Abstract
γδ T cells are primarily found in the gastrointestinal mucosa and play an important role in the first line of defense against viral, bacterial, and fungal pathogens. We sought to examine the impact of human immunodeficiency virus type 1 (HIV-1) infection on mucosal as well as peripheral blood γδ T-cell populations. Our results demonstrate that HIV-1 infection is associated with significant expansion of Vδ1 and contraction of Vδ2 cell populations in both the mucosa and peripheral blood. Such changes were observed during acute HIV-1 infection and persisted throughout the chronic phase, without apparent reversion after treatment with highly active antiretroviral therapy (HAART). Despite an increase in the expression of CCR9 and CD103 mucosal homing receptors on peripheral blood γδ T cells in infected individuals, mucosal and peripheral blood γδ T cells appeared to be distinct populations, as reflected by distinct CDR3 length polymorphisms and sequences in the two compartments. Although the underlying mechanism responsible for triggering the expansion of Vδ1 γδ T cells remains unknown, HIV-1 infection appears to have a dramatic impact on γδ T cells, which could have important implications for HIV-1 pathogenesis.
γδ T cells are minor constituents in the peripheral blood but provide a sizable contribution to the immune compartment of the gastrointestinal mucosa, likely representing the first defense against pathogens crossing this surface. In the mucosa, they constitute up to 50% of all lymphocytes in the intraepithelial compartment and approximately 10% of lymphocytes in the lamina propria (26, 44). Mucosal γδ T cells are ideally situated to contribute to the earliest stages of the immune response against infection through epithelial surfaces and are believed to link the innate and acquired immune responses. In addition, γδ T cells influence gastrointestinal epithelial cell proliferation and differentiation (27) and development of mucosal immunoglobulin A-producing B cells and play a role in oral tolerance (15).
γδ T cells recognize soluble protein and nonprotein antigens, though the mechanism by which these antigens are recognized remains enigmatic. Unlike αβ T cells, γδ T cells recognize antigens via their T-cell receptor (TCR) in a major histocompatibility complex (MHC)-independent manner. The γδ TCR recognizes intact proteins in a fashion similar to that in which antibodies recognize antigens. γδ T cells employ a distinct set of variable (V), diversity (D), and joining (J) regions. Though the potential of γδ TCR diversity is, in theory, greater than that of αβ T cells (7), reduced combinatorial somatic recombination results in restricted γδ TCR diversity. Despite a restriction in receptor diversity, the lack of antigenic processing or MHC restriction permits recognition of a wide variety of native and foreign antigens. Though there are six known γ and six known δ chains, peripheral blood γδ T cells in healthy individuals predominantly express the Vδ2 and Vγ9 TCR variable segments (8).
The physiologic role of γδ T cells has not been completely elucidated, though evidence suggests that γδ T cells are involved in protection against infectious pathogens and play a role in tumor immunosurveillance, and γδ T cells have been implicated in the pathogenesis of autoimmune disease. A variety of stimuli appear to be capable of generating a γδ cell response. The Vγ9Vδ2 T cells that predominate in the blood of healthy subjects recognize phosphorylated, nonpeptidic microbial metabolites produced by mycobacteria as well as a variety of other pathogens. Vδ1 T cells, the dominant population in the mucosal epithelial layer, recognize MHC class I-related receptors, MHC class I chain-related A and B (MICA and MICB), which are stress induced on intestinal epithelial cells (18). Though the mechanisms of antigen recognition differ, the effector functions of γδ T cells are analogous to those of αβ T cells. Once activated, γδ T cells exert cytotoxicity via the perforin-granzyme pathway or through induction of apoptosis via Fas/Fas-ligand interactions (6). These cells can also produce a variety of cytokines and chemokines, depending on the stimulatory signal. Different pathogens have been shown to induce expansion of specific subsets of γδ T cells. For instance, expansion of Vδ2 cells occurs during infection by mycobacterial species, Toxoplasma gondii, Listeria monocytogenes, Epstein-Barr virus (EBV), Plasmodium, visceral Leishmania, and Salmonella, while expansion of Vδ1 cells has been shown to occur during infection with Borrelia burgdorferi, cytomegalovirus, and human immunodeficiency virus type 1 (HIV-1) (22). In each case, whether γδ T-cell expansion reflects specific reactivity against microbial antigens or is an indirect effect of immune modulation is not known.
γδ T cells have been implicated in the immune response to a number of viruses, including herpes simplex virus, EBV, and HIV-1 (30). Coculture of Vγ9Vδ2 γδ T cells with HIV-1-infected lymphocytes has been reported to result in the elaboration of HIV-1-suppressive soluble mediators and a cytotoxic response (9, 37, 46) against the infected cells. HIV-1 infection has been associated with a significant decrease in the peripheral blood Vγ9Vδ2 cell population and with an expansion in the population of Vδ1 cells such that they become the dominant population of γδ T cells (2). Vδ1 predominance also occurs in the bone marrow of HIV-1-infected subjects but does not appear in either the blood or bone marrow of patients chronically infected with most other viruses (40). While fairly specific for infection with HIV-1, the predominance of Vδ1 cells has not been associated with the presence of opportunistic infections (1, 41).
There is considerable heterogeneity of the γδ T-cell populations across body compartments in terms of cellular phenotype, nature of the antigen recognized, and effector functions employed (7). While phenotypic changes in γδ subsets of the peripheral blood have been described, the effect of HIV-1 infection on colonic mucosal γδ subsets has not previously been examined. Since the gastrointestinal mucosa harbors the majority of the body's pool of γδ T cells, which may play an important role in transmucosal HIV-1 infection and the control of HIV-1 replication, it is important that the effect of HIV-1 on these cells be examined. The principal aim of this study was to examine the effect of HIV-1 infection on the γδ T-cell population in the gastrointestinal mucosa and peripheral blood of HIV-1-infected subjects during acute and chronic HIV-1 infection as well as before and after treatment with highly active antiretroviral therapy (HAART). Our results demonstrate that HIV-1 infection is associated with significant expansion of Vδ1 and contraction of Vδ2 γδ T-cell populations in the colonic mucosa as well as in the peripheral blood. These changes are noted during acute HIV-1 infection and, despite HAART, persist into the chronic phase of infection without reversion. Though Vδ1 population expansion and Vδ2 population contraction are observed in the mucosa and blood, these compartments contain distinct Vδ1 and Vδ2 γδ T-cell populations. Though HIV-1 infection is associated with significant changes in the blood and mucosal γδ T-cell populations, the factors driving these changes remain unknown.
MATERIALS AND METHODS
Patients and sample acquisition.
Peripheral blood and rectosigmoid colonic mucosal tissue were collected from HIV-1-seropositive and -seronegative subjects. Endoscopic biopsies were obtained from the colon from macroscopically normal mucosa at a standardized site in the rectosigmoid region 30 cm from the anal margin. This site was chosen for all sampling to avoid potential regional variation and the potential for confounding effects from infectious or traumatic proctitis that would be expected to occur distal to this location. Biopsies were taken using large-cup endoscopic-biopsy forceps (Microvasive Radial Jaw; Boston Scientific, Boston, Mass.) (outside diameter, 3.3 mm) and (i) immediately placed in tissue culture medium (RPMI 1640 supplemented with 10% fetal calf serum [BioWhittaker, Walkersville, Md.]), (ii) placed into 2-ml prelabeled cryovials (Nalgene, Rochester, N.Y.) and frozen in liquid nitrogen, or (iii) placed in formalin for routine hematoxylin and eosin staining to exclude confounding infectious processes. Phlebotomy was undertaken immediately prior to endoscopy; blood was collected in EDTA-containing tubes.
Within 10 minutes from acquisition, mucosal mononuclear cells (MMCs) were mechanically isolated (using a Medimachine apparatus [Dako Cytomation, Carpinteria, Calif.] per the manufacturer's protocol) from mucosal biopsies. Immediately after isolation, cells were resuspended in phosphate-buffered saline containing antibodies for use in flow cytometry. Utilizing 7-AAD (Calbiochem, San Diego, Calif.) to assess cell viability by flow cytometry has routinely revealed more than 90% live MMCs. Peripheral blood mononuclear cells (PBMCs) were prepared by centrifugation on a Ficoll-Hypaque density gradient (Nycomed Pharma AS, Oslo, Norway). As before, PBMCs are stained for flow cytometry immediately after isolation. Informed consent was obtained from all subjects, and the study was approved by the Human Subjects Protection Committee at the Manhattan Veterans' Administration Hospital and The Rockefeller University Hospital.
Flow cytometry.
Cell surface expression of lymphocyte antigens was identified by monoclonal-antibody staining of freshly isolated PBMCs and MMCs followed by flow cytometry using a FACSCalibur (Becton Dickinson Immunocytometry Systems [BDIS], Mountain View, Calif.), with analysis performed using CellQuest software (BDIS). Monoclonal antibodies used in this study included anti-human CD3-PE-Cy5 (clone UCHT1), anti-human CD4-phycoerythrin (clone RPA-T4), anti-human CD8-fluorescein isothiocyanate (FITC) (clone RPA-T8), anti-human CD103-phycoerythrin (clone Ber-ACT8) (BDIS), anti-human γδ TCR-allophycocyanin (clone B1) and anti-human αβ TCR (clone T10B9.1A-31) (Pharmingen, San Diego, Calif.), anti-human Vδ1-FITC (clone TS8.2) (Endogen, Woburn, Mass.), anti-human Vδ2-FITC (clone IMMU 389), and anti-human CCR9-FITC (clone 112509) (R & D Systems, Minneapolis, Minn.). Lymphocytes (initially identified by their forward- and side-scatter characteristics) were then subjected to phenotypic analysis. To determine the percentages of αβ and γδ T cells in the T-cell population, gated lymphocytes were examined for expression of CD3 and, finally, CD3+ lymphocytes were analyzed for expression of αβ or γδ TCR. Phenotypic characterization of these populations with regard to CCR9 and CD103 could then be performed using four-color flow cytometry. To determine the relative percentages of Vδ1, Vδ2, and γδ T cells, isolated MMCs and PBMCs were first examined for expression of the γδ TCR. After gating on the γδ TCR+ cells, expression of the δ-elements was quantified.
PCR analysis of CDR3 size distribution.
CDR3 length polymorphism analysis uses the size heterogeneity of the CDR3 antigen recognition domain to characterize the diversity of the TCR repertoire within the Vδ1 and Vδ2 γδ T-cell populations. The CDR3 size distribution pattern of a defined Vδ transcript provides information with regard to antigenic restriction, such that a monoclonal Vδ pattern characterized by a single peak of the CDR3 distribution pattern indicates recognition of a single conventional antigen. A polyclonal response is represented by a multipeaked CDR3 size pattern.
PBMCs and mucosal biopsies were kept frozen in liquid nitrogen until use. Using an RNeasy extract kit (QIAGEN, Valencia, Calif.), total RNA was prepared from 1 × 106 to 5 × 106 frozen PBMCs or two biopsies. Total DNase-treated RNA (1 μg) was reverse transcribed according to the manufacturer's instructions. In brief, 1 μg of total DNase-treated RNA in 20 μl of distilled water was heated for 5 min at 80°C, transferred onto ice, combined with reverse-transcription mixture containing 6 μl of 5× reaction buffer (250 mM Tris [pH 8.3], 375 mM KCl, 15 mM MgCl2), 1.5 μl of deoxynucleoside triphosphates (10 mM each), 0.6 μl of RNasin, 1 μl of random hexamers (Pharmacia) (1 mM), and 2 μl of Moloney murine leukemia virus reverse transcriptase (200 U/μl; Gibco-BRL), and incubated for 1 h at 42°C.
PCR was performed under the following conditions: one cycle of denaturation at 95°C for 10 min followed by 35 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 1 min, with a final extension of 10 min at 72°C. For the CDR3 regions of Vδ1 and Vδ2, primer pair Vδ1a and δC1a and primer pair Vδ2a and δC1a, respectively, were used in the first round of PCR and primer pair Vδ1b and δC1b and primer pair Vδ2b and δC1b, respectively, were used in the second round of PCR. The δC1b primer was labeled with fluorescein (6-FAM) so that the length polymorphism of the final fluorescent PCR products could be analyzed. The sequences for the primer are as follows: for Vδ1a, 5′-TATTCGCCAGGGTTCTGATGAACAG; for Vδ2a, 5′TCAACTGGTACAGGAAGACCCAAGG; for δC1a, 5′-GAATTCCTTCACCAGACAAGCGAC; for Vδ1b, 5′-CAGCCTTACAGCTAGAAGATTCAGC; for Vδ2b, 5′-GCACCATCAGAGAGAGATGAAGGG; and for δC1b, 5′-AAACGGATGGTTTGGTATGAGGCTG. The PCR products were size separated on a 6% denaturing polyacrylamide gel on an automated sequencer (Prism 377; Applied Biosystems, Foster City, Calif.). The length polymorphism of the CDR3 region of the Vδ1 and Vδ2 chains was analyzed by GeneScan 3.1 (Applied Biosystems).
TCR-δ chain sequence analysis.
PCR-amplified products of CDR3 regions of Vδ1 and Vδ2 chains were cloned into a TA vector (Invitrogen, Carlsbad, Calif.) for sequence analysis. The samples were then run on a 6% denaturing polyacrylamide gel on an automated sequencer (Prism 377; Applied Biosystems). Nucleotide sequences were aligned using a Bioedit Biologic sequence editor (Tom Hall, North Carolina State University) and then translated into putative amino acid sequences. Comparative studies were performed with sequences from rectal biopsies and peripheral blood.
Statistical methodology.
Values are expressed as means ± standard deviations. Using a paired t test, statistical comparisons were made between PBMCs and MMCs from individuals. Using a two-sample, equal variance (homoscedastic) t test, statistical comparisons were made between HIV-1-infected and control subjects. Using SPSS 11.0 for Windows software (SPSS, Chicago, Ill.), Pearson correlation coefficients were calculated to evaluate correlations between variables; all reported P values were two sided at the 0.05 significance level.
RESULTS
Patient characteristics.
We examined MMCs obtained from the rectal mucosa and PBMCs obtained from the peripheral blood of HIV-1-seropositive and -seronegative subjects (Table 1). In all, 71 HIV-1-seropositive and 47 HIV-1-seronegative subjects were studied, although endoscopy was performed on only 44 subjects (19 HIV-1-seronegative and 25 HIV-1-seropositive subjects). Histopathological examination did not identify pathological mucosal inflammation in any subject, though many biopsies from HIV-1-seropositive subjects were described as showing nonspecific mild chronic inflammation. Opportunistic infections were not identified in any subject within 3 months proximate to the study period. Only five HIV-1-infected subjects had a past history of opportunistic infection, and the opportunistic infections (by cytomegalovirus colitis and cryptosporidial enteritis) involved the gastrointestinal mucosa in only two subjects. A total of 4 of 28 (14.3%) subjects who had been treated with HAART and 5 of 10 (50%) untreated subjects had a CD4 cell count of less than 200 cells/mm3. Among the 28 HIV-1-infected subjects who were receiving HAART, 9 (32.1%) had plasma levels of HIV-1 RNA that were below the level of detection; the other subjects had recently initiated therapy (25%) or had detectable viremia despite chronic HAART (42.9%) (defined as a regimen consisting of at least three antiretroviral medications, including a protease inhibitor and two reverse transcriptase inhibitors).
TABLE 1.
Patient demographics
| Patient group | No. of patients | Male/ female | Racea | Age (yr) | No. (mean ± SD) of CD4 cells/mm3 (range) | PVLd (mean ± SD) (log 10) (range) | Duration (mean ± SD) of HIV infection (mo) (range) | Duration (mean ± SD) of HAART at time of study (mo) (range) |
|---|---|---|---|---|---|---|---|---|
| HIV-1 seropositive | 71 | 63/8 | 40W, 13H, 17AA, 1A | 43.7 ± 11.1 (20-71) | 422.9 ± 275.6 (7-1,201) | 5.2 ± 5.6 (UD-6.26) | <3 | 9.6 ± 14.1 (0-56) |
| Acute | 33 | 30/3 | 24W, 5H, 4AA | 39.5 ± 8.3 (24-64) | 523.5 ± 240.5 (27-1,164) | 5.6 ± 5.7 (3.51-6.26) | NAc | Pretreatment |
| Chronic | 38 | 33/5 | 16W, 8H, 13AA, 1A | 47.3 ± 12.1 (23-75) | 328.4 ± 276.1 (7-874) | 4.2 ± 4.4 (UD-4.96) | 23.8 ± 18.1 (8-56) | 18.2 ± 14.9 (0-56) |
| Treated | 28 | 24/4 | 15W, 5H, 7AA, 1A | 47.0 ± 11.9 (33-62) | 373.0 ± 248.2 (24-1,201) | 3.7 ± 2.4 (UD-4.67) | 32.4 ± 12.7 (15-56) | 24.6 ± 11.8 (13-56) |
| Untreated | 10 | 9/1 | 1W, 3H, 6AA | 48.3 ± 13.0 (23-66) | 234.3 ± 285.6 (7-874) | 4.7 ± 4.5 (4.19-4.96) | 58.7 ± 37.5 (8-120) | 0 |
| HIV-1 seronegative | 47 | 32/15 | 21W, 4H, 9AA, 13A | 43.8 ± 17.9 (22-82) | NDb | NA | NA | NA |
W, White; H, Hispanic; AA, African American; A, Asian; UD, undetectable.
ND, not done.
NA, not applicable.
PVL, plasma viral load.
HIV-1 infection is associated with a significant increase in mucosal but not peripheral γδ T-cell populations.
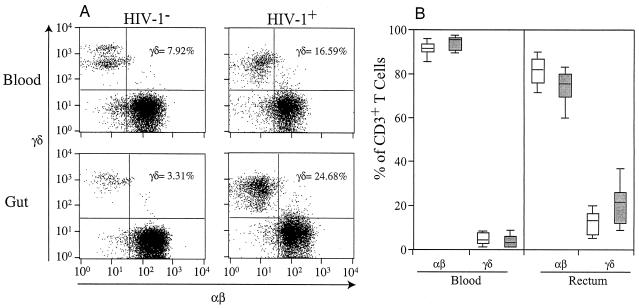
Using flow cytometry, MMCs mechanically isolated from rectosigmoid biopsies of HIV-1-infected and uninfected subjects, as well as PBMCs isolated by density gradient separation, were examined. In both HIV-1-infected and uninfected cohorts, the percentage of mucosal T cells expressing the γδ TCR was significantly higher than the percentage of peripheral blood T cells expressing the γδ TCR (P < 0.001 for both HIV-1-infected and uninfected cohorts). HIV-1 infection was associated with a significant increase in the percentage of mucosal T cells that express the γδ TCR (23.3% ± 15.6% for HIV-1-seropositive subjects versus 13.2% ± 5.2% for HIV-1-seronegative subjects; P = 0.03) (Fig. 1). This phenomenon was not observed when percentages of PBMCs were examined (4.9% ± 2.6% for HIV-1-seropositive subjects versus 4.3% ± 3.1% for HIV-1-seronegative subjects; P = 0.58) (Fig. 1). Although many of the HIV-1-infected subjects had detectable levels of HIV-1 RNA in their plasma, there was no discernible increase in the percentage of γδ T cells in the peripheral blood of these individuals compared with that seen with HIV-1-seronegative subjects or HIV-1-seropositive subjects with undetectable levels of HIV-1 RNA in their plasma.
FIG. 1.
Comparison of percentages of peripheral blood and mucosal αβ and γδ T cells in HIV-1-seronegative (HIV-1−) and -seropositive (HIV-1+) subjects. PBMCs and MMCs from the rectosigmoid region of HIV-1-seronegative (n = 47 for the study of PBMCs and n = 19 for the study of rectal biopsies) and -seropositive (n = 71 for the study of PBMCs and n = 25 for the study of rectal biopsies) subjects were analyzed by flow cytometry. (A) Representative flow cytometry plots outlining αβ TCR-FITC cell levels on the x axis and γδ TCR-allophycocyanin on the y axis for both PBMCs and MMCs. (B) Box plot showing cell types and anatomic compartments studied (x axis) and percentages of CD3+ T cells that express either the αβ or γδ TCR (y axis). In these plots, the boxes extend from the first to the third quartiles, enclosing the middle 50% of the data. The middle line within each box indicates the median of the data, whereas the vertical line extends from the 10th to the 90th percentile. This graph shows that for both HIV-1-seronegative (open boxes) and -seropositive (shaded boxes) subjects, a higher percentage of MMCs than PBMCs expressed the γδ TCR. Further, while HIV-1 infection was not associated with changes in the percentages of αβ or γδ T cells in the peripheral blood, there was a significant increase in mucosal γδ T-cell populations of HIV-1-seropositive compared with HIV-1-seronegative subjects.
HIV-1 infection is associated with significant expansion of Vδ1 and contraction of Vδ2 γδ T-cell populations in both the mucosa and peripheral blood of acutely and chronically HIV-1-infected subjects.
Having shown that HIV-1 infection is associated with an increased percentage of mucosal γδ T cells, we sought to determine whether this expansion is seen predominantly in the Vδ1 subset, in similarity to what has been observed with the blood of HIV-1-infected individuals.
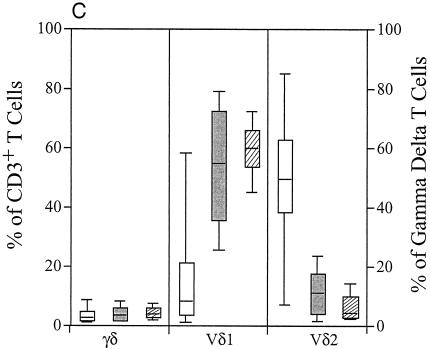
As has been previously described (1, 2) and as confirmed by the present study, the colonic mucosal γδ T-cell populations of healthy subjects contained significantly more Vδ1-expressing (P = 0.05) and significantly fewer Vδ2-expressing (P = 0.01) γδ T cells than those of their circulating counterparts. HIV-1 infection was associated with a significant increase in the percentage of γδ T cells that express the Vδ1 chain in both the blood and gastrointestinal mucosa (Fig. 2A). The percentage of the Vδ1-expressing cell population seen increased from 26.6% ± 21.2% in the blood of HIV-1-uninfected subjects to 59.3% ± 14.8% in the blood in those subjects who were chronically HIV-1 infected (P < 0.002) and from 41.8% ± 13.3% to 59.0% ± 17.7% in the gastrointestinal mucosa (P < 0.001) in these subjects. This observed increase in the percentage of the Vδ1 γδ T-cell population was seen concurrently with decreases in the percentage of the Vδ2-expressing population in both the blood (42.2% ± 20.7% to 2.6% ± 3.3%; P < 0.001) and gastrointestinal mucosa (18.8% ± 6.2% to 10.2% ± 7.7%; P < 0.001) of HIV-1-infected subjects.
FIG. 2.
Assessment of Vδ1 and Vδ2 changes in the blood and mucosa of HIV-1-infected subjects. (A) Results from comparisons of Vδ1 and Vδ2 expression for the PBMCs and MMCs of HIV-1-seronegative subjects (open boxes) (n = 27 for study of PBMCs and n = 19 for the study of rectal biopsies) and of those subjects chronically infected with HIV-1 (shaded boxes) (n = 14 for the study of PBMCs and rectal biopsies). The graph shows that the blood and gastrointestinal mucosa of HIV-1-infected subjects is characterized by a significantly higher percentage of γδ T cells expressing Vδ1 and a significantly smaller percentage expressing Vδ2 compared with that of the HIV-1-seronegative subjects. (B) Comparison of the absolute numbers of total, Vδ1, and Vδ2 γδ T cells present in 1 ml of peripheral blood of 10 HIV-1-seronegative (open boxes) and 10 chronically HIV-1-infected subjects (shaded boxes). Supporting the findings depicted in panel A, this graph shows that HIV-1 infection is associated with a numerical increase in Vδ1 γδ T cells and with a numerical decrease in Vδ2 γδ T cells in 1 ml of blood compared to the results seen for HIV-1-seronegative subjects. The total numbers of γδ T cells were not statistically different between groups. (C) PBMCs from HIV-1-seronegative (open boxes) (n = 27) and chronically HIV-1-infected (hatched boxes) subjects (n = 14) were compared with those from HIV-1-infected subjects (shaded boxes) in the acute infection period (i.e., prior to the development of positive anti-HIV-1 ELISA examinations) (n = 33). The percentages of cells stained for γδ TCR were quantified, and after gating was performed on those cells expressing the γδ TCR, the percentages expressing Vδ1 and Vδ2 were determined. This graph shows that in similarity to the changes in αβ T-cell populations, the alterations in γδ T-cell populations are seen as early as the acute period of HIV-1 infection.
These results illustrate changes in the percentage of γδ T-cell populations expressing Vδ1 and Vδ2. To show that the absolute number of Vδ1 γδ T cells was increased and that the absolute number of Vδ2 γδ T cells was decreased in the blood of HIV-1-infected subjects, we examined γδ TCR staining on all of the lymphocytes that were isolated from 1 ml of blood of HIV-1-infected (n = 10) and uninfected (n = 10) subjects (Fig. 2B). While there was no statistically significant difference in the absolute number of PBMCs expressing the γδ TCR between HIV-1-seropositive (15,938.7 ± 9,490.5 cells/ml) and HIV-1-seronegative (19,015.9 ± 12,665.7 cells/ml) subjects (P = 0.55), significant differences in the Vδ1 and Vδ2 subsets were seen. The absolute number of Vδ1 cells (10,506.1 ± 8,771.8 cells) in 1 ml of blood from HIV-1-seropositive subjects was significantly increased compared to that from HIV-1-seronegative subjects (3,380.6 ± 2,078.7 cells/ml) (P = 0.04). Further, there was an absolute decrease in Vδ2 γδ T cells in 1 ml of blood from HIV-1-seropositive subjects (693.0 ± 535.1 cells) compared to 1 ml of blood from HIV-1-seronegative subjects (7,163.2 ± 8,309.5 cells) (P = 0.04). There was no correlation between the absolute CD4 cell count or quantity of HIV-1 RNA in the plasma of the HIV-1-infected subjects and the percentage of blood or mucosal γδ T cells or the percentage of Vδ1 or Vδ2 γδ T cells in the blood or gastrointestinal mucosa (data not shown). Furthermore, there was no significant correlation between the absolute numbers of Vδ1 and Vδ2 γδ T cells in the blood of HIV-1-infected subjects (correlation coefficient = 0.037).
Having shown that the blood of chronically HIV-1-infected subjects is characterized by expansion of Vδ1 γδ T-cell populations and contraction of Vδ2 γδ T-cell populations, we sought to determine whether these changes are observed during the earliest identifiable stage of HIV-1 infection. We examined γδ T cells from the blood of 33 HIV-1-infected subjects who were identified during acute HIV-1 infection. These subjects were viremic but had not yet developed anti-HIV-1 serum antibodies or had an evolving humoral immune response. Temporally similar to the changes that were noted in the total populations of CD4- and CD8-expressing αβ T cells (data not shown), peripheral blood γδ T-cell population changes were seen during early HIV-1 infection (Fig. 2C). While acute HIV-1 infection was not associated with an increase in the percentage of peripheral blood γδ T cells, a statistically significant increase in the Vδ1 subset and decrease in the Vδ2 subset populations can be seen at this early point after HIV-1 infection (P < 0.001 for changes seen in primary HIV-1 infection compared with those seen with HIV-1-seronegative subjects). Further, the percentage of Vδ1-expressing γδ T cells in the blood of primary infection subjects (53.9% ± 21.6%) was not statistically different from that of subjects chronically infected with HIV-1 (59.3% ± 14.8%; P = 0.236). The contraction of Vδ2 cell populations was also observed during acute infection (12.4% ± 7.8%) and remained low during the chronic phase of infection (2.6% ± 3.3%; P = 0.01).
Treatment of HIV-1 with HAART is not associated with reversal of the skewed percentages in γδ T-cell population subsets.
To indirectly assess whether persistent viremia is responsible for γδ T-cell proliferation, we examined the effect of suppressive antiretroviral therapy by comparing γδ T-cell phenotypes in the mucosal and blood compartments of untreated HIV-1-seropositive subjects to those of subjects who had been receiving effective HAART for at least 1 year (mean, 19.3 months).
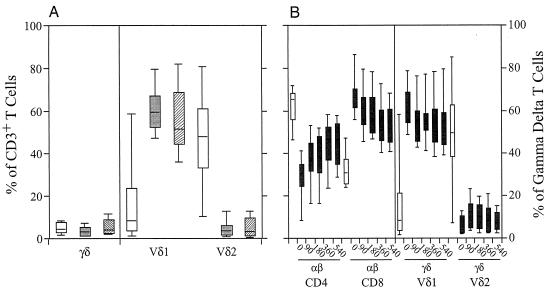
Despite significant suppression of HIV-1 replication, rendering plasma levels of HIV-1 RNA undetectable (plasma level of HIV-1 RNA, fewer than 50 copies/ml) for a mean duration of 16.4 months, the phenotypic skewing seen in the γδ T-cell populations remained unchanged; the gastrointestinal mucosa and blood of treated HIV-1-infected subjects contained a predominance of Vδ1 cells, while Vδ2 γδ T cells represented a minority (Fig. 3A). While a significantly higher percentage of Vδ1 γδ T cells was seen in the peripheral blood of effectively treated (55.8% ± 17.1%) and untreated (59.9% ± 9.7%) HIV-1-infected subjects than in the peripheral blood of HIV-1 seronegative subjects (26.6% ± 21.2%) (P < 0.001 for each comparison), there were no significant differences between the two HIV-1-seropositive groups (P = 0.418). Likewise, with regard to the percentage of Vδ2-expressing γδ T cells in the blood, there was no significant difference between effectively treated (5.1% ± 5.1%) and untreated (5.0% ± 4.3%) HIV-1-seropositive subjects (P = 0.993) but the blood of subjects in both groups contained a significantly reduced percentage of Vδ2 cells compared with that of HIV-1-seronegative subjects (42.2% ± 20.7%; P < 0.001 for each comparison).
FIG. 3.
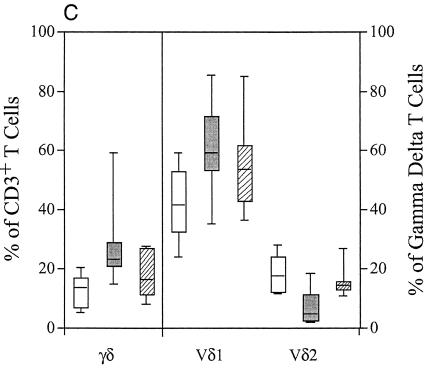
Determination of the effect of suppressive HIV-1 treatment on γδ T-cell population changes. (A) Results from a comparison of Vδ1 and Vδ2 expression on PBMCs of HIV-1-seronegative subjects (open boxes) (n = 47), of chronically HIV-1-infected subjects who had not been treated with HAART (shaded boxes) (n = 10), and of HIV-1-infected subjects whose antiretroviral treatment regimens had rendered their plasma levels of HIV-1 RNA undetectable (hatched boxes) (n = 28). The graph shows that effective treatment of HIV-1 infection with suppressive HAART did not normalize the γδ T-cell population changes seen with HIV-1 infection. (B) PBMCs from HIV-1-infected subjects identified and treated for 540 days after diagnosis during the chronic infection period (shaded boxes) (n = 8) were compared with those from HIV-1-seronegative subjects (closed boxes) (n = 47). On the left side of the graph, the percentages of cells staining for the αβ TCR (FITC) when examined for expression of CD4 (phycoerythrin) and CD8 (allophycocyanin) are indicated. On the right side of the graph, the percentages of γδ T cells expressing Vδ1 and Vδ2 are indicated. This graph shows that despite suppressive antiretroviral treatment for 540 days, the changes in Vδ1 and Vδ2 γδ T-cell populations did not normalize compared with those of HIV-1-seronegative subjects. (C) Comparison of Vδ1 and Vδ2 expression on MMCs of HIV-1-seronegative subjects (open boxes) (n = 19), of chronically HIV-1-infected subjects who had not received HAART (shaded boxes) (n = 9), and of those whose antiretroviral treatment regimens had rendered their plasma levels of HIV-1 RNA undetectable (hatched boxes) (n = 8). In comparison to the lack of reversibility of the phenotypic changes seen when blood γδ T-cell populations were studied, effective viral suppression with therapy did appear to result in partial normalization of the phenotypic changes in gastrointestinal γδ T-cell populations. For each box plot, the x axis outlines the T-cell phenotype while the y axis outlines the percentage of the lymphocyte population with that phenotype.
For eight HIV-1-infected subjects identified during the chronic infection period, longitudinal data from the initiation date of antiretroviral medications to day 540 on therapy are available (Fig. 3B). On diagnosis, the mean plasma HIV-1 RNA level was 5.49 ± 4.61 log copies/ml and the mean CD4 cell count was 501.5 ± 243.9. Seven of the eight subjects had undetectable levels of HIV-1 RNA by day 90. In all, over the 540 days of study only two subjects showed an elevation of plasma HIV-1 RNA levels into the detectable range. In this cohort, effective treatment of HIV-1 with HAART was associated with a trend toward normalization of CD4 and CD8+ αβ T-cell percentages in the peripheral blood of these subjects such that within 360 days after the initiation of therapy, both CD4 and CD8 cell percentages were significantly different (P = 0.03 for each) from their pretherapy baseline values. In comparison, treatment did not impact upon the phenotypic changes in the γδ T-cell population; despite 540 days of an effective antiretroviral regimen, there were no significant decreases in the percentage of Vδ1 (P = 0.179) or increases in the percentage of Vδ2 (P = 0.459) γδ T cells compared with their pretreatment values.
Compared with the observed persistent peripheral blood γδ T-cell population changes in HIV-1-infected subjects receiving effective HAART, mucosal γδ T-cell population changes modestly normalize in HIV-1-infected subjects receiving effective HAART for more than 1 year. The gastrointestinal mucosa of HIV-1-seropositive subjects who were not receiving suppressive HAART was found to contain a significantly higher percentage of γδ T cells than the mucosa of HIV-1-seronegative subjects (29.6% ± 19.2% versus 13.2% ± 5.7%; P = 0.015). Treatment of HIV-1 with suppressive HAART was associated with a reduction of mucosal γδ T cells to 16.3% ± 8.3%, a difference which approached significance when compared with that of untreated HIV-1-infected subjects (P = 0.087) (Fig. 3C). Further, the percentage of mucosal Vδ1-expressing γδ T cells in HAART-treated subjects (56.7% ± 17.1%) was slightly, though insignificantly, decreased compared to that seen in untreated HIV-1-seropositive subjects (61.4% ± 20.2%; P = 0.40). Treatment of HIV-1 with suppressive HAART was associated with a rise in the percentage of mucosal Vδ2 γδ T cells to 13.8% ± 7.8%, though this was not significantly different from that seen in the untreated population (18.8% ± 6.2%; P = 0.22).
Expression of mucosal homing receptors is increased among peripheral γδ T cells of HIV-1-infected subjects.
To explain the expanded peripheral populations of Vδ1 γδ T cells, we hypothesized that increased trafficking of γδ T cells from the periphery to the mucosa in response to heightened mucosal HIV-1 replication or overflow from the mucosal compartment to the periphery is responsible. To examine this hypothesis, we quantified the expression of mucosal homing receptors on peripheral blood γδ T cells. γδ and αβ T cells were analyzed for the expression of CCR9, a receptor that specifically directs lymphocytes to the small bowel (28, 36), and of CD103 (αEβ7), which is associated with lymphocytes destined for the mucosal epithelium throughout the gastrointestinal tract. CD103 was present on 64% of the MMCs isolated from rectal mucosal biopsies, while consistent with the colonic derivation of the biopsies, only 5.7% of the MMCS expressed CCR9 (data not shown). In comparison, a minority of PBMCs expressed either of these mucosal homing markers. As shown in Fig. 4, a higher percentage of blood γδ T cells of HIV-1-infected subjects (3.5% ± 2.7%) expressed either CCR9 or CD103 than those of HIV-1-uninfected subjects (1.2% ± 1.4%; P = 0.01). In comparison, the increased percentage of αβ T cells from HIV-1-infected subjects expressing either CCR9 or CD103 (2.8% ± 4.1%) did not reach statistical significance compared to those from HIV-1-seronegative subjects (1.6% ± 0.7%, P = 0.32). While expression of both CCR9 and CD103 was increased for γδ T cells from HIV-1-infected subjects compared with that seen with seronegative subjects, significance was only reached for expression of CCR9 (2.4% ± 1.7% versus 0.6% ± 0.7%; P = 0.02). In comparison, neither the percentage of CCR9 nor of CD103 was significantly increased for αβ T cells of patients infected with HIV-1 compared with that seen with uninfected subjects. Significant increases in the percentage of γδ T cells expressing mucosal homing receptors were seen in both treated and untreated HIV-1-infected subjects compared with that seen with HIV-1 seronegative subjects, though there were no significant differences in the expression of these receptors on PBMCs of these HIV-1-infected cohorts (data not shown). Further, though this analysis was performed on total γδ T-cell populations in blood samples, we have noted that the increase in CCR9 expression is limited to the Vδ2 γδ T-cell population (data not shown). The percentage of peripheral blood γδ T cells expressing either CCR9 or CD103 was not significantly correlated with the CD4 cell count or the plasma level of HIV-1 RNA (data not shown).
FIG. 4.
Comparison of expression of mucosal homing receptors CCR9 and CD103 on blood αβ (right panel) and γδ (left panel) T cells of HIV-1-seronegative and chronically infected subjects. PBMCs derived from HIV-1-seronegative subjects (open boxes) (n = 10) and subjects chronically infected with HIV-1 (shaded boxes) (n = 16) were prepared by Ficoll-Hypaque gradient and studied using flow cytometry. As shown, while there was no increase in the expression of mucosal lymphocyte homing receptors on αβ T cells of HIV-1-infected subjects, the expression of CCR9 and CCR9 or CD103 was increased for the total population of peripheral blood γδ T cells of HIV-1-infected subjects. For each box plot, the x axis outlines the T-lymphocyte phenotype while the y axis outlines the percentage of the lymphocyte population with that phenotype.
Peripheral blood and mucosas harbor distinct populations of γδ T cells.
To determine the clonality of γδ T-cell populations, we utilized CDR3 length polymorphism analysis (spectratyping) of the Vδ1 and Vδ2 elements for eight HIV-1-seropositive subjects who were identified during the chronic infection period. Figure 5 shows profiles of CDR3 length polymorphisms of Vδ1 and Vδ2 chains from the blood and rectal mucosa of eight HIV-1-infected subjects at the time of diagnosis during chronic HIV-1 infection. The profiles reveal dramatic differences in the CDR3 regions of the two anatomic compartments of each subject for both Vδ1 and Vδ2. Further, extensive length polymorphism in the CDR3 region for both Vδ1 and Vδ2 in each compartment was seen, likely reflecting a polyclonal response to infection. In addition, since there is no commonality between the CDR3 length patterns of the samples derived from the eight subjects, the antigens recognized by these cells may differ between individuals. This is significantly different from what has been reported for healthy individuals, for whom identical and stable CDR3 patterns have been described as being present throughout the colon and rectum (21, 24). Sequencing of the CDR3 region of the Vδ1 and Vδ2 γδ T cells of the blood and gastrointestinal mucosa of six HIV-1-infected subjects confirmed considerable heterogeneity between these compartments (data not shown).
FIG. 5.
Analysis of CDR3 length polymorphisms of Vδ1 and Vδ2 γδ T cells of chronically HIV-1-infected subjects. Two endoscopically obtained rectosigmoid biopsies and 1 × 106 to 5 × 106 frozen PBMCs were used for total RNA extraction. Single-strand cDNA was synthesized from the RNA, and analysis of CDR3 size distribution of Vδ1-Cδ rearrangements was performed using a Vδ1 primer and a Cδ antisense primer. Using an internal Cδ1 primer with a fluorescent dye bound to the amino-2 group of the primer, amplification products were subjected to a runoff elongation cycle and analyzed. The results of CDR3 length polymorphism analysis of the Vδ1 and Vδ2 regions of RNA derived from PBMCs and rectal biopsies from eight HIV-1-infected subjects at the time of diagnosis are shown. As shown, the spectratypes revealed dramatic differences in the CDR3 regions between the two anatomic compartments of each subject for both Vδ1 and Vδ2. The plots reveal extensive length polymorphism in the CDR3 region for both Vδ1 and Vδ2 in each compartment. The x axis of these plots reveals the CDR3 length, while the y axis shows fluorescence intensity in arbitrary units.
Treatment with HAART is associated with variable changes in the CDR3 length polymorphism profile.
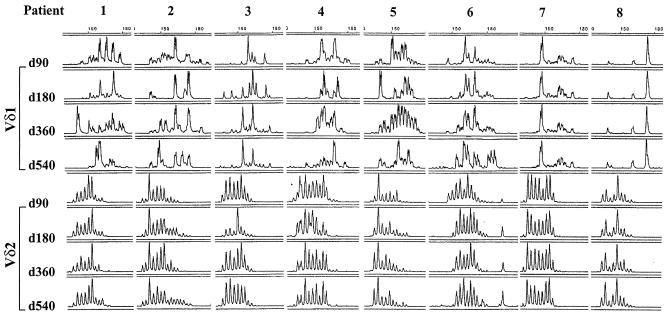
Having shown the HIV-1-infected subjects exhibited marked heterogeneity in the Vδ1 CDR3 region and that treatment with HAART is not associated with normalization of the percentages of Vδ1 and Vδ2 peripheral blood γδ T cells, we sought to examine whether suppression of HIV-1 replication with HAART would be associated with a restriction or expansion of the CDR3 diversity of Vδ1 and Vδ2 γδ T cells, as might be expected with removal of an inciting HIV-1-related antigen. We examined changes in the CDR3 length polymorphism profile in RNA from PBMCs extracted from the eight chronically HIV-1-infected subjects at 90, 180, 360, and 540 days after the initiation of HAART. Figure 6 demonstrates that despite 540 days of effective HAART, there was no appreciable restriction of Vδ1 or Vδ2 diversity. For the majority of these subjects, the Vδ1 CDR3 length polymorphism profile differed over time without discernible restriction or expansion of diversity. Patients 7 and 8, on the other hand, demonstrated a stable oligoclonal response over the duration of the study. In comparison with the Vδ1 profiles, the Vδ2 profiles of each of the patients appeared to be quite stable over the 540 days of treatment.
FIG. 6.
Determination of the effect of suppressive antiretroviral therapy on diversity of the Vδ1 and Vδ2 CDR3 of blood γδ T cells. The results of longitudinal CDR3 length polymorphism analysis of the Vδ1 and Vδ2 regions of RNA derived from eight chronically HIV-1-infected subjects at 90 to 540 days after diagnosis and initiation of antiretroviral therapy are shown. As shown, there was no appreciable restriction of the Vδ1 diversity despite the administration of suppressive antiretroviral medications. For the majority of these subjects, the Vδ1 CDR3 length polymorphism profiles differed over time without a discernible pattern to suggest restriction or expansion of diversity whereas the Vδ2 profiles of each of the eight patients appeared to be quite stable over the duration of treatment. The x axis of these plots reveals the CDR3 length, while the y axis shows fluorescence intensity in arbitrary units.
DISCUSSION
There is considerable heterogeneity of the γδ T-cell population in the body, with differing effector functions in diverse tissue distributions (7). While the effect of HIV-1 infection on peripheral blood γδ T cells has been studied by some investigators, little examination of the effects of HIV-1 on the more populous gastrointestinal mucosal γδ T cells has been undertaken. We undertook this study to assess whether HIV-1 infection is associated with phenotypic alteration of the mucosal and blood γδ T-cell populations and to determine whether these changes could be reversed with effective antiviral therapy. We found that while HIV-1-infected subjects did not exhibit an increase in the percentage of peripheral blood T cells expressing the γδ TCR, there was an expansion in the percentage of mucosal T cells that expressed the γδ TCR. In both the blood and mucosa of HIV-1-infected subjects, there was an expansion in the percentage of the Vδ1 and contraction in the percentage of the Vδ2 cell subsets. This study is the first to show that these phenotypic changes of the peripheral γδ T-cell population are observed during the acute HIV-1 infection period, persist during the chronic phase of infection, and are not reversed by prolonged suppression of HIV-1 replication with HAART. Expression of CCR9 and CD103 mucosal homing receptors was increased among peripheral blood γδ T cells of HIV-1-infected subjects, but as determined on the basis of analysis of CDR3 length polymorphism profiles and sequencing of the CDR3 region of Vδ1 and Vδ2 γδ T cells, mucosal and peripheral blood populations in HIV-1-infected subjects are distinct and polyclonal. It is thus interesting that HIV-1 infection is associated with expansion of the Vδ1 population and contraction of the Vδ2 γδ T-cell population in both the mucosa and peripheral blood, despite the genetic differences in the cells of these two compartments.
The most salient finding of this study was the striking and irreversible increase in the Vδ1 γδ T-cell population coupled with the dramatic decline in the Vδ2 cell population, which was seen in both the gastrointestinal mucosa and blood of HIV-1-infected subjects. These changes have previously been described for blood γδ T cells of HIV-1-infected subjects (2) and have also been shown to occur in the bone marrow (40), lungs (1), and duodenum (34) of HIV-1-infected subjects. While the increases in Vδ1 T-cell cell population could potentially represent a homeostatic compensation in response to the declines in Vδ2 γδ T-cell population (or vice versa), the lack of a negative correlation between these subsets among the HIV-1-infected subjects speaks against this probability. Still, the small number of subjects analyzed may limit the accuracy of this assessment. To date, the mechanisms responsible for Vδ1 population expansion and Vδ2 population reduction have not been elucidated.
The expansion of the Vδ1 cell population might have occurred in response to multiple antigens, since we observed that this expansion was not associated with preferred Vγ gene segments. Vδ1 cells might therefore have been acting in a manner similar to that seen in response to superantigens. The results of other studies that have examined the junctional diversity of Vδ1 and Vδ2 γδ T cells in HIV-1-infected individuals have also suggested that the changes are not due to clonal expansion of Vδ1 cells or to clonal deletion of Vδ2 cells (23). The expanded Vδ1 γδ T-cell populations seen in HIV-1 infection have also been shown to express a number of Vγ receptors, further arguing against a clonal expansion of this cell population (50). The numerical changes in Vδ1 T cells may be reflective of a polyclonal γδ T-cell response associated with some aspect of HIV-1 pathogenesis rather than against a single or a few specific antigens (49). Given that the polyclonal depletion of the Vδ2 γδ T cells has been noted to be more evident in subjects with opportunistic infections, infectious or inflammatory cofactors may also be involved in γδ T-cell population changes (5).
It is tempting to envisage that the increases in mucosal and peripheral Vδ1 γδ T-cell populations reflect the expansion of a population reactive against a soluble HIV-1 antigen. This would explain the alteration in γδ population phenotypic changes observed during the acute HIV-1 infection period and in those chronically infected subjects who had not been receiving therapy. However, the lack of normalization of the γδ population changes with suppressive HAART refutes this, providing contradictory evidence that Vδ1 γδ T-cell expansion is unrelated to the extent of HIV-1 replication and, thus, is unlikely to be due to the presence of an HIV-1-associated antigen in the blood or mucosa, though antigenemia may still be present despite the lack of detection of HIV-1 RNA.
Rather than responding to the presence of a viral antigen, the expansion of the Vδ1 cells may be in response to the induced expression of a cellular membrane ligand. EBV-transformed B cells from HIV-1-seropositive, but not from HIV-1-seronegative, subjects have been shown to be capable of activating Vδ1 cells (20). Recent data suggesting that expanded Vδ1 γδ T-cell populations may also destroy HIV-1-uninfected as well as -infected CD4+ cells (43) provide further evidence against reactivity to a viral antigen and stronger evidence for reactivity to a cellular ligand. Given that they are predominantly located in the mucosal epithelium in healthy subjects, it is not surprising that Vδ1 γδ T cells recognize MHC-class I-related receptors (MICA and MICB) induced by stress or infection of intestinal epithelial cells (18). Due to its large population of CCR5- and CXCR4-expressing activated CD4+ lymphocytes, the gastrointestinal mucosal compartment supports heightened HIV-1 replication (39, 45), and the concomitant mucosal inflammation (35) could conceivably result in increased expression of MICA and MICB, triggering local Vδ1 cell expansion. Other ligands recognized by these cells likely also belong to the class of stress-induced host factors, such as heat shock proteins (3, 42) or UL16 binding proteins (10). Our data provide some clues that may be utilized in the search for this antigen. The responsible ligand appears to be expressed early during HIV-1 infection and is not lost despite effective suppression of viral replication. Identification of this target of Vδ1 cells may permit discovery of treatment modalities that can slow the progression of disease by the salvage of CD4 T cells.
The possibility of increased mucosal recruitment of γδ T cells in response to HIV-1-associated mucosal inflammation is supported by our finding of increased expression of CCR9 or CD103 on peripheral blood γδ T cells. The converse may also be true; Boullier et al. suggested that the increase in the peripheral Vδ1 population might reflect increased trafficking from the tissues into the circulation due to cytokine gradients (6). In fact, other mucosal inflammatory states, such as inflammatory bowel disease, have been shown to be associated with an increased population of peripheral Vδ1 γδ T cells (16). Whether mucosa-derived lymphocytes migrating through the bloodstream retain expression of mucosal homing receptors is unknown. Therefore, it is not possible to ascertain the site of origin of the increased Vδ1 cells or their direction of migration.
One mechanism possibly responsible for the loss of Vδ2 γδ T cells is infection and destruction by HIV-1 itself. Some authors have found that a significant percentage of γδ T cells express CD4 (11). In a recent study, Imlach et al. showed that a large proportion of γδ lymphocytes expressed the chemokine receptors CCR5 and CXCR4 and that CD4+ γδ T cells could be productively infected by HIV-1 (25). Using quantitative PCR for HIV-1 proviral sequences, they showed that there is a substantial infection of γδ lymphocytes, contributing 3 to 45% of the total HIV-1 proviral load in blood. In another recent study, Gurney et al. showed that a subset of developing thymic γδ T cells expressing CD4, CXCR4, and CCR5 are susceptible to HIV-1 infection. These cells could be productively infected by both CXCR4- and CCR5-tropic HIV-1 virus, which could cause depletion of CD4+ γδ T cells (19). While both of these studies clearly show that γδ T cells can express CD4 and functional HIV-1 coreceptors, they did not assess whether these receptors were expressed preferentially on Vδ1 or Vδ2 γδ T cells and whether preferential infection of Vδ2 γδ T cells could contribute to their loss in HIV-1-infected subjects.
The clinical significance of changes in the Vδ1/Vδ2 ratio of the mucosa and blood of HIV-1-infected subjects remains unclear. The Vγ9/Vδ2 γδ T cells that predominate in the blood of healthy subjects and are severely depleted in HIV-1-infected subjects are believed to recognize a number of naturally occurring and synthetic nonpeptide phosphoantigens expressed by an array of pathogens, including mycobacteria, Plasmodium falciparum (4), and Francisella tularensis (17), as well as certain lymphoma cells (14). In particular, HIV-1 has been shown by Enders et al. to be associated with a polyclonal depletion of a specific subset of Vγ9δ2 cells expressing the Jγ1.2 segment (12). This cellular population is believed to represent the primary population of circulating γδ T cells that recognize the nonpeptide antigens that are expressed by many pathogens (33).
Whether the loss of these peripheral blood γδ T cells may increase susceptibility to systemic opportunistic infections and neoplasia has not been determined. Given that the gastrointestinal mucosa represents the primary site of residence of γδ T cells, where they function as vital immune effectors and influence epithelial cell homeostasis (27), it is likely that mucosal changes in γδ T-cell populations are immunologically important. Despite their likely reactivity to host cellular antigens, in accordance with their activation state, cytolytic activity against infected cells, and the increased production of gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) in patients with systemic viral infections, it is likely that one of the functions of γδ T cells is the elimination of virus-infected cells (20, 47). In comparison with αβ T cells, the antiviral response of γδ T cells is not dependent upon prior exposure to viral antigens. In fact, one study showed that 40% of Vγ9/Vδ2 cells from healthy donors can lyse HIV-1-infected cells (46). These cells could exert important antiviral activity through rapid polyclonal responses.
Stimulation of γδ T cells by virally infected cells results in cellular proliferation and cytolysis through the perforin-granzyme pathway. However, not all γδ T cells reactive to virus-infected cells are directly cytotoxic. Others mediate their effect by elaboration of soluble mediators. In addition to production of IFN-γ and TNF-α, which contribute to elimination of virally infected cells, activated γδ T cells also produce HIV-1-suppressive β-chemokines (29). Other populations of γδ T cells involved in viral immune responses in humans serve an immunoregulatory function. These cells, through the release of cytokines and lymphokines, modulate the Th1 and Th2 pathways of αβ T-cell helper-cell differentiation (13, 48). Type I (Th1) cytokine (IFN-γ, interleukin-2, and interleukin-12) production is strongly associated with T-cell activation and macrophage stimulation in attacks on intracellular pathogens. Skewing of the T-cell response to either Th1 or Th2 activation has significant consequences for the clearance of pathogens. Vδ1 cells, as a whole, appear to secrete less IFN-γ than do Vδ2 cells (11). Therefore, the increase in peripheral and mucosal Vδ1 cell populations may ultimately result in decreasing clearance of HIV-1 and other intracellular pathogens by decreasing the Th1 response.
Decreased pathogen clearance by γδ T cells of HIV-1-infected subjects has been observed in vitro. One study showed that the Vγ9Vδ2 cells that remain in the blood after HIV-1 infection appear to be incapable of proliferation to their nonpeptidic ligands, though they can still produce IFN-γ and TNF-α (38). Though we showed that HAART is not associated with a reversal of the decline in Vδ2 cell populations, it may reverse the anergy exhibited by the Vγ9Vδ2 cells (32). An important study by Martini et al. revealed that while acute HIV-1 replication during a structured treatment interruption is associated with a significant decline in the number of Vγ9δ2 effector γδ T cells and the functional anergy of these cells, resumption of HAART was able to reverse these changes (31). Since γδ T cells have recently been shown to be capable of reacting against and destroying HIV-1-infected and uninfected CD4+ cells in HIV-1-infected individuals, expansion of Vδ1 cell populations may also represent an important immunologic cofactor in disease progression (43). At this stage of investigation, the physiological consequences of Vδ1 expansion remain unknown and any of the consequences proposed above may prove true.
The lymphocyte response to viral pathogens involves not only B cells and αβ T cells but also the γδ T-cell population. The results we present may have significant clinical implications. Given the importance of the mucosal compartment in HIV-1 pathogenesis, further study to elucidate the significance of the changes observed here is critical. Efforts to strengthen the innate γδ T-cell immune response against HIV-1 may have important implications for both treatment strategies and prevention of transmission through mucosal surfaces (29). Future work should define the ligand for the expanded population of Vδ1 cells, examine the cause of Vδ2 population reduction, and more closely examine the clinical and immunologic ramifications of the described changes. In addition, given the importance of the gastrointestinal mucosa, future work should include this important compartment in studies of γδ T-cell pathogenesis in HIV-1.
Acknowledgments
This work was supported in part by grants AI-01668 and AI-46964 from the National Institute of Allergy and Infectious Diseases.
We are indebted to the General Clinical Research Center of The Rockefeller University (M01-RR00102) and the Acute HIV Infection and Early Disease Research Program (AIEDRP) (AI-41534).
REFERENCES
- 1.Agostini, C., R. Zambello, L. Trentin, A. Cerutti, P. Bulian, C. Crivellaro, A. Cipriani, and G. Semenzato. 1994. Gamma delta T cell receptor subsets in the lung of patients with HIV-1 infection. Cell. Immunol. 153:194-205. [DOI] [PubMed] [Google Scholar]
- 2.Autran, B., F. Triebel, C. Katlama, W. Rozenbaum, T. Hercend, and P. Debre. 1989. T cell receptor gamma/delta+ lymphocyte subsets during HIV infection. Clin. Exp. Immunol. 75:206-210. [PMC free article] [PubMed] [Google Scholar]
- 3.Battistini, L., M. Salvetti, G. Ristori, M. Falcone, C. S. Raine, and C. F. Brosnan. 1995. Gamma delta T cell receptor analysis supports a role for HSP 70 selection of lymphocytes in multiple sclerosis lesions. Mol. Med. 1:554-562. [PMC free article] [PubMed] [Google Scholar]
- 4.Behr, C., R. Poupot, M. A. Peyrat, Y. Poquet, P. Constant, P. Dubois, M. Bonneville, and J. J. Fournie. 1996. Plasmodium falciparum stimuli for human γδ T cells are related to phosphorylated antigens of mycobacteria. Infect. Immun. 64:2892-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boullier, S., M. Cochet, F. Poccia, and M. L. Gougeon. 1995. CDR3-independent gamma delta V delta 1+ T cell expansion in the peripheral blood of HIV-infected persons. J. Immunol. 154:1418-1431. [PubMed] [Google Scholar]
- 6.Boullier, S., G. Dadaglio, A. Lafeuillade, T. Debord, and M. L. Gougeon. 1997. V delta 1 T cells expanded in the blood throughout HIV infection display a cytotoxic activity and are primed for TNF-alpha and IFN-gamma production but are not selected in lymph nodes. J. Immunol. 159:3629-3637. [PubMed] [Google Scholar]
- 7.Carding, S. R., and P. J. Egan. 2002. γδ T cells: functional plasticity and heterogeneity. Nat. Rev. Immunol. 2:336-345. [DOI] [PubMed] [Google Scholar]
- 8.Ciccone, E., O. Viale, C. Bottino, D. Pende, N. Migone, G. Casorati, G. Tambussi, A. Moretta, and L. Moretta. 1988. Antigen recognition by human T cell receptor gamma-positive lymphocytes. Specific lysis of allogeneic cells after activation in mixed lymphocyte culture. J. Exp. Med. 167:1517-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cipriani, B., G. Borsellino, F. Poccia, R. Placido, D. Tramonti, S. Bach, L. Battistini, and C. F. Brosnan. 2000. Activation of C-C beta-chemokines in human peripheral blood γδ T cells by isopentenyl pyrophosphate and regulation by cytokines. Blood 95:39-47. [PubMed] [Google Scholar]
- 10.Cosman, D., J. Mullberg, C. L. Sutherland, W. Chin, R. Armitage, W. Fanslow, M. Kubin, and N. J. Chalupny. 2001. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity 14:123-133. [DOI] [PubMed] [Google Scholar]
- 11.De Rosa, S. C., D. K. Mitra, N. Watanabe, L. A. Herzenberg, L. A. Herzenberg, and M. Roederer. 2001. Vδ1 and Vδ2 γδ T cells express distinct surface markers and might be developmentally distinct lineages. J. Leukoc. Biol. 70:518-526. [PubMed] [Google Scholar]
- 12.Enders, P. J., C. Yin, F. Martini, P. S. Evans, N. Propp, F. Poccia, and C. D. Pauza. 2003. HIV-mediated γδ T cell depletion is specific for Vγ2+ cells expressing the Jγ1.2 segment. AIDS Res. Hum. Retrovir. 19:21-29. [DOI] [PubMed] [Google Scholar]
- 13.Ferrick, D. A., M. D. Schrenzel, T. Mulvania, B. Hsieh, W. G. Ferlin, and H. Lepper. 1995. Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma delta T cells in vivo. Nature 373:255-257. [DOI] [PubMed] [Google Scholar]
- 14.Fisch, P., M. Malkovsky, S. Kovats, E. Sturm, E. Braakman, B. S. Klein, S. D. Voss, L. W. Morrissey, R. DeMars, W. J. Welch, et al. 1990. Recognition by human V gamma 9/V delta 2 T cells of a GroEL homolog on Daudi Burkitt's lymphoma cells. Science 250:1269-1273. [DOI] [PubMed] [Google Scholar]
- 15.Fujihashi, K., J. R. McGhee, M. Yamamoto, T. Hiroi, and H. Kiyono. 1996. Role of gamma delta T cells in the regulation of mucosal IgA response and oral tolerance. Ann. N. Y. Acad. Sci. 778:55-63. [DOI] [PubMed] [Google Scholar]
- 16.Giacomelli, R., I. Parzanese, G. Frieri, A. Passacantando, F. Pizzuto, T. Pimpo, P. Cipriani, A. Viscido, R. Caprilli, and G. Tonietti. 1994. Increase of circulating gamma/delta T lymphocytes in the peripheral blood of patients affected by active inflammatory bowel disease. Clin. Exp. Immunol. 98:83-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gougeon, M. L., S. Boullier, V. Colizzi, and F. Poccia. 1999. NKR-mediated control of γδ T-cell immunity to viruses. Microbes Infect. 1:219-226. [DOI] [PubMed] [Google Scholar]
- 18.Groh, V., A. Steinle, S. Bauer, and T. Spies. 1998. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science 279:1737-1740. [DOI] [PubMed] [Google Scholar]
- 19.Gurney, K. B., O. O. Yang, S. B. Wilson, and C. H. Uittenbogaart. 2002. TCR gamma delta+ and CD161+ thymocytes express HIV-1 in the SCID-hu mouse, potentially contributing to immune dysfunction in HIV infection. J. Immunol. 169:5338-5346. [DOI] [PubMed] [Google Scholar]
- 20.Hacker, G., S. Kromer, M. Falk, K. Heeg, H. Wagner, and K. Pfeffer. 1992. V delta 1+ subset of human gamma delta T cells responds to ligands expressed by EBV-infected Burkitt lymphoma cells and transformed B lymphocytes. J. Immunol. 149:3984-3989. [PubMed] [Google Scholar]
- 21.Hata, S., M. Clabby, P. Devlin, H. Spits, J. E. De Vries, and M. S. Krangel. 1989. Diversity and organization of human T cell receptor delta variable gene segments. J. Exp. Med. 169:41-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayday, A. C. 2000. γδ cells: a right time and a right place for a conserved third way of protection. Annu. Rev. Immunol. 18:975-1026. [DOI] [PubMed] [Google Scholar]
- 23.Hinz, T., D. Wesch, K. Friese, A. Reckziegel, B. Arden, and D. Kabelitz. 1994. T cell receptor gamma delta repertoire in HIV-1-infected individuals. Eur. J. Immunol. 24:3044-3049. [DOI] [PubMed] [Google Scholar]
- 24.Holtmeier, W., Y. Chowers, A. Lumeng, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1995. The delta T cell receptor repertoire in human colon and peripheral blood is oligoclonal irrespective of V region usage. J. Clin. Investig. 96:1108-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imlach, S., C. Leen, J. E. Bell, and P. Simmonds. 2003. Phenotypic analysis of peripheral blood γδ T lymphocytes and their targeting by human immunodeficiency virus type 1 in vivo. Virology 305:415-427. [DOI] [PubMed] [Google Scholar]
- 26.James, S. P., C. Fiocchi, A. S. Graeff, and W. Strober. 1986. Phenotypic analysis of lamina propria lymphocytes. Predominance of helper-inducer and cytolytic T-cell phenotypes and deficiency of suppressor-inducer phenotypes in Crohn's disease and control patients. Gastroenterology 91:1483-1489. [PubMed] [Google Scholar]
- 27.Komano, H., Y. Fujiura, M. Kawaguchi, S. Matsumoto, Y. Hashimoto, S. Obana, P. Mombaerts, S. Tonegawa, H. Yamamoto, S. Itohara, et al. 1995. Homeostatic regulation of intestinal epithelia by intraepithelial gamma delta T cells. Proc. Natl. Acad. Sci. USA 92:6147-6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunkel, E. J., J. J. Campbell, G. Haraldsen, J. Pan, J. Boisvert, A. I. Roberts, E. C. Ebert, M. A. Vierra, S. B. Goodman, M. C. Genovese, A. J. Wardlaw, H. B. Greenberg, C. M. Parker, E. C. Butcher, D. P. Andrew, and W. W. Agace. 2000. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J. Exp. Med. 192:761-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehner, T., E. Mitchell, L. Bergmeier, M. Singh, R. Spallek, M. Cranage, G. Hall, M. Dennis, F. Villinger, and Y. Wang. 2000. The role of γδ T cells in generating antiviral factors and beta-chemokines in protection against mucosal simian immunodeficiency virus infection. Eur. J. Immunol. 30:2245-2256. [DOI] [PubMed] [Google Scholar]
- 30.Maccario, R., P. Comoli, E. Percivalle, D. Montagna, F. Locatelli, and G. Gerna. 1995. Herpes simplex virus-specific human cytotoxic T-cell colonies expressing either gamma delta or alpha beta T-cell receptor: role of accessory molecules on HLA-unrestricted killing of virus-infected targets. Immunology 85:49-56. [PMC free article] [PubMed] [Google Scholar]
- 31.Martini, F., F. Poccia, D. Goletti, S. Carrara, D. Vincenti, G. D'Offizi, C. Agrati, G. Ippolito, V. Colizzi, L. P. Pucillo, and C. Montesano. 2002. Acute human immunodeficiency virus replication causes a rapid and persistent impairment of Vγ9Vδ2 T cells in chronically infected patients undergoing structured treatment interruption. J. Infect. Dis. 186:847-850. [DOI] [PubMed] [Google Scholar]
- 32.Martini, F., R. Urso, C. Gioia, A. De Felici, P. Narciso, A. Amendola, M. G. Paglia, V. Colizzi, and F. Poccia. 2000. γδ T-cell anergy in human immunodeficiency virus-infected persons with opportunistic infections and recovery after highly active antiretroviral therapy. Immunology 100:481-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyagawa, F., Y. Tanaka, S. Yamashita, B. Mikami, K. Danno, M. Uehara, and N. Minato. 2001. Essential contribution of germline-encoded lysine residues in Jγ1.2 segment to the recognition of nonpeptide antigens by human γδ T cells. J. Immunol. 167:6773-6779. [DOI] [PubMed] [Google Scholar]
- 34.Nilssen, D. E., F. Müller, O. Øktedalen, S. S. Frøland, O. Fausa, T. S. Halstensen, and P. Brandtzaeg. 1996. Intraepithelial γ/δ T cells in duodenal mucosa are related to the immune state and survival time in AIDS. J. Virol. 70:3545-3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olsson, J., M. Poles, A. L. Spetz, J. Elliott, L. Hultin, J. Giorgi, J. Andersson, and P. Anton. 2000. Human immunodeficiency virus type 1 infection is associated with significant mucosal inflammation characterized by increased expression of CCR5, CXCR4, and beta-chemokines. J. Infect. Dis. 182:1625-1635. [DOI] [PubMed] [Google Scholar]
- 36.Papadakis, K. A., J. Prehn, V. Nelson, L. Cheng, S. W. Binder, P. D. Ponath, D. P. Andrew, and S. R. Targan. 2000. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J. Immunol. 165:5069-5076. [DOI] [PubMed] [Google Scholar]
- 37.Poccia, F., L. Battistini, B. Cipriani, G. Mancino, F. Martini, M. L. Gougeon, and V. Colizzi. 1999. Phosphoantigen-reactive Vγ9Vδ2 T lymphocytes suppress in vitro human immunodeficiency virus type 1 replication by cell-released antiviral factors including CC chemokines. J. Infect. Dis. 180:858-861. [DOI] [PubMed] [Google Scholar]
- 38.Poccia, F., S. Boullier, H. Lecoeur, M. Cochet, Y. Poquet, V. Colizzi, J. J. Fournie, and M. L. Gougeon. 1996. Peripheral V gamma 9/V delta 2 T cell deletion and anergy to nonpeptidic mycobacterial antigens in asymptomatic HIV-1-infected persons. J. Immunol. 157:449-461. [PubMed] [Google Scholar]
- 39.Poles, M. A., J. Elliott, P. Taing, P. A. Anton, and I. S. Chen. 2001. A preponderance of CCR5+ CXCR4+ mononuclear cells enhances gastrointestinal mucosal susceptibility to human immunodeficiency virus type 1 infection. J. Virol. 75:8390-8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossol, R., J. M. Dobmeyer, T. S. Dobmeyer, S. A. Klein, S. Rossol, D. Wesch, D. Hoelzer, D. Kabelitz, and E. B. Helm. 1998. Increase in Vδ1+ γδ T cells in the peripheral blood and bone marrow as a selective feature of HIV-1 but not other virus infections. Br. J. Haematol. 100:728-734. [DOI] [PubMed] [Google Scholar]
- 41.Sciammas, R., and J. A. Bluestone. 1999. TCRγδ cells and viruses. Microbes Infect. 1:203-212. [DOI] [PubMed] [Google Scholar]
- 42.Selin, L. K., S. Stewart, C. Shen, H. Q. Mao, and J. A. Wilkins. 1992. Reactivity of gamma delta T cells induced by the tumour cell line RPMI 8226: functional heterogeneity of clonal populations and role of GroEL heat shock proteins. Scand. J. Immunol. 36:107-117. [DOI] [PubMed] [Google Scholar]
- 43.Sindhu, S. T. A. K., R. Ahmad, R. Morisset, A. Ahmad, and J. Menezes. 2003. Peripheral blood cytotoxic γδ T lymphocytes from patients with human immunodeficiency virus type 1 infection and AIDS lyse uninfected CD4+ T cells, and their cytocidal potential correlates with viral load. J. Virol. 77:1848-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Targan, S. R., R. L. Deem, M. Liu, S. Wang, and A. Nel. 1995. Definition of a lamina propria T cell responsive state. Enhanced cytokine responsiveness of T cells stimulated through the CD2 pathway. J. Immunol. 154:664-675. [PubMed] [Google Scholar]
- 45.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 46.Wallace, M., S. R. Bartz, W. L. Chang, D. A. Mackenzie, C. D. Pauza, and M. Malkovsky. 1996. Gamma delta T lymphocyte responses to HIV. Clin. Exp. Immunol. 103:177-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallace, M., M. Malkovsky, and S. R. Carding. 1995. Gamma/delta T lymphocytes in viral infections. J. Leukoc. Biol. 58:277-283. [DOI] [PubMed] [Google Scholar]
- 48.Wen, L., D. F. Barber, W. Pao, F. S. Wong, M. J. Owen, and A. Hayday. 1998. Primary gamma delta cell clones can be defined phenotypically and functionally as Th1/Th2 cells and illustrate the association of CD4 with Th2 differentiation. J. Immunol. 160:1965-1974. [PubMed] [Google Scholar]
- 49.Wesch, D., T. Hinz, and D. Kabelitz. 1998. Analysis of the TCR Vγ repertoire in healthy donors and HIV-1-infected individuals. Int. Immunol. 10:1067-1075. [DOI] [PubMed] [Google Scholar]
- 50.Wesch, D., D. Kabelitz, K. Friese, and K. Pechhold. 1996. Mycobacteria-reactive gamma delta T cells in HIV-infected individuals: lack of V gamma 9 cell responsiveness is due to deficiency of antigen-specific CD4 T helper type 1 cells. Eur. J. Immunol. 26:557-562. [DOI] [PubMed] [Google Scholar]