Abstract
Lassa virus is an enveloped virus with glycoprotein spikes on its surface. It contains an RNA ambisense genome that encodes the glycoprotein precursor GP-C, the nucleoprotein NP, the polymerase L, and the Z protein. Here we demonstrate that the Lassa virus Z protein (i) is abundant in viral particles, (ii) is strongly membrane associated, (iii) is sufficient in the absence of all other viral proteins to release enveloped particles, and (iv) contains two late domains, PTAP and PPXY, necessary for the release of virus-like particles. Our data provide evidence that Z is the Lassa virus matrix protein that is the driving force for virus particle release.
Lassa virus belongs to the large family of Arenaviridae, including the closely related Lymphocytic choriomeningitis virus (LCMV) and other important human pathogens like Guanarito virus, Junin virus, and Machupo virus. Lassa virus is the etiologic agent of a hemorrhagic fever endemic in West Africa, where annually up to 100,000 cases of clinically apparent Lassa fever occur. Up to 20% of these patients develop hemorrhagic manifestations with a total mortality of 10 to 15% (28, 29). In recent years this disease has been increasingly exported from regions where it is endemic to other parts of the world (36).
Lassa virus consists of a helical nucleocapsid containing a bisegmented RNA genome surrounded by a lipid bilayer with integrated glycoprotein spikes. Each single-stranded RNA encodes two viral genes in an ambisense coding strategy separated by an intergenic region. The small RNA encodes the nucleoprotein NP (60 kDa) and the immature glycoprotein precursor pre-GP-C (80 kDa), which is cotranslationally cleaved by signal peptidase into GP-C (75 kDa) and a stable signal peptide of 58 amino acids (aa) (10). GP-C is cleaved posttranslationally by subtilase SKI-1/S1P into the N-terminal subunit GP-1 (40 kDa) and the membrane-bound subunit GP-2 (35 kDa). Both subunits are incorporated in virus particles (24, 25). The large RNA segment encodes the RNA-dependent RNA polymerase L (∼200 kDa) and the Z protein with a length of 99 amino acids and a molecular mass of approximately 11 kDa (33, 35).
During the last few years, the role of the arenavirus Z protein has been elucidated in respect to virus replication. The Z protein of Lassa virus and LCMV contains a RING motif and was shown to have zinc-binding activity (34). Most of the information so far indicates a regulatory role of Z, as the LCMV Z protein was shown to bind to the promyelotic leukemia protein and to relocate nuclear structures formed by the promyelotic leukemia protein (2, 4). The LCMV Z protein has also been reported to interact with the nuclear fraction of the ribosomal protein P0 and with the eukaryotic translation initiation factor eIF4E (3, 7). Furthermore, the Z protein of Tacaribe virus is implicated in RNA synthesis and genome replication in the early stage of infection (13). In contrast, the Z protein of LCMV was not required for RNA replication and transcription in a LCMV minigenome system. Moreover, the Z protein exerted a potent inhibitory effect on intracellular transcription and replication of the LCMV minigenome in a dose-dependent manner (9). In addition to the regulatory role of Z during virus replication, the Z protein was suggested as a structural component of the virus particle (5, 34). Furthermore, a hypothesis was formulated that Z might be the arenavirus counterpart of matrix proteins found in other negative-strand RNA viruses (22).
Here we investigated the role of the Lassa virus Z protein for assembly and release of virus-like particles. We demonstrate that solitary expressed Z protein is strongly membrane associated like typical matrix proteins of other lipid-enveloped RNA viruses. Moreover, we show that expression of recombinant Z protein in the absence of other viral proteins is sufficient for the release of lipid-enveloped virus-like particles with an efficiency similar to that observed from Lassa virus-infected cells. The Z protein contains two so-called late domains close to the C terminus of Z. Late domains were originally identified in the Gag proteins of a number of retroviruses and in the matrix proteins of rhabdo- and filoviruses and interact with cellular target proteins to promote virus particle release by membrane fission (11, 12, 15, 17, 18, 26, 27, 37, 38). Mutation of two separate tetrapeptide motifs of Z suspected as late domains significantly reduced the release of virus-like particles. We therefore suggest that the Z protein is a matrix protein containing two late domains that are involved in the fission of virus particles from the plasma membrane.
(T.S. and R.E. performed this work in partial fulfillment of the requirements for a Ph.D. degree from the Philipps-Universität Marburg.)
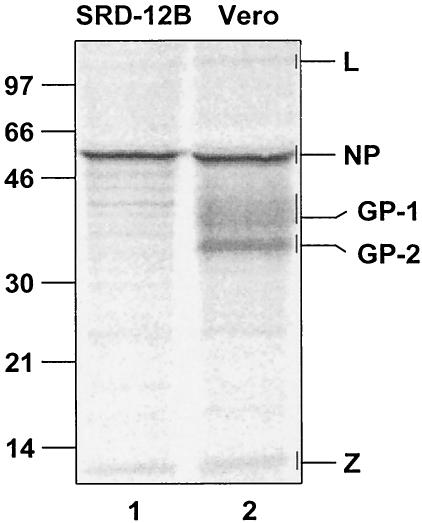
Quantification of Lassa virus Z protein in virus particles.
In order to determine the amounts of L, NP, GP-1, GP-2, and Z proteins in Lassa virus particles, Vero and SRD-12B cells were infected with Lassa virus and were incubated with [35S]methionine-cysteine. The supernatant of the cells was freed from cellular debris, and virus was purified as described earlier (25). Viral proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were quantified (Fig. 1, lane 2). The intensity of the bands of radioactively labeled protein was calculated on the basis of methionine and cysteine content. The analysis resulted in a L:NP:GP-1:GP-2:Z ratio of approximately 1:160:60:60:20. These data show that the Z protein is sufficiently present in virus particles for building up a matrix structure beneath the lipid layer of the viral envelope. It is noteworthy that the ratio of NP to Z (160:20) was comparable to Lassa virus-like particles devoid of glycoproteins that were released from SKI-1/S1P-deficient SRD-12B cells (Fig. 1, lane 1) (25). Our findings of the Lassa virus protein composition are comparable to those of LCMV (34).
FIG. 1.
Quantification of Lassa virus proteins. Vero and SRD cells were infected with Lassa virus at a multiplicity of infection of 1 before cells were labeled with 800 μCi of [35S]methonine-cysteine (Premix; Amersham-Buchler) 24 h postinfection for 48 h. Virions of cell culture supernatants were precleared by centrifugation at 1,000 × g for 10 min and were then passed through a 20% sucrose cushion by UC at 20,000 rpm for 2 h (SW28 rotor; Beckman). Viral proteins were subjected to SDS-PAGE on 12% polyacrylamide gels and were quantified by using a FUJI BAS 1000 BioImaging analyzer system (raytest). Masses are given in kilodaltons on left.
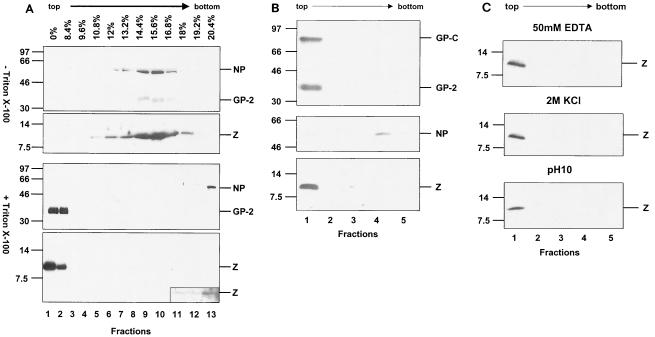
Membrane association of Lassa virus Z protein.
Lassa virus was propagated in Vero cells, cell supernatant was freed from cellular debris, and viral particles were pelleted through a 20% sucrose cushion as described before (25). Virus particles were either treated with 1% Triton X-100 to dissolve the lipid envelope or were left untreated. Viral material was loaded on iodixanol gradients and was subjected to velocity ultracentrifugation (UC) (24). Fractions were collected from the top of the gradient, and aliquots were subjected to SDS-PAGE with subsequent immunoblotting and were analyzed for Lassa virus-specific proteins by using antibodies against GP-2, NP and Z proteins (Fig. 2A). As expected, NP, GP-2, and Z were predominantly found in fractions 8 and 9, whereas protein of Triton X-100-treated material behaves differently. Membrane-bound GP-2 was present in the top fractions 1 and 2 of the gradient, whereas NP was found in the bottom fraction (fraction 13). Interestingly, the majority of Z was present in the top fractions 1 and 2, indicating a membrane-binding capacity of Z protein. Furthermore, a very faint band of Z appeared in the bottom fraction when the immunochemical luminescence reaction was extended (Fig. 2A, insertion). This observation could be due to a small soluble fraction of Z protein or could indicate an interaction of a small fraction of Z protein with NP.
FIG. 2.
Membrane association of Lassa virus Z protein. (A) Purified Lassa virus was left untreated (upper panel) or was treated with 1% Triton X-100, loaded on iodixanol (Sigma) gradients, and subjected to UC (1.5 h at 4°C, 41,000 rpm, SW41; Beckman). Aliquots of the fractions of both gradients were subjected to SDS-PAGE. Viral proteins were subsequently immunoblotted and were detected with the antisera anti-GP-2, anti-NP, and anti-Z (24). Antisera against NP and Z were raised in rabbits as described previously (24) by using peptides derived from Lassa virus strain Josiah NP (aa 53 to 76) and from the Z protein (aa 2 to 16). (B) Membrane association of solitarily expressed Z. Vero cells were transfected with the beta-actin promoter-driven pCAGGS vector (30) with insertions encoding Z, GP-C, and NP. Forty-eight hours posttransfection, cells were disrupted in 20 mM Tris-HCl, pH 7.4, by using a Dounce homogenizer and were centrifuged (10 min, 700 × g). Supernatant containing membranes and cytosol was adjusted with OptiPrep (Sigma) to a final concentration of 35% (wt/wt) and was overlaid with 30% OptiPrep in TNE and subsequently with TNE lacking OptiPrep. Samples were then centrifuged to equilibrium at 165,000 × g for 4 h at 4°C in an SW55 rotor (Beckman). Fractions of 0.5 ml were collected from the top of the gradient after centrifugation. Aliquots were subjected to SDS-PAGE followed by immunoblotting using specific antisera against GP-C, NP, and Z. (C) Detachment studies of membrane-bound Z protein. Aliquots of fraction 1 from the membrane flotation described in legend for panel B were either treated with 2 M KCl or with 50 mM EDTA for 1 h at room temperature or were treated for 1 h at 4°C with 1 M sodium bicarbonate (pH 10) that was neutralized with 1 M Tris-HCl (pH 6.8). Aliquots then were subjected to a second round of flotation analysis and were subsequently analyzed by SDS-PAGE followed by immunoblotting. Masses are given in kilodaltons on left of panels A through C.
In order to investigate whether Z alone bound efficiently to cellular membranes in mammalian cells or whether an interaction with other viral proteins, for example with GP-2, was necessary for membrane binding, flotation analysis of solitary expressed Z protein was performed. Lassa virus Z protein and, as controls, NP and GP-C were individually transiently expressed in Vero cells as described earlier (1, 25). Cells expressing the respective viral proteins were disrupted, and the postnuclear supernatant was subjected to flotation. Cellular membranes and associated proteins float to the OptiPrep-TNE (25 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 5 mM EDTA) interface during UC. The integral membrane protein GP-C and its subunit GP-2 were detected in the OptiPrep-TNE interface (fraction 1), whereas NP was detected in high-density fractions 4 and 5 (Fig. 2B). Interestingly, the Z protein floated into the interface between 30% OptiPrep and TNE (fraction 1), indicating that the Z protein is associated with cellular membranes. These experiments show that Z alone possesses an intrinsic membrane-binding capacity.
The nature and strength of the membrane binding of Z were examined by various well-established membrane detachment procedures by using either sodium bicarbonate buffer at pH 10 or 2 M KCl or 50 mM EDTA before the treated samples were subjected to flotation analysis as described previously (20, 21). However, none of these treatments, which characteristically remove most peripheral membrane proteins, disrupted the association of Z protein with membranes (Fig. 2C). Similar membrane-binding characteristics have been described before for matrix proteins of several viruses, such as vesicular stomatitis virus, Sendai virus, influenza virus, and measles virus (6, 8, 21, 32). In contrast, the Borna disease virus matrix protein can be removed from biological membranes by the treatments mentioned above (20).
Release of Z protein containing enveloped particles.
A huge body of evidence implicates viral matrix proteins in the formation and release of viral particles (14). We recently showed that virus particles containing RNA, NP, L, and Z but devoid of glycoprotein are released from cells lacking the GP-C-processing protease SKI-1/S1P. To examine whether the Z protein is the driving force for budding and release of virus-like particles, Z was expressed recombinantly in Vero cells in the absence of other viral proteins. Supernatants of Z- and, as a control, NP-transfected cells were freed from cellular debris and were subjected to UC through a 20% sucrose cushion as described previously (25). The UC-pelleted material was analyzed by SDS-PAGE, followed by immunoblotting with Z- and NP-specific antisera, respectively. The Z protein was detected in the UC-pelleted fraction, while solitarily expressed NP was not detected, although both viral proteins were expressed at comparable levels as shown by immunoblot detection of intracellular NP and Z (Fig. 3A). In order to prove that Z is released in an enveloped particle, we employed a protease protection assay as described before (25). Therefore, UC-pelleted material from supernatants of transfected cells was either treated with proteinase K or with proteinase K in the presence of Triton X-100 or was left untreated. Treatment with proteinase K alone had no effect on the Z protein, whereas the addition of Triton X-100 destroyed the lipid envelope and therefore enabled the degradation of Z by proteinase K (Fig. 3B). These results clearly demonstrate that Z induces the production of Z-containing enveloped particles.
FIG. 3.
Analysis of released Z-protein-containing particles. (A) Vero cell cultures were transfected with pCAGGS encoding Z and, as a control, NP. Cellular supernatants were collected, freed from cellular debris (3,000 × g, 10 min), and pelleted through a 20% sucrose cushion by UC. The UC pellet was dissolved in 100 μl of SDS-PAGE sample buffer, and transfected cells were dissolved in 500 μl of SDS-PAGE sample buffer. Twenty-microliter samples were subjected to SDS-PAGE followed by immunoblotting. NP and Z were visualized by specific immune reactions. C, cell lysate; S, pelleted material from the supernatant. (B) Protease protection assay for the identification of lipid-enveloped Z-containing particles. Aliquots of UC-pelleted Z were either treated with 0.1 μg of proteinase K alone per μl or proteinase K with 1% Triton X-100 for 30 min at 37°C or were left untreated. Samples then were analyzed by SDS-PAGE followed by immunoblotting. Masses are given in kilodaltons on left of both panels A and B.
Identification of two late domains.
The primary sequence of Z reveals two putative late domains, PTAP (amino acids 81 to 84) and PPPY (amino acids 94 to 97) (Fig. 4A). In order to prove which of both motifs or whether both motifs play a role in the pinching off of enveloped particles from the plasma membrane, deletion and substitution mutants of the two potential late domains were generated by recombinant mutagenesis (Fig. 4B). Wild-type Z protein and the respective mutants of Z protein were transiently expressed in Vero cells with the pCAGGS vector and were radioactively labeled. In addition, Vero cells and SRD-12B cells were infected with Lassa virus and were radioactively labeled. Z protein of released particles and intracellular Z protein were immunoprecipitated, subjected to SDS-PAGE, and quantified. The percentage of released Z protein in comparison to Z protein expressed in cells was calculated. Interestingly, the amount of Z released as enveloped particles after recombinant expression is comparable to the release of Z protein-containing virus particles after Lassa virus infection of Vero cells or of virus-like particles released from infected SKI-1/S1P-deficient SRD-12B cells (Fig. 5A). The percentage of released wild-type Z particles after transfection was set to 100%, and the ratio of released mutant Z protein is shown as the relative percentage compared to wild-type Z protein. Truncation of the C terminus of Z by 6, 15, or 30 amino acids reduced the amount of released Z particles by almost 90% (Fig. 5B). In order to show the significance of each late domain in the Z protein, the PTAP and PPPY tetrapeptides were mutated. Mutant 81ATAA84 containing a functional PPPY motif but a mutated PTAP motif still releases Z particles from the transfected cells up to 50% compared to wild-type Z particles, indicating that the PTAP motif also plays a role for particle release, although the effect is not as strong as the one of the PPPY motif. Z protein with a mutated PPPY motif (mutant 94-97A) and an intact PTAP motif is released only to about 10%, indicating that the PPPY motif is the dominant factor for virus release. In order to prove whether a single point mutation within the PPPY motif is sufficient to inhibit particle release, tyrosine 97 was replaced by alanine. This mutation decreased Z particle release to a level comparable to that of the deletion mutants. Mutation of both domains (mutant 81-84/94-97A) had no additional effect on the release of virus-like particles. In conclusion, our mutational analyses clearly demonstrate that both motifs, PTAP and PPPY, are functionally competent for efficient release of enveloped Z particles, indicating that both motifs are essential for an efficient release of Lassa virus particles. However, the PPPY motif plays a dominant role in this process.
FIG. 4.
Lassa virus wild-type Z protein and mutants. (A) The amino acid sequence of Lassa virus Z protein, strain Josiah, is shown in one-letter code (NCBI accession number NC_004296/004297). Amino acids associated with particular structures and possible functions are shown in boldfaced single letters. The cysteine and histidine residues that are proposed to form the RING domain are underlined, the two tetrapeptides postulated as late domains are doubly underlined, and the hydrophobic peptide (aa 50 to 61) is indicated with a dotted line. (B) Wild-type Z, deletion, and substitution mutants used in this study are shown schematically.
FIG. 5.
Release of Lassa virus, wild-type Z particles, and Z particles with mutated late domains. Vero and SRD-12B cells were infected with Lassa virus at a multiplicity of infection of 1. In addition, Vero cell cultures were transfected with pCAGGS encoding wild-type and mutated Z, respectively. Forty-eight hours postinfection and 24 h posttransfection, respectively, cells were metabolically labeled with [35S]methionine-cysteine for 4 and 8 h. Collected cells and cell supernatants were clarified from cell debris by centrifugation. Z protein was immunoprecipitated by using a precipitation buffer containing detergents, subjected to SDS-PAGE, and quantified by using a BioImaging analyzer system. Wild-type and mutated Z was tested in at least three independent experiments differing within 10%. Mean values are shown. (A) The release of Lassa virus and virus-like particles after infection of Vero and SRD-12B cells or with wild-type Z protein-transfected cells was quantified, and the ratio of Z protein detected in the supernatant to cellular Z protein was calculated (Tina software). (B) The ratio of released wild-type (wt) Z protein was set to 100%, and the ratio of released mutant Z protein is shown as the relative percentage compared to wild-type Z protein.
Interestingly, an obvious counterpart of a matrix protein has not yet been identified for arenaviruses, although cross-linking studies with LCMV virions have shown complex formation between NP and Z, suggesting a possible role of Z in virion morphogenesis (34). Here we provide evidence that the Lassa virus Z protein plays the dominant role for virus budding. We demonstrated that Lassa virus Z protein is abundant in virus particles. It possesses an intrinsic lipid membrane-binding capacity, as was demonstrated for many matrix proteins of lipid-enveloped viruses. Solitary expression of Z revealed a strong membrane association that is resistant to EDTA or high-pH or high-salt-concentration conditions. Similar results were described for matrix proteins of many negative single-stranded RNA viruses. The vesicular stomatitis virus matrix protein, for example, has been reported to penetrate the phospholipid membrane as deep as nine carbon residues of membrane lipids, while other matrix proteins need myristylation or use internal sequences forming basic patches of beta sheets or contain hydrophobic domains in their secondary structure to mediate membrane association (23). The nature of the interaction of the Z protein with lipid membranes is not known, but a combination of basic and hydrophobic residues could be responsible for this membrane-binding capacity. Our observations that Z protein expressed in the absence of other viral proteins and after virus infection is immunocytochemically visualized in characteristic cytoplasmic patches and only in small amounts near the cell surface are in agreement with studies of other viral matrix proteins, including VP40 of Marburg virus and Ebola virus (data not shown) (16, 19, 38). The similarity of intracellular distribution of Z protein to those of other well-known viral matrix proteins further supports our view that Z functions as a viral matrix protein.
A number of viral matrix proteins contain late domains that were shown to be essential for the budding of these viruses. Three classes of late domains have been identified as PTAP, PPXY, and YXXL motifs. They can be moved from its original location or can even be interchanged between different matrix proteins without loss of function (12, 31). Of the known Z proteins of the arenaviruses, only the Z protein of Lassa virus contains two late domains, the PTAP and PPXY motif. Of the other published arenavirus Z protein sequences, the Z sequences of LCMV strains Armstrong and Mx also contain a PPPY motif but not a PTAP motif, whereas the WE strain possesses only a PPY motif close to the C terminus that does not fulfill the known criteria for a late domain. In contrast, the Z protein of the New World arenavirus Pichinde contains only a PSAP motif, whereas Tacaribe virus does not contain a characteristic proline-rich motif. These sequence deviations between different arenaviruses are not understood but might influence the different pathogenicity within the virus family. For Lassa virus, our data clearly show that the PTAP and PPXY motif is required for efficient release of Z protein-containing particles. Mutation of only one of these two motifs resulted in a clear decrease in release of particles, although mutation of the C-terminal PPPY motif shows a much stronger effect. The existence of two synergistic or redundant late domains as described before for some other viral matrix protein could prove to become a common theme for virus release.
In conclusion, the structural function of the Z protein as a matrix protein has been demonstrated for Lassa virus and is suggested for all other arenaviruses.
Acknowledgments
T.S. and R.E. contributed equally to this work.
Peptides were synthesized and kindly provided by M. Krause, Institut für Molekularbiologie und Tumorforschung, Universität Marburg.
This work was supported by the Deutsche Forschungsgemeinschaft, Sachbeihilfe Ga 282/4-1, and SFB 286 TP1. T.S. was supported by the Graduiertenkolleg “Protein function at the atomic level” and the “Studienstiftung des deutschen Volkes,” Marburg. R.E. was supported by the FAZIT-Stiftung, Frankfurt, Germany.
REFERENCES
- 1.Beyer, W. R., D. Popplau, W. Garten, D. von Laer, and O. Lenz. 2003. Endoproteolytic processing of the lymphocytic choriomeningitis virus glycoprotein by the subtilase SKI-1/S1P. J. Virol. 77:2866-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borden, K. L. B., E. J. Campbell Dwyer, and M. S. Salvato. 1998. An arenavirus RING (zinc-binding) protein binds the oncoprotein promyelocyte leukemia protein (PML) and relocates PML nuclear bodies to the cytoplasm. J. Virol. 72:758-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borden, K. L. B., E. J. Campbelldwyer, G. W. Carlile, M. Djavani, and M. S. Salvato. 1998. Two RING finger proteins, the oncoprotein PML and the arenavirus Z protein, colocalize with the nuclear fraction of the ribosomal P proteins. J. Virol. 72:3819-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borden, K. L., E. J. CampbellDwyer, and M. S. Salvato. 1997. The promyelocytic leukemia protein PML has a pro-apoptotic activity mediated through its RING domain. FEBS Lett. 418:30-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchmeier, M. J. 2002. Arenaviruses: protein structure and function, p. 159-173. In M. B. A. Oldstone (ed.), Arenaviruses, vol. I. Springer, Berlin, Germany. [DOI] [PubMed]
- 6.Caldwell, S. E., and D. S. Lyles. 1986. Dissociation of newly synthesized Sendai viral proteins from the cytoplasmic surface of isolated plasma membranes of infected cells. J. Virol. 57:678-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell Dwyer, E. J., H. Lai, R. C. MacDonald, M. S. Salvato, and K. L. B. Borden. 2000. The lymphocytic choriomeningitis virus RING protein Z associates with eukaryotic initiation factor 4E and selectively represses translation in a RING-dependent manner. J. Virol. 74:3293-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chong, L. D., and J. K. Rose. 1993. Membrane association of functional vesicular stomatitis virus matrix protein in vivo. J. Virol. 67:407-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornu, T. I., and J. C. de la Torre. 2001. RING finger Z protein of lymphocytic choriomeningitis virus (LCMV) inhibits transcription and RNA replication of an LCMV S-segment minigenome. J. Virol. 75:9415-9426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eichler, R., O. Lenz, T. Strecker, and W. Garten. 2003. Signal peptide of Lassa virus glycoprotein GP-C exhibits an unusual length. FEBS Lett. 538:203-206. [DOI] [PubMed] [Google Scholar]
- 11.Freed, E. O. 2003. The HIV-TSG101 interface: recent advances in a budding field. Trends Microbiol. 11:56-59. [DOI] [PubMed] [Google Scholar]
- 12.Freed, E. O. 2002. Viral late domains. J. Virol. 76:4679-4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcin, D., S. Rochat, and D. Kolakofsky. 1993. The Tacaribe arenavirus small zinc finger protein is required for both mRNA synthesis and genome replication. J. Virol. 67:807-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garoff, H., R. Hewson, and D.-J. E. Opstelten. 1998. Virus maturation by budding. Microbiol. Mol. Biol. Rev. 62:1171-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garrus, J. E., U. K. von Schwedler, O. W. Pornillos, S. G. Morham, K. H. Zavitz, H. E. Wang, D. A. Wettstein, K. M. Stray, M. Cote, R. L. Rich, D. G. Myszka, and W. I. Sundquist. 2001. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107:55-65. [DOI] [PubMed] [Google Scholar]
- 16.Harty, R. N., M. E. Brown, G. Wang, J. Huibregtse, and F. P. Hayes. 2000. A PPxY motif within the VP40 protein of Ebola virus interacts physically and functionally with a ubiquitin ligase: implications for filovirus budding. Proc. Natl. Acad. Sci. USA 97:13871-13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harty, R. N., J. Paragas, M. Sudol, and P. Palese. 1999. A proline-rich motif within the matrix protein of vesicular stomatitis virus and rabies virus interacts with WW domains of cellular proteins: implications for viral budding. J. Virol. 73:2921-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jayakar, H. R., K. G. Murti, and M. A. Whitt. 2000. Mutations in the PPPY motif of vesicular stomatitis virus matrix protein reduce virus budding by inhibiting a late step in virion release. J. Virol. 74:9818-9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolesnikova, L., H. Bugany, H. D. Klenk, and S. Becker. 2002. VP40, the matrix protein of Marburg virus, is associated with membranes of the late endosomal compartment. J. Virol. 76:1825-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraus, I., M. Eickmann, S. Kiermayer, H. Scheffczik, M. Fluess, J. A. Richt, and W. Garten. 2001. Open reading frame III of Borna disease virus encodes a nonglycosylated matrix protein. J. Virol. 75:12098-12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kretzschmar, E., M. Bui, and J. K. Rose. 1996. Membrane association of influenza virus matrix protein does not require specific hydrophobic domains or the viral glycoproteins. Virology 220:37-45. [DOI] [PubMed] [Google Scholar]
- 22.Lee, K. J., and J. de la Torre. 2002. Reverse genetics of Arenaviruses, p. 175-193. In M. B. A. Oldstone (ed.), Arenaviruses. Springer, Berlin, Germany. [DOI] [PubMed]
- 23.Lenard, J. 1996. Negative-strand virus M and retrovirus MA proteins: all in a family? Virology 216:289-298. [DOI] [PubMed] [Google Scholar]
- 24.Lenz, O., J. ter Meulen, H. Feldmann, H. D. Klenk, and W. Garten. 2000. Identification of a novel consensus sequence at the cleavage site of the Lassa virus glycoprotein. J. Virol. 74:11418-11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenz, O., J. ter Meulen, H. D. Klenk, N. G. Seidah, and W. Garten. 2001. The Lassa virus glycoprotein precursor GP-C is proteolytically processed by subtilase SKI-1/S1P. Proc. Natl. Acad. Sci. USA 98:12701-12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Licata, J. M., M. Simpson-Holley, N. T. Wright, Z. Han, J. Paragas, and R. N. Harty. 2003. Overlapping motifs (PTAP and PPEY) within the Ebola virus VP40 protein function independently as late budding domains: involvement of host proteins TSG101 and VPS-4. J. Virol. 77:1812-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin-Serrano, J., T. Zang, and P. D. Bieniasz. 2001. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat. Med. 7:1313-1319. [DOI] [PubMed] [Google Scholar]
- 28.McCormick, J. B., I. J. King, P. A. Webb, K. M. Johnson, R. O'Sullivan, E. S. Smith, S. Trippel, and T. C. Tong. 1987. A case-control study of the clinical diagnosis and course of Lassa fever. J. Infect. Dis. 155:445-455. [DOI] [PubMed] [Google Scholar]
- 29.McCormick, J. B., P. A. Webb, J. W. Krebs, K. M. Johnson, and E. S. Smith. 1987. A prospective study of the epidemiology and ecology of Lassa fever. J. Infect. Dis. 155:437-444. [DOI] [PubMed] [Google Scholar]
- 30.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 31.Pornillos, O., J. E. Garrus, and W. I. Sundquist. 2002. Mechanisms of enveloped RNA virus budding. Trends Cell Biol. 12:569-579. [DOI] [PubMed] [Google Scholar]
- 32.Riedl, P., M. Moll, H. D. Klenk, and A. Maisner. 2002. Measles virus matrix protein is not cotransported with the viral glycoproteins but requires virus infection for efficient surface targeting. Virus Res. 83:1-12. [DOI] [PubMed] [Google Scholar]
- 33.Salvato, M., E. Shimomaye, and M. B. Oldstone. 1989. The primary structure of the lymphocytic choriomeningitis virus L gene encodes a putative RNA polymerase. Virology 169:377-384. [DOI] [PubMed] [Google Scholar]
- 34.Salvato, M. S., K. J. Schweighofer, J. Burns, and E. M. Shimomaye. 1992. Biochemical and immunological evidence that the 11 kDa zinc-binding protein of lymphocytic choriomeningitis virus is a structural component of the virus. Virus Res. 22:185-198. [DOI] [PubMed] [Google Scholar]
- 35.Salvato, M. S., and E. M. Shimomaye. 1989. The completed sequence of lymphocytic choriomeningitis virus reveals a unique RNA structure and a gene for a zinc finger protein. Virology 173:1-10. [DOI] [PubMed] [Google Scholar]
- 36.ter Meulen, J., O. Lenz, L. Koivogui, N. Magassouba, S. K. Kaushik, R. Lewis, and W. Aldis. 2001. Short communication: Lassa fever in Sierra Leone: UN peacekeepers are at risk. Trop. Med. Int. Health 6:83-84. [DOI] [PubMed] [Google Scholar]
- 37.Timmins, J., G. Schoehn, S. Ricard-Blum, S. Scianimanico, T. Vernet, R. W. Ruigrok, and W. Weissenhorn. 2003. Ebola virus matrix protein VP40 interaction with human cellular factors Tsg101 and Nedd4. J. Mol. Biol. 326:493-502. [DOI] [PubMed] [Google Scholar]
- 38.Timmins, J., S. Scianimanico, G. Schoehn, and W. Weissenhorn. 2001. Vesicular release of ebola virus matrix protein VP40. Virology 283:1-6. [DOI] [PubMed] [Google Scholar]