, was assayed in duplicate in transfection and Western blot analyses, and mean values of ratios of Gag-Pol versus Gag from the duplicates were used to compare frameshifting efficiencies.
Prediction of mRNA secondary structure.
The mRNA structures were predicted using the computer program RNAstructure 3.71 (http://128.151.176.70/RNAstructure.html), a Windows implementation of the Zuker algorithm for RNA secondary structure prediction based on free energy minimization written by David H. Mathews (23).
RESULTS
Identification of the slippery sequence necessary for EIAV Gag-Pol frameshifting.
Figure 1 schematically depicts the proposed EIAV slippery sequence and two possible regulatory RNA structures previously predicted by the lowest free energy of folding (stem-loop structure [18]) or by sequence comparison with other viruses (pseudoknot structure [31]). We initially assessed the EIAV slippery sequence to confirm its putative role in frameshifting and Gag-Pol expression. An A→T transition within the proposed slippery sequence was generated to introduce a stop codon into the gag-pol reading frame while keeping the gag reading frame intact. The introduced stop codon was designed to block the synthesis of full-length Gag-Pol, given that the predicted slippery sequence was the site of frameshifting. Subsequently, the designed mutant should be replication deficient due to lack of functional Gag-Pol proteins. As shown in Fig. 2A, the mutant EIAVuk carrying the A→T mutation was indeed completely replication deficient. When transfected into the Cos-1 cells, the CMVuk pro− A→T mutant produced only Gag protein; no Gag-Pol polyprotein was detected with highly reactive reference immune serum in Western blotting (Fig. 2B and C). These results confirmed the site of Gag-Pol frameshifting and demonstrated that a stop codon introduced into the gag-pol reading frame completely abolished Gag-Pol synthesis.
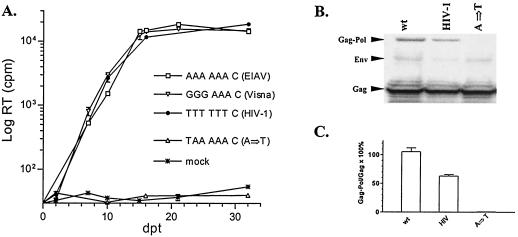
FIG. 2.
Replication profiles and Gag-Pol expression of chimeric EIAV containing variant slippery sequences. (A) EIAVuk provirus containing the indicated mutations was transfected into ED cells. Supernatant medium of each transfected sample was collected at the indicated days posttransfection (dpt). RT activity of the collected supernatant was assayed as a measure of virus production from transfected cells. Slippery sequences and the origin of each mutant are indicated. Duplicates of each mutant were transfected, and the presented data are representative of three independent experiments. (B) Cellular lysates of Cos-1 cells transfected with CMVuk pro− proviruses carrying the indicated mutants were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 3 to 8% gradient Tris-acetate gel (see Materials and Methods). EIAV-specific proteins were identified by immunoblotting with a reference immune serum from a naturally infected horse (8, 24). The data represent at least duplicate experiments. (C) Digitally quantified frameshifting efficiencies of the mutants from the Western blotting, with the mean value of duplicate wild-type controls set as 100%, using a Kodak imaging station (see Materials and Methods). The data presented here were calculated from at least duplicate samples.
It has been reported that different slippery sequences confer slightly different levels of frameshifting in an in vitro assay system and that the frameshifting efficiency of a particular slippery sequence is also influenced by the flanking sequence (15). To examine the biological impact of different slippery sequences on viral replication, the confirmed EIAV slippery sequence was replaced with slippery sequences of HIV-1 or visna virus, whose sequences are completely different from the EIAV slippery sequence, and replication properties of the resultant chimeric proviruses were evaluated (Fig. 2A). Interestingly, the replication kinetics of these two chimeric EIAVuk proviruses were indistinguishable from that of the wild-type EIAVuk, indicating that heterologous slippery sequences can successfully support EIAV replication. The frameshifting efficiency of the chimeric mutant carrying the HIV-1 slippery sequence was also compared with that of the wild type in a Western blotting assay (Fig. 2B and C). To quantify frameshifting efficiency, the ratio of Gag-Pol to Gag from the wild type was set as 100%, and the same ratios from the mutants were compared with those of the wild-type control. As summarized in Fig. 2B and C, the chimeric mutant carrying the HIV-1 slippery sequence displayed about one-half of the level of translational frameshifting observed with the wild-type control. These observations confirm the previous report that frameshifting efficiency of a specific slippery sequence is affected by downstream regulatory sequences (15). However, the replication property of the mutant was not impaired (Fig. 2A), indicating that as low as 50% of wild-type frameshifting is sufficient to translate enough Gag-Pol proteins for EIAV replication. Thus, the wild-type EIAV genomic sequence appears to generate twice the amount of Gag-Pol polyprotein required for the assembly of replication-competent progeny virions.
Characterization of the function of a GC-paired stretch in EIAV Gag-Pol frameshifting.
Based on the predicted RNA structures, there is a characteristic 5-bp GC stretch nine nucleotides downstream of the slippery sequence in both the predicted pseudoknot and stem-loop structural models (Fig. 1). To test the function of this paired region in Gag-Pol translational frameshifting, two specific mutants were generated. First, the mRNA sequence on one side of the GC-paired segment was mutated to disrupt the base pairing (Fig. 3A, unpaired). Evaluation of the replication competence of the unpaired mutant in transfected ED cells revealed that the alteration of the GC pairing completely blocked detectable replication (Fig. 3B). Analysis of the translational frameshifting efficiency of the unpaired mutant further revealed a fivefold reduction in Gag-Pol production compared to the wild type in transfected Cos-1 cells (Fig. 3C and D). Assuming that about 5% of ribosomes undergo frameshifting during translation of wild-type EIAV genomic RNA, the observed fivefold reduction in frameshifting efficiency correlates with an overall 1% of ribosomal frameshifting, a rate that is similar to the basal level of frameshifting conferred by the slippery sequence alone. Thus, these data demonstrated that disruption of the GC-paired segment markedly suppressed EIAV Gag-Pol frameshifting and revealed a critical role for the GC-paired segment in enhancing Gag-Pol frameshifting by about fivefold above the frameshifting levels mediated by the slippery sequence alone.
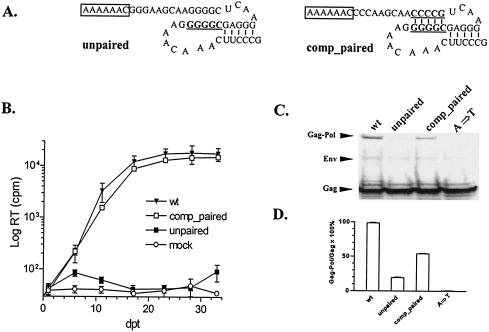
FIG. 3.
Function of GC-paired stretch in EIAV translational frameshifting and viral replication. (A) Predicted secondary structures of mutants altering the GC-paired segment. The slippery sequences are boxed, and mutated nucleotides are in bold type and underlined. (B) Replication profiles of EIAV mutants in ED cells transfected with proviral constructs described above for panel A compared to those of wild-type virus and a replication-defective slippery sequence mutant (A→T in Fig. 1). (C) Gag and Gag-Pol protein expressed in Cos-1 cells transfected with CMVuk pro− proviruses containing the indicated mutants, using procedures described in the legend for Fig. 2. (D) Relative frameshifting efficiencies of the mutants compared with wild-type control quantified as outlined in the legend for Fig. 2. The data are representative of three independent experiments.
To further evaluate the role of the GC-paired segment in frameshifting, a complementary mutation was made to restore base pairing (Fig. 3A, comp_paired). Replication competency of the comp_paired mutant in transfected ED cells (Fig. 3B) demonstrated the necessity of the intact GC-base-paired segment during viral replication. Analysis of Gag-Pol translational frameshifting efficiency revealed levels of Gag-Pol production that were about 50% of wild-type levels (Fig. 3C and D). Therefore, the crucial role of this RNA base pairing is further emphasized by the observation that RNA sequence alterations in the comp_paired mutant did not affect viral replication, despite the fact that these mutations altered the amino acid sequence of the C terminus of NC protein and the N terminus of preprotease encoded by this genomic segment. These observations define the GC-base-paired segment as a crucial structural determinant of translational frameshifting in EIAV.
Testing of the stem-loop structural model for EIAV frameshifting.
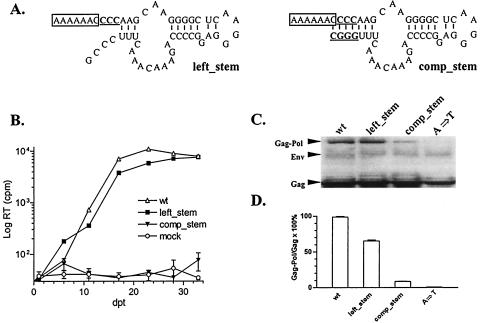
In addition to the GC-paired stretch examined in the preceding section, there is another predicted paired stem immediately following the EIAV slippery sequence in the proposed stem-loop structure, but not in the pseudoknot structure (Fig. 1). To test the biological relevance of this second stem in frameshifting, the GGG sequence immediately following the slippery sequence was mutated to CCC to disrupt the predicted stem structure, resulting in the left_stem mutant (Fig. 4A). Interestingly, the left_stem mutant replicated as well as the wild-type virus in transfected ED cells (Fig. 4B) and showed about 60% of the wild-type level of frameshifting (Fig. 4C and D). The data indicated that the predicted paired stem structure is not required for EIAV replication. To further test the stem-loop model, we generated a second mutant in which the opposite side of the stem was mutated complementarily to restore the base pairing in the stem structure, namely, comp_stem (Fig. 4A). Since the cleavage site between NC and p9 of the Gag polyprotein was altered in the comp_stem mutant, it is expected that this mutant would be replication deficient, as complete polyprotein processing is required for the production of infectious virus (25, 34) (Fig. 4B). Surprisingly, the comp_stem mutant expressed only about 5% of Gag-Pol polyproteins compared with the wild-type control (Fig. 4C and D), indicating that restored base pairing in the stem-loop structure dramatically reduced frameshifting efficiency. The free energy (ΔG) of the comp_stem mutant folded into the predicted stem-loop structure is 15% higher than the wild type folded into the same stem-loop structure (RNA structure 3.71 [data not shown]), suggesting that the comp_stem mutant may be thermodynamically unfavorable and fold into an alternative structure with the lowest free energy but that is not functional for inducing frameshifting. Nevertheless, these data indicate that formation of a paired stem downstream of the EIAV slippery sequence is not required for sufficient Gag-Pol frameshifting to support viral replication and that restored base pairing in the comp_stem mutant abolished Gag-Pol frameshifting. Thus, these observations argue against the proposed stem-loop as the structural model for the RNA segment mediating Gag-Pol translational frameshifting in EIAV.
FIG. 4.
Role of the predicted stem-loop structure in EIAV Gag-Pol frameshifting. (A) Predicted secondary structures of EIAV mutants. The slippery sequences are boxed, and mutated nucleotides are in bold type and underlined. (B) Replication profiles of EIAV proviral mutants defined in panel A after transfection of ED cells using procedures described in the legend for Fig. 2. (C) Analyses of Gag and Gag-Pol polyprotein expression in Cos 1 cells transfected with the indicated mutant constructs and compared to wild-type or a replication-defective (A→T) slippery sequence mutant. (D) The relative frameshifting efficiency of each mutant was analyzed as described in the legend for Fig. 2D. The data are representative of three independent experiments.
Testing of the pseudoknot structural model for EIAV frameshifting.
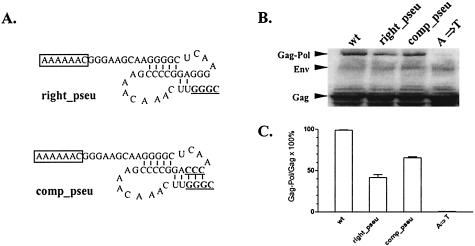
The preceding data indirectly support the pseudoknot structure model, as the left_stem mutant that maintained the predicted pseudoknot while disrupting the predicted stem-loop was replication competent. To test the pseudoknot model directly, we next evaluated two additional mutants directed at critical predicted pseudoknot motifs. As shown in Fig. 5A, the right_pseu mutant disrupted base pairing that contributes to the formation of the predicted pseudoknot, and the comp_pseu mutant compensated the right_pseu mutation to restore the pseudoknot structure. Due to the fact that both mutations contain a mutated cleavage site between NC and p9 of Gag, it was not feasible to examine the effects of the pseudoknot mutants on viral replication. Therefore, we only assessed the Gag-Pol frameshifting efficiencies of these mutants in transfected Cos-1 cells, as summarized in Fig. 5C and D. These data demonstrated that disruption of the predicted pseudoknot in the right_pseu mutant reduced Gag-Pol frameshifting levels to 35% of that observed in wild-type virus, indicating that the amounts Gag-Pol proteins expressed from the right_pseu mutant were reduced by twofold compared with the wild-type control. Restoration of the pseudoknot in the comp_pseu mutant rescued the translational frameshifting efficiency to 60% of wild-type level. Thus, these data indicate that mutations in sequence motifs integral to the pseudoknot model markedly affect Gag-Pol translational frameshifting efficiency. While it is not possible to directly correlate levels of Gag-Pol frameshifting with viral replication potential, it is interesting that the frameshifting mutants (e.g., comp_paired and left_stem) described above with 50% frameshifting efficiency replicated with similar kinetics to wild-type virus. Therefore, it is reasonable to predict that the levels of Gag-Pol frameshifting produced by the comp_pseu mutant could sustain viral replication, while the lower levels of frameshifting observed with right_pseu would be inadequate to support virus replication.
FIG. 5.
Function of the pseudoknot structure in EIAV translational frameshifting. (A) Predicted pseudoknot structures of indicated EIAV mutants. The slippery sequences are boxed, and mutated nucleotides are in bold type and underlined. (B) EIAV Gag and Gag-Pol polyprotein expression in Cos-1 cells transfected with CMVuk pro− proviruses containing the indicated mutants, as described in legends for previous figures. (C) Relative translational frameshifting efficiencies of the mutants compared with the wild type, calculated as described in the legend for Fig. 2. The data are representative of duplicate experiments.
DISCUSSION
In this report we have for the first time experimentally defined the genetic determinants of EIAV genomic RNA that are essential for Gag-Pol ribosomal frameshifting. Using a combination of assays for viral Gag-Pol protein expression and for viral replication, we were able to quantify the influence of selected RNA mutations on translational frameshifting efficiency and to correlate these results with replication competence. In this manner, we have defined several critical RNA sequence motifs that affect EIAV Gag-Pol frameshifting, including a slippery sequence, a GC-paired stretch, and a pseudoknot structure.
Comparisons of EIAV RNA sequences with other retroviruses have predicted an AAA AAA C slippery sequence in the EIAV genome (18). Using our protein expression and proviral replication assays, we demonstrated that introduction of a termination codon within the slippery sequence inhibited Gag-Pol, but not Gag, protein expression and completely inhibited virus replication in transfected ED cells (Fig. 2). Thus, these data confirmed the critical role of the predicted slippery sequence in EIAV Gag-Pol polyprotein expression and viral replication. In addition, we demonstrated that structurally distinct heterologous slippery sequences from either HIV-1 or visna virus substituted into the EIAV provirus supported virus replication at levels similar to the wild-type virus (Fig. 2). An informative observation in these latter studies was that the level of EIAV Gag-Pol expression produced by the HIV-1 slippery sequences was about one-half of the level observed with the wild-type slippery sequence. Therefore, these data indicate that the levels of Gag-Pol expression observed during EIAV replication are in fact in at least twofold excess of the minimum level required to support viral replication, providing a surplus of Gag-Pol protein for incorporation into progeny virions.
In general, Gag-Pol expression during retroviral replication is believed to occur at a frequency of about 5% (1 of 20 ribosome translations) compared to Gag protein translation, with only about 5 to 10 Gag-Pol polyproteins being incorporated into budding virions containing several thousand copies of Gag polyprotein. The present studies demonstrate that a 50% reduction in frameshifting is compatible with virus replication (the HIV-1 in Fig. 2, comp_paired in Fig. 3, and left_stem in Fig. 4) and that an 80% reduction in frameshifting abolishes viral replication (unpaired in Fig. 3). While there appears to be only a difference of about 30% frameshifting efficiency from the replication-competent (50% of frameshifting) to replication-deficient (20% frameshifting) phenotypes, the level of Gag-Pol expression was determined to be one-half of the wild type with 50% frameshifting and only one-fifth of the wild type with 20% frameshifting efficiency, representing a 2.5-fold reduction in terms of the amount of expressed Gag-Pol. The present observations that wild-type levels of viral replication were supported by one-half of wild-type Gag-Pol expression may indicate specific mechanisms that retroviruses have evolved with to survive in the presence of slightly imbalanced amounts (i.e., 50% of the wild-type frameshifting) of Gag-Pol proteins. However, if the level of Gag-Pol proteins were reduced by fourfold (i.e., 20% of the wild-type frameshifting), the amounts of Gag-Pol proteins for viral replication would be under the minimal threshold and cause a deficiency in viral replication.
The second EIAV genetic determinant identified for −1 Gag-Pol translational frameshifting was a distinct 5-base GC-paired segment downstream of the slippery sequence. Disruption of the base pairing reduced Gag-Pol polyprotein expression to only 20% of the wild-type level and completely inhibited proviral replication (Fig. 3). Confirming the role of the GC-paired segment was the observation that restoration of the base pairing by complementary mutations rescued proviral replication to wild-type levels with a concomitant increase in Gag-Pol polyprotein expression to about 50% of wild-type levels, or about 1 of 40 (2.5%) ribosomes translating Gag-Pol from the genomic RNA. These data provide two novel insights into the regulation and role of EIAV Gag-Pol translational frameshifting. First, the five-base-paired stretch identified in EIAV is substantially shorter than stem structures found in the previously identified stem-loop model, which are around 9 to 12 bases long (14, 19, 21). Second, the fact that 2.5% of Gag-Pol polyprotein expression was sufficient for EIAV replication suggests that a level of translational frameshifting of 1 of 40 ribosomes translating the Gag-Pol proteins can be tolerated for productive viral replication.
The information on EIAV Gag-Pol frameshifting was previously limited to structural modeling studies that predicted either a pseudoknot (31) or a stem-loop (18) conformation, based on comparisons to other retroviruses or computer modeling. The present studies were designed to test these two predicted models by examining the effects of mutations on sequence motifs distinct for each predicted structure (Fig. 4 and 5). The results of these studies evidently are consistent with the pseudoknot model and argue against the stem-loop model for Gag-Pol frameshifting. As summarized in Fig. 4, disruption of a predicted left stem base pairing unique to the stem-loop structure did not affect proviral replication of this variant, while introduction of this predicted base pairing in a second variant (comp_stem) completely inhibited virus replication and Gag-Pol polyprotein expression. The comp_stem mutation was designed to disrupt base pairings essential for the formation of the pseudoknot structure and to simultaneously facilitate formation of the predicted stem-loop structure. In the absence of the pseudoknot structure, the frameshifting efficiency of the comp_stem mutant was reduced by 20-fold. These data indicated an incompatibility of the stem-loop structure with replication-competent virus, suggesting the alternative pseudoknot structure as the appropriate conformation. While the location of the characteristic base pairing that contributed to the pseudoknot structure interfered with Gag processing and precluded examination of replication properties of variant proviruses, assays of Gag-Pol expression clearly revealed the influence of pseudoknot mutations on translational frameshifting efficiency (Fig. 5). Mutations that disrupted pseudoknot base pairing markedly reduced Gag-Pol polyprotein expression by twofold, while complementary mutations to restore the pseudoknot base pairing rescued Gag-Pol expression. Thus, these data are consistent with the pseudoknot organization as a structural basis for EIAV Gag-Pol translational frameshifting.
It is interesting that recent crystallographic studies on translational complexes have revealed that when tRNA molecules decode a slippery sequence in a translational ribosome complex, the frameshifting regulatory structure is proximal to the translational complex with a spacer passing through the ribosomal tunnel in single-stranded form (26). This structural model suggests that a single-stranded mRNA spacer between the slippery sequence and the regulatory RNA structure is essential for programmed −1 ribosomal frameshifting. In this regard, the EIAV pseudoknot structure, but not the stem-loop structure, uniquely provides this single-stranded spacer region. These structural considerations would predict that mutations eliminating the single-stranded spacer (e.g., comp_stem in Fig. 4) would inhibit translational frameshifting, as observed in these EIAV studies.
Among the structural determinants of EIAV translational frameshifting identified in the present studies, there appears to be a hierarchy of regulatory control. The GC-paired stretch appears to be the major determinant of translational frameshifting, while the pseudoknot motif performs a secondary regulatory role. These two structural determinants then may provide a coarse and fine control, respectively, on translational frameshifting to regulate Gag-Pol polyprotein expression. These observations are consistent with recent studies indicating fine regulatory roles of mRNA structure on Gag-Pol translational frameshifting efficiency. For example, Gag-Pol translational frameshifting in Rous sarcoma virus genomic RNA was predominantly mediated by a complex stem-loop structure, while a pseudoknot structure fine-tuned the frameshifting efficiency (22). Recently, an intramolecular triplex RNA structure has been indicated in regulating HIV-1 Gag-Pol translational frameshifting (10), in contrast to previous studies indicating a hairpin structure downstream of the HIV-1 slippery sequence as sufficient to induce adequate translational frameshifting (7). Also, a base pairing interaction bridging a 4-kb-long stretch has been shown to regulate translational frameshifting of Barley yellow dwarf virus mRNA (2). Taken together, these data indicate complex RNA structural interactions in modulating protein expression via translational frameshifting.
The EIAV slippery sequence and the associated regulatory structures are located within the C terminus of the NC coding sequence (Fig. 1). Due to the fact that the gag reading frame overlaps with the gag-pol reading frame, our mutations to change mRNA structure resulted in concomitant alterations of amino acids at the C terminus of NC within Gag and in the preprotease sequence within Gag-Pol. However, the mutants made in this study were replication competent as long as the RNA signals (slippery sequences and regulatory structural elements) for frameshifting were maintained without mutating the cleavage site between NC and p9. These results suggested that viruses might have evolved to tolerate alteration of amino acids in this region to accommodate RNA structures essential for frameshifting. Recently, it was reported that murine leukemia virus with its readthrough signal replaced by the HIV-1 frameshifting signal remains replication competent despite an extra 44 nucleotides that were inserted into the N terminus of the preprotease (6).
Translational frameshifting represents an effective strategy for genetic economy in retroviruses that allows differential expression of proteins from overlapping nucleotide sequences in a single mRNA. This strategy eliminates the need for mRNA splicing in which transcriptional controls are required to regulate ultimate protein expression levels. To achieve the appropriate levels of translation and protein expression from the overlapping genes by translational frameshifting, it appears that retroviruses rely on an ordered hierarchy of RNA structures that provide coarse and fine modulation of ribosomal frameshifting. Given the importance of these structural determinants for retroviral replication, these genetic elements may provide novel conserved targets for antivirals based on small inhibitory RNA or other modalities.
Acknowledgments
This work was supported by grant number R01 CA49296 from the National Cancer Institute of the National Institutes of Health to R. C. Montelaro. Chaoping Chen was supported as a Postdoctoral Trainee in the Molecular Microbial Persistence and Pathogenesis training program funded by National Institutes of Health grant T32 AI49820 from the National Institute of Allergy and Infectious Diseases.
We thank Feng Li for critical discussions and production of the CMVuk pro− mutant, and we acknowledge the DNA Sequencing Core of the University of Pittsburgh for their technical assistance.
REFERENCES
- 1.Aupeix-Scheidler, K., S. Chabas, L. Bidou, J. P. Rousset, M. Leng, and J. J. Toulme. 2000. Inhibition of in vitro and ex vivo translation by a transplatin-modified oligo(2′-O-methylribonucleotide) directed against the HIV-1 gag-pol frameshift signal. Nucleic Acids Res. 28:438-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry, J. K., and W. A. Miller. 2002. A −1 ribosomal frameshift element that requires base pairing across four kilobases suggests a mechanism of regulating ribosome and replicase traffic on a viral RNA. Proc. Natl. Acad. Sci. USA 99:11133-11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bidou, L., G. Stahl, B. Grima, H. Liu, M. Cassan, and J. P. Rousset. 1997. In vivo HIV-1 frameshifting efficiency is directly related to the stability of the stem-loop stimulatory signal. RNA 3:1153-1158. [PMC free article] [PubMed] [Google Scholar]
- 4.Brierley, I. 1993. Probing the mechanism of ribosomal frameshifting on viral RNAs. Biochem. Soc. Trans. 21:822-826. [DOI] [PubMed] [Google Scholar]
- 5.Brierley, I. 1995. Ribosomal frameshifting viral RNAs. J. Gen. Virol. 76:1885-1892. [DOI] [PubMed] [Google Scholar]
- 6.Brunelle, M. N., L. Brakier-Gingras, and G. Lemay. 2003. Replacement of murine leukemia virus readthrough mechanism by human immunodeficiency virus frameshift allows synthesis of viral proteins and virus replication. J. Virol. 77:3345-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunelle, M. N., C. Payant, G. Lemay, and L. Brakier-Gingras. 1999. Expression of the human immunodeficiency virus frameshift signal in a bacterial cell-free system: influence of an interaction between the ribosome and a stem-loop structure downstream from the slippery site. Nucleic Acids Res. 27:4783-4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, C., F. Li, and R. C. Montelaro. 2001. Functional roles of equine infectious anemia virus Gag p9 in viral budding and infection. J. Virol. 75:9762-9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook, R. F., C. Leroux, S. J. Cook, S. L. Berger, D. L. Lichtenstein, N. N. Ghabrial, R. C. Montelaro, and C. J. Issel. 1998. Development and characterization of an in vivo pathogenic molecular clone of equine infectious anemia virus. J. Virol. 72:1383-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinman, J. D., S. Richter, E. P. Plant, R. C. Taylor, A. B. Hammell, and T. M. Rana. 2002. The frameshift signal of HIV-1 involves a potential intramolecular triplex RNA structure. Proc. Natl. Acad. Sci. USA 99:5331-5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du, Z., J. A. Holland, M. R. Hansen, D. P. Giedroc, and D. W. Hoffman. 1997. Base-pairings within the RNA pseudoknot associated with the simian retrovirus-1 gag-pro frameshift site. J. Mol. Biol. 270:464-470. [DOI] [PubMed] [Google Scholar]
- 12.Giedroc, D. P., C. A. Theimer, and P. L. Nixon. 2000. Structure, stability and function of RNA pseudoknots involved in stimulating ribosomal frameshifting. J. Mol. Biol. 298:167-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hung, M., P. Patel, S. Davis, and S. R. Green. 1998. Importance of ribosomal frameshifting for human immunodeficiency virus type 1 particle assembly and replication. J. Virol. 72:4819-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, K. H., and S. A. Lommel. 1998. Sequence element required for efficient −1 ribosomal frameshifting in red clover necrotic mosaic dianthovirus. Virology 250:50-59. [DOI] [PubMed] [Google Scholar]
- 15.Kim, Y. G., S. Maas, and A. Rich. 2001. Comparative mutational analysis of cis-acting RNA signals for translational frameshifting in HIV-1 and HTLV-2. Nucleic Acids Res. 29:1125-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, Y. G., L. Su, S. Maas, A. O'Neill, and A. Rich. 1999. Specific mutations in a viral RNA pseudoknot drastically change ribosomal frameshifting efficiency. Proc. Natl. Acad. Sci. USA 96:14234-14239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kontos, H., S. Napthine, and I. Brierley. 2001. Ribosomal pausing at a frameshifter RNA pseudoknot is sensitive to reading phase but shows little correlation with frameshift efficiency. Mol. Cell. Biol. 21:8657-8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le, S. Y., B. A. Shapiro, J. H. Chen, R. Nussinov, and J. V. Maizel. 1991. RNA pseudoknots downstream of the frameshift sites of retroviruses. Genet. Anal. Tech. Appl. 8:191-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, L., A. L. Wang, and C. C. Wang. 2001. Structural analysis of the −1 ribosomal frameshift elements in giardiavirus mRNA. J. Virol. 75:10612-10622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lichtenstein, D. L., K. E. Rushlow, R. F. Cook, M. L. Raabe, C. J. Swardson, G. J. Kociba, C. J. Issel, and R. C. Montelaro. 1995. Replication in vitro and in vivo of an equine infectious anemia virus mutant deficient in dUTPase activity. J. Virol. 69:2881-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lucchesi, J., K. Makelainen, A. Merits, T. Tamm, and K. Makinen. 2000. Regulation of −1 ribosomal frameshifting directed by cocksfoot mottle sobemovirus genome. Eur. J. Biochem. 267:3523-3529. [DOI] [PubMed] [Google Scholar]
- 22.Marczinke, B., R. Fisher, M. Vidakovic, A. J. Bloys, and I. Brierley. 1998. Secondary structure and mutational analysis of the ribosomal frameshift signal of Rous sarcoma virus. J. Mol. Biol. 284:205-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathews, D. H., J. Sabina, M. Zuker, and D. H. Turner. 1999. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J. Mol. Biol. 288:911-940. [DOI] [PubMed] [Google Scholar]
- 24.Montelaro, R. C., B. Parekh, A. Orrego, and C. J. Issel. 1984. Antigenic variation during persistent infection by equine infectious anemia virus, a retrovirus. J. Biol. Chem. 259:10539-10544. [PubMed] [Google Scholar]
- 25.Pettit, S. C., M. D. Moody, R. S. Wehbie, A. H. Kaplan, P. V. Nantermet, C. A. Klein, and R. Swanstrom. 1994. The p2 domain of human immunodeficiency virus type 1 Gag regulates sequential proteolytic processing and is required to produce fully infectious virions. J. Virol. 68:8017-8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plant, E. P., K. L. Jacobs, J. W. Harger, A. Meskauskas, J. L. Jacobs, J. L. Baxter, A. N. Petrov, and J. D. Dinman. 2003. The 9-A solution: how mRNA pseudoknots promote efficient programmed −1 ribosomal frameshifting. RNA 9:168-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen, L. X., and I. Tinoco, Jr. 1995. The structure of an RNA pseudoknot that causes efficient frameshifting in mouse mammary tumor virus. J. Mol. Biol. 247:963-978. [DOI] [PubMed] [Google Scholar]
- 28.Stephens, R. M., J. W. Casey, and N. R. Rice. 1986. Equine infectious anemia virus gag and pol genes: relatedness to visna and AIDS virus. Science 231:589-594. [DOI] [PubMed] [Google Scholar]
- 29.Swanstrom, R., and J. W. Wills. 1997. Synthesis, assembly, and processing of viral proteins, p. 263-334. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [PubMed]
- 30.Telenti, A., R. Martinez, M. Munoz, G. Bleiber, G. Greub, D. Sanglard, and S. Peters. 2002. Analysis of natural variants of the human immunodeficiency virus type 1 gag-pol frameshift stem-loop structure. J. Virol. 76:7868-7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ten Dam, E. B., C. W. Pleij, and L. Bosch. 1990. RNA pseudoknots: translational frameshifting and readthrough on viral RNAs. Virus Genes 4:121-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang, Y., N. M. Wills, Z. Du, A. Rangan, J. F. Atkins, R. F. Gesteland, and D. W. Hoffman. 2002. Comparative studies of frameshifting and nonframeshifting RNA pseudoknots: a mutational and NMR investigation of pseudoknots derived from the bacteriophage T2 gene 32 mRNA and the retroviral gag-pro frameshift site. RNA 8:981-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wills, N. M., R. F. Gesteland, and J. F. Atkins. 1991. Evidence that a downstream pseudoknot is required for translational read-through of the Moloney murine leukemia virus gag stop codon. Proc. Natl. Acad. Sci. USA 88:6991-6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu, X. F., Z. Matsuda, Q. C. Yu, T. H. Lee, and M. Essex. 1995. Role of the C terminus Gag protein in human immunodeficiency virus type 1 virion assembly and maturation. J. Gen. Virol. 76:3171-3179. [DOI] [PubMed] [Google Scholar]