Abstract
We recently reported that immunostimulatory oligodeoxynucleotides (CpG oligodeoxynucleotides [CpG-ODN]) were effective in postexposure treatment of retrovirus-induced disease (A. R. M. Olbrich et al., J. Virol. 76:11397-11404, 2002). We now show that the timing of treatment is a critical factor in treatment efficacy. In stark contrast to the success of postexposure treatments, we found that CpG treatment of susceptible mice prior to Friend retrovirus infection accelerated the development of virus-induced erythroleukemia. Furthermore, 70.8% of mice that were resistant to Friend virus-induced leukemia developed disease after inoculation of CpG-ODN before infection. The CpG pretreatment of these mice enhanced viral loads in their spleens and blood compared to controls that received ODN without CpG motifs. The main target cells of Friend virus, erythroid precursor cells and B cells, proliferated after CpG-ODN inoculation and provided an enlarged target cell population for viral infection. Our present findings together with our previous report demonstrate that CpG-ODN treatment of viral infections may be a double-edged sword that can result in an effective therapy but also in an acceleration of disease progression depending on the time point of treatment.
The treatment of mice with synthetic oligodeoxynucleotides containing unmethylated CpG motifs (CpG-ODN) has been shown to have curative effects in allergy models (26), experimental cancer models (1), and infectious diseases (20, 22, 27). In particular, the ability of CpG-ODN to promote Th1-type responses and activate several cell populations of the immune system has been associated with the therapeutic effect of CpG-ODN in these models (17). In several recently published mouse studies CpG-ODN have been reported to be very effective against tumor challenges or infections with microbes when they were injected prior to inoculation of the disease-inducing agent (1, 6, 19, 24). Thus, a nonspecific priming of the immune system by CpG-ODN seems to enhance resistance to different challenges in animals. These data suggest that CpG-ODN might be an interesting substance for inducing paraimmunity in animals or individuals at risk of acquiring viral diseases. Paraimmunity-inducing drugs, which are based on poxvirus antigen preparations, are currently used for nonspecific vaccination of livestock and companion animals.
In a recent publication CpG-ODN have been reported to induce protective paraimmunity in a mouse model of genital herpesvirus infection (7). In sharp contrast to these findings we show here that CpG-ODN treatment prior to infection of mice with Friend retrovirus (FV) can accelerate virus-induced disease. FV is a retrovirus complex comprised of a replication-competent helper virus called Friend murine leukemia virus, which is nonpathogenic in adult mice, and a replication-defective but pathogenic component called spleen focus-forming virus (13). Infection of adult susceptible mice with FV complex induces severe splenomegaly, which is followed within several weeks by the development of lethal erythroleukemia (11, 25). The major histocompatibility complex genotype of the infected animal strongly influences the initial immune response of a mouse against the virus and its susceptibility to FV. For example, mice of the H-2b/b haplotype are resistant to FV-induced disease because they mount lymphocyte responses that appear earlier and are of higher magnitude than those of mice with an H-2a/b haplotype, which are susceptible to FV-mediated leukemia (8, 21). Immunity to FV is associated with a Th1-type immune response, including the production of gamma interferon and the activation of T cells (4, 21). Since CpG-ODN shift the immune system toward a Th1-dominated response, it was possible that CpG-ODN pretreatment of FV-susceptible mice might induce resistance. On the other hand, CpG-ODN have been shown to promote proliferation of a number of different cell populations of the hematopoietic system (16, 23). Since initial FV infection and viral spread depend on proliferating hematopoietic cells (14, 15), it was also possible that CpG pretreatment could enlarge the target cell pool for the virus and thus enhance virulence.
To test for these two possible effects of CpG-ODN, susceptible (B10.A × A.BY)F1 mice (H-2a/b) or resistant (B10 × A.BY)F1 mice (H-2b/b) were injected with 15 nmol (95 μg) of CpG-ODN 1668 (27) or control ODN containing no CpG motif on days −12, −4, and 0 before FV infection. These were the same dose of CpG-ODN and the same frequency of injections that were used previously for the successful postexposure treatment of FV infection (20). After CpG-ODN inoculation mice were injected intravenously on day 0 with 3,000 spleen focus-forming units of pathogenic FV complex (2).
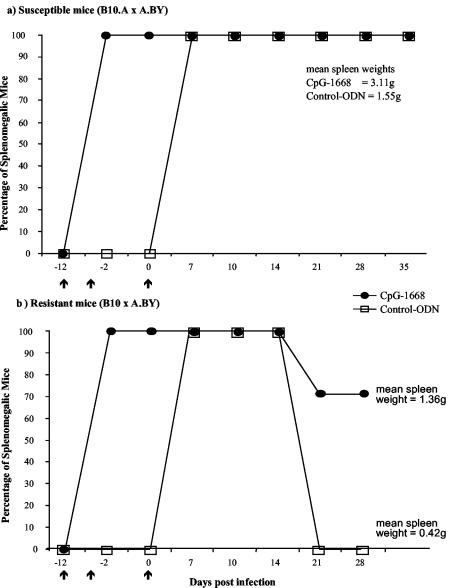
Figure 1a shows that all susceptible (B10.A × A.BY)F1 mice injected with control ODN developed long-term splenomegaly after FV infection indicative of fatal erythroleukemia. In the treated group of animals the immunostimulative CpG-ODN induced a mild splenomegaly (spleen weights increased from 0.15 g to 0.35 g) prior to infection with FV. This CpG-induced splenomegaly was only temporary and disappeared at around day 10 after CpG-ODN inoculation if mice were not infected with FV (data not shown). However, similarly to the control animals 100% of the mice infected with FV after treatment with CpG-ODN developed a severe and sustained enlargement of the spleen (with spleen weights between 2.44 and 3.98 g at day 28 postinfection). Interestingly, the mean spleen weight at 28 days postinfection was significantly higher in the CpG-ODN-treated group than in the control group (Fig. 1a), indicating that CpG-ODN treatment prior to infection had a negative effect on the progression of FV-induced erythroleukemia.
FIG. 1.
Effect of CpG-ODN pretreatment on disease progression of FV-infected susceptible and resistant mice. Adult age-matched female mice were inoculated intravenously with 3,000 spleen focus-forming units of FV (2). Intraperitoneal injections of 15 nmol (95 μg) of CpG-ODN or control ODN without the CpG motif were administered on days −12 and −4 and at −1 h with respect to infection (arrows). The same or a comparable dose of CpG-ODN has been used in other experimental therapies of infectious diseases and has never been documented to be toxic for mice (7, 20, 24). Phosphothioate-modified single-stranded ODN were used in our experiments (MWG-Biotech AG, Ebersberg, Germany). The immunostimulatory sequence was 5′-TCCATGACGTTCCTGATGCT-3′ (CpG-ODN), whereas the control ODN had an inverted CG motif (5′-TCCATGAGCTTCCTGATGCT-3′). Splenomegaly induction and progression were monitored before and after FV infection by spleen palpation as described elsewhere (10). Mild splenomegaly (spleen weights between 0.3 and 0.4 g) prior to FV infection was induced by the immunostimulatory effect of the CpG-ODN. An enlargement of the spleen was measurable for only about 10 days in uninfected, CpG-treated mice. (a) CpG-ODN treatment was performed on susceptible (B10.A × A.BY)F1 mice prior to FV infection. For CpG-treated mice, n = 7; for control-ODN-injected mice, n = 7. Mean spleen weights for each group at 43 days post-FV infection are given in the figure. The spleen weights were significantly different between the two groups by the Mann-Whitney test (P = 0.007). (b) CpG-ODN treatment was performed on resistant (B10 × A.BY)F1 mice prior to FV infection. For CpG-treated mice, n = 14; for control-ODN-injected mice, n = 9. Results are from two independent experiments, which gave similar results. The difference in splenomegaly between the two groups was statistically significant by Fisher's exact test (P = 0.0016). Mean spleen weights for each group at 28 days post-FV infection are given in the figure. The spleen weights were significantly different between the two groups by the Mann-Whitney test (P = 0.0046).
To further investigate whether CpG-ODN treatment before infection could render resistant mice susceptible to FV-induced leukemia, (B10 × A.BY)F1 mice (H-2b/b) were treated with ODN before FV challenge. Whereas the infected mice that were pretreated with control ODN recovered from acute splenomegaly and did not develop erythroleukemia, the percentage of leukemic animals was 70.8% in the CpG-ODN-pretreated group (Fig. 1b). The mean spleen weight at 28 days postinfection was also significantly higher in this group than in the control animals (Fig. 1b). Three injections with 95 μg of CpG-ODN prior to FV infection were necessary for the induction of a sustained splenomegaly in resistant mice, since a single treatment with 95 μg of CpG-ODN on day −4 only temporarily induced severe splenomegaly and did not induce leukemia (data not shown).
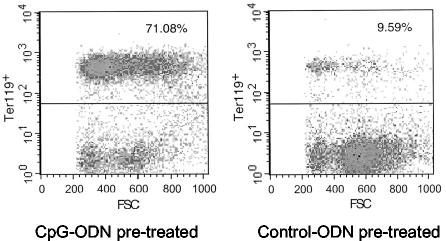
To determine if sustained splenomegaly in (B10 × A.BY)F1 mice inoculated with the higher dose of CpG was a typical FV-induced erythroleukemia, spleen cell suspensions were stained with an erythroid cell marker (Ter119) and analyzed by flow cytometry. Figure 2 shows a representative staining of spleen cells from an FV-infected, recovered (B10 × A.BY)F1 mouse compared to that of an FV-infected, splenomegalic mouse that was pretreated with CpG-ODN. The CpG-ODN pretreatment caused a shift from 9.59% erythroid cells in the spleen of the control mouse to 71.08% positive cells in the treated animal. The findings indicate that the severe splenomegaly in the group of CpG-ODN-treated mice was due to the massive proliferation of cells in the erythroid lineage. Since the analysis was done at 28 days postinfection, this proliferation of Ter119-positive cells was due to the massive replication of FV and not induced by the CpG-ODN stimulation of the extramedullary hemopoiesis, which lasted only up to 2 weeks in uninfected mice (data not shown).
FIG. 2.
Flow cytometric analysis of erythroid cells in FV-infected mice treated with CpG-ODN or control ODN prior to infection. At 28 days post-FV infection nucleated spleen cells from resistant (B10 × A.BY)F1 mice were stained with the erythroid cell surface marker Ter119 (12). (Left) Spleen cells from a representative resistant (B10 × A.BY)F1 mouse that was injected with CpG-ODN prior to infection. This mouse did not recover from FV-induced splenomegaly and had a grossly enlarged spleen with 2.9 × 109 total cells and a weight of 2 g (an untreated, uninfected mouse has typically about 1 × 108 to 2 × 108 total spleen cells and a spleen weight of 0.15 g). (Right) Spleen cells from a representative (B10 × A.BY)F1 mouse that was injected with control ODN prior to infection. This mouse had recovered from early FV-induced splenomegaly and presented with a slightly enlarged spleen containing 3.8 × 108 total cells and with a weight of 0.32 g.
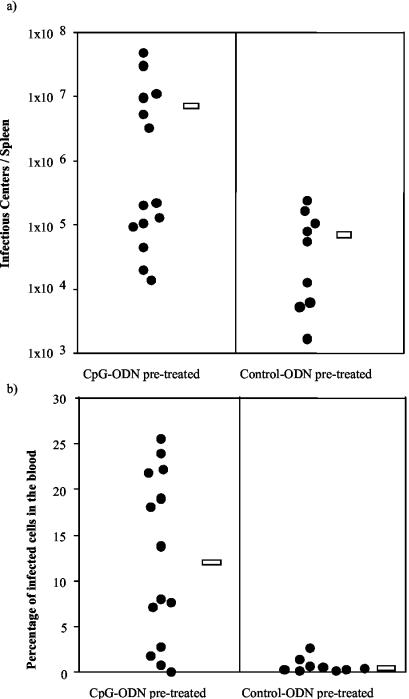
In order to ascertain whether CpG-ODN treatment of resistant (B10 × A.BY)F1 mice prior to FV infection was associated with enhanced viral loads, we measured the level of FV infection in spleen and blood of the animals. At 4 weeks postinfection the control mice harbored significantly fewer infectious centers in their spleens than did mice pretreated with CpG-ODN (Fig. 3a). A significant difference between the groups was also seen in the level of infected blood cells, with CpG-ODN-pretreated mice showing 16.2-times-higher infection levels than the control animals (Fig. 3b). Thus, treatment with CpG-ODN prior to FV infection facilitated viral replication in vivo.
FIG. 3.
Levels of FV infection in the spleen and blood of ODN-pretreated mice. Cells from spleens (a) and blood (b) of resistant (B10 × A.BY)F1 mice taken at 28 days after FV infection were used to determine levels of FV infection as described previously (4). In the group of mice that were treated with CpG-ODN prior to infection (left) the four animals with the lowest viral loads in spleen and blood had recovered from acute splenomegaly at 28 days postinfection. In the control-ODN group (right) all animals recovered from early splenomegaly. (a) Number of infectious centers in the spleen. Infectious centers from spleens were detected by titrations of single-cell suspensions onto susceptible Mus dunni cells as described previously (3). The mean number of infectious centers per spleen in the CpG-ODN-pretreated mice was 7.9 × 106 (open bar on left) and for the control group was 7.6 × 104 (open bar on right). The differences between the groups of treated and untreated mice were statistically significant by the Mann-Whitney test (P = 0.0182). (b) Percentages of blood cells expressing cell surface viral antigen. For the quantification of FV-infected blood cells, single-cell suspensions of nucleated, live cells were analyzed by flow cytometry. To detect FV infection, cells were stained with tissue culture supernatant containing Friend murine leukemia virus glycosylated Gag-specific monoclonal antibody 34 (5). The mean percentage of blood infection for the group of CpG-ODN-pretreated mice was 12.31% (open bar on left) and for the control group was 0.76% (open bar on right). The differences between the groups of treated and control mice were statistically significant by the Mann-Whitney test (P = 0.0015).
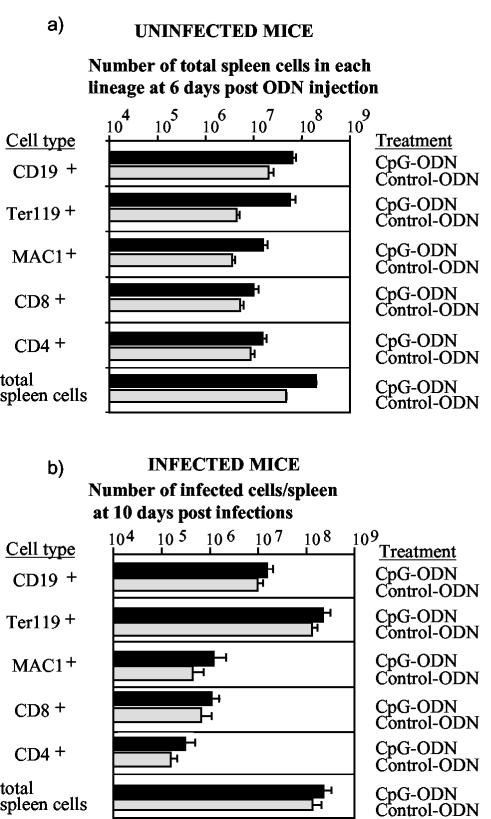
To identify the cell subsets that were involved in this enhanced virus replication, we determined infection levels in different spleen cell subpopulations. We had previously shown that FV is capable of infecting a number of different cell types of the hematopoietic system. The main target cells of the virus are erythroid precursor cells (Ter119+), B cells (CD19+), and monocytic cells (MAC1+) (5, 9). In a first set of experiments we determined the effect of CpG-ODN treatment on proliferation and activation of different cell subsets of the hematopoietic system in uninfected mice. CpG-ODN inoculation significantly increased the number of CD19+, Ter119+, MAC1+, CD4+, and CD8+ spleen cells in comparison to control-ODN-treated mice (Fig. 4a). This increase was most pronounced for the CD19+, MAC1+, and Ter119+ cell subsets. Thus, the numbers of potential FV target cells were increased due to CpG-ODN treatment. In addition, similarly to what has been described elsewhere (18), we found that monocytes (enhanced major histocompatibility complex class II expression), B cells, and T cells (enhanced CD69 expression) were activated by CpG-ODN in vivo (data not shown). The enlarged target cell pool of dividing cells is a likely explanation for increased FV replication after CpG-ODN pretreatment of resistant mice. To test this, we compared the number of FV-infected cells in each spleen cell subset between CpG-ODN- and control-ODN-pretreated mice at 10 days postinfection. The mean total number of infected spleen cells in the group of mice that received the CpG-ODN was 2.43 × 108, whereas it was only 1.43 × 108 in the group of mice that were injected with control ODN prior to FV inoculation (Fig. 4b). The absolute number of infected cells was increased in all analyzed spleen cell populations after CpG-ODN pretreatment (Fig. 4b). A statistically significant increase in viral load was found in Ter119+ erythroid precursor cells, CD19+ B cells, and CD4+ T cells. More than 99% of the total increase in infected spleen cell numbers measured after CpG-ODN injection was due to higher infection levels in the Ter119+ and CD19+ cells. Thus, the proliferative effect of CpG-ODN on B cells and erythroid precursor cells was likely the mechanism of FV-induced leukemia in resistant mice.
FIG. 4.
Effect of CpG-ODN on proliferation and FV infection of spleen cell subpopulations. (a) Total numbers of cells in different spleen cell subpopulations are shown. Uninfected mice were treated with CpG-ODN (n = 10) or control ODN (n = 12), and their spleen cells were analyzed by flow cytometry at 6 days posttreatment. The different ODN treatments of the animals are indicated in the figure. The error bars represent standard errors of the means. The differences between the groups of treated and control mice were statistically significant for all analyzed spleen cell subpopulations by the Mann-Whitney test (P < 0.004). (b) The log10 geometric mean number of FV-infected cells in each splenic subpopulation from resistant (B10 × A.BY)F1 mice was calculated by multiplying the total number of nucleated cells per spleen by the percentage of monoclonal antibody 34-positive cells of each lineage divided by 100. The spleen cells were taken at 10 days after FV infection to investigate the effect of CpG-ODN treatment on early virus replication, which determines disease progression. Cells were analyzed by two-color flow cytometry as described previously (5). The different ODN treatments prior to FV infection of the animals are indicated in the figure. The error bars represent standard errors of the means. The differences between the infection levels were not statistically significant for CD8+ cells (P = 0.10) and MAC1+ (CD11b) cells (P = 0.06), whereas the differences were significant by the Mann-Whitney test for CD4+ cells (P = 0.02), CD19+ cells (P = 0.01), and Ter119+ cells (P = 0.04). The number of animals investigated in both groups was n = 8.
Our present findings together with our previous report on successful postexposure treatment of a retrovirus-induced disease demonstrate that CpG-ODN treatment of viral infections may be a double-edged sword that can result in an effective therapy but also in an acceleration of disease progression. The critical parameter seems to be the time of treatment in relation to the time point of virus infection. Although pretreatment with CpG-ODN has been reported to induce resistance to challenges with tumor cells, Leishmania major, and herpes simplex virus type 2 (1, 6, 7, 19, 24), it had the opposite effect after type C retrovirus challenge. The reason for this different outcome of CpG-ODN treatment is most likely that they not only stimulate the immune system but also activate the target cells of FV. As a consequence the virus spreads too fast to be controlled by the immune system. Such a phenomenon might play an especially important role in viral infections with C-type retroviruses, like FV, because these viruses are dependent on activated and proliferating cells in order to infect their target cells and integrate into the cellular genome (14). However, many other viruses also infect cells of the hematopoietic system, e.g., human immunodeficiency virus, human T-cell leukemia virus type 1, measles virus, hepatitis B and C viruses, and many more, and their target cells are stimulated by CpG-ODN. Thus, a CpG-ODN treatment could potentially lead to enhanced virulence in several different viral infections when applied at the wrong time point pre- or postinfection. With this information at hand researchers and clinicians should carefully evaluate the antiviral potential of CpG-ODN that has recently been described for two different model virus infections (20, 22). If used under the right conditions, CpG-ODN should be a powerful substance for antiviral therapy in the future.
Acknowledgments
The work was supported by a grant to U.D. from the Deutsche Forschungsgemeinschaft (Di 714/6-1).
We thank Kim Hasenkrug (Rocky Mountain Laboratories) for helpful discussions and critical reading of the manuscript.
REFERENCES
- 1.Blazar, B. R., A. M. Krieg, and P. A. Taylor. 2001. Synthetic unmethylated cytosine-phosphate-guanosine oligodeoxynucleotides are potent stimulators of antileukemia responses in naive and bone marrow transplant recipients. Blood 98:1217-1225. [DOI] [PubMed] [Google Scholar]
- 2.Dittmer, U., D. M. Brooks, and K. J. Hasenkrug. 1998. Characterization of a live-attenuated retroviral vaccine demonstrates protection via immune mechanisms. J. Virol. 72:6554-6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dittmer, U., D. M. Brooks, and K. J. Hasenkrug. 1999. Requirement for multiple lymphocyte subsets in protection against retroviral infection by a live attenuated vaccine. Nat. Med. 5:189-193. [DOI] [PubMed] [Google Scholar]
- 4.Dittmer, U., K. E. Peterson, R. Messer, I. M. Stromnes, B. Race, and K. J. Hasenkrug. 2001. Role of interleukin-4 (IL-4), IL-12, and gamma interferon in primary and vaccine-primed immune responses to Friend retrovirus infection. J. Virol. 75:654-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dittmer, U., B. Race, K. E. Peterson, I. M. Stromnes, R. J. Messer, and K. J. Hasenkrug. 2002. Essential roles for CD8+ T cells and gamma interferon in protection of mice against retrovirus-induced immunosuppression. J. Virol. 76:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hafner, M., R. Zawatzky, C. Hirtreiter, W. A. Buurman, B. Echtenacher, T. Hehlgans, and D. N. Mannel. 2001. Antimetastatic effect of CpG DNA mediated by type I IFN. Cancer Res. 61:5523-5528. [PubMed] [Google Scholar]
- 7.Harandi, A. M., K. Eriksson, and J. Holmgren. 2003. A protective role of locally administered immunostimulatory CpG oligodeoxynucleotide in a mouse model of genital herpes infection. J. Virol. 77:953-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasenkrug, K. J., and U. Dittmer. 2000. The role of CD4 and CD8 T cells in recovery and protection from retroviral infection: lessons from the Friend virus model. Virology 272:244-249. [DOI] [PubMed] [Google Scholar]
- 9.Hasenkrug, K. J., D. M. Brooks, and U. Dittmer. 1998. Critical role for CD4+ T cells in controlling retrovirus replication and spread in persistently infected mice. J. Virol. 72:6559-6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasenkrug, K. J., D. M. Brooks, M. N. Robertson, R. V. Srinivas, and B. Chesebro. 1998. Immunoprotective determinants in Friend murine leukemia virus envelope protein. Virology 248:66-73. [DOI] [PubMed] [Google Scholar]
- 11.Hoatlin, M. E., and D. Kabat. 1995. Host-range control of a retroviral disease: Friend erythroleukemia. Trends Microbiol. 3:51-57. [DOI] [PubMed] [Google Scholar]
- 12.Ikuta, K., T. Kina, I. MacNeil, N. Uchida, B. Peault, Y. H. Chien, and I. L. Weissman. 1990. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell 62:863-874. [DOI] [PubMed] [Google Scholar]
- 13.Kabat, D. 1989. Molecular biology of Friend viral erythroleukemia. Curr. Top. Microbiol. Immunol. 148:1-42. [DOI] [PubMed] [Google Scholar]
- 14.Kost, T. A., M. J. Koury, and S. B. Krantz. 1981. Mature erythroid burst-forming units are target cells for Friend virus-induced erythroid bursts. Virology 108:309-317. [DOI] [PubMed] [Google Scholar]
- 15.Kost, T. A., M. J. Koury, W. D. Hankins, and S. B. Krantz. 1979. Target cells for Friend virus-induced erythroid bursts in vitro. Cell 18:145-152. [DOI] [PubMed] [Google Scholar]
- 16.Krieg, A. M., A. K. Yi, S. Matson, T. J. Waldschmidt, G. A. Bishop, R. Teasdale, G. A. Koretzky, and D. M. Klinman. 1995. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374:546-549. [DOI] [PubMed] [Google Scholar]
- 17.Krieg, A. M. 2001. Now I know my CpGs. Trends Microbiol. 9:249-252. [DOI] [PubMed] [Google Scholar]
- 18.Krieg, A. M. 2002. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20:709-760. [DOI] [PubMed] [Google Scholar]
- 19.Lipford, G. B., T. Sparwasser, S. Zimmermann, K. Heeg, and H. Wagner. 2000. CpG-DNA-mediated transient lymphadenopathy is associated with a state of Th1 predisposition to antigen-driven responses. J. Immunol. 165:1228-1235. [DOI] [PubMed] [Google Scholar]
- 20.Olbrich, A. R. M., S. Schimmer, K. Heeg, K. Schepers, T. N. M. Schumacher, and U. Dittmer. 2002. Effective postexposure treatment of retrovirus-induced disease with immunostimulatory DNA containing CpG motifs. J. Virol. 76:11397-11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson, K. E., M. Iwashiro, K. J. Hasenkrug, and B. Chesebro. 2000. Major histocompatibility complex class I gene controls the generation of gamma interferon-producing CD4+ and CD8+ T cells important for recovery from Friend retrovirus-induced leukemia. J. Virol. 74:5363-5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pyles, R. B., D. Higgins, C. Chalk, A. Zalar, J. Eiden, C. Brown, G. Van Nest, and L. R. Stanberry. 2002. Use of immunostimulatory sequence-containing oligonucleotides as topical therapy for genital herpes simplex virus type 2 infection. J. Virol. 76:11387-11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sparwasser, T., L. Hultner, E. S. Koch, A. Luz, G. B. Lipford, and H. Wagner. 1999. Immunostimulatory CpG-oligodeoxynucleotides cause extramedullary murine hemopoiesis. J. Immunol. 162:2368-2374. [PubMed] [Google Scholar]
- 24.Stacey, K. J., and J. M. Blackwell. 1999. Immunostimulatory DNA as an adjuvant in vaccination against Leishmania major. Infect. Immun. 67:3719-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wendling, F., and P. E. Tambourin. 1978. Oncogenicity of Friend-virus-infected cells: determination of origin of spleen colonies by the H-2 antigens as genetic markers. Int. J. Cancer 22:479-486. [DOI] [PubMed] [Google Scholar]
- 26.Wild, J. S., and S. Sur. 2001. CpG oligonucleotide modulation of allergic inflammation. Allergy 56:365-376. [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann, S., O. Egeter, S. Hausmann, G. B. Lipford, M. Rocken, H. Wagner, and K. Heeg. 1998. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J. Immunol. 160:3627-3630. [PubMed] [Google Scholar]