Abstract
Efficient proteolytic release of nonstructural protein 3 (NS3) from the viral polyprotein is considered to be crucial for the cytopathogenicity of pestiviruses. Here we describe a novel cytopathogenic (cp) bovine viral diarrhea virus strain (BVDV CP8) with a complex insertion composed of viral and cell-derived sequences, including two fragments of the cellular J-domain protein Jiv (J-domain protein interacting with viral protein) located in the N-terminal region of the polyprotein. BVDV CP8 expresses a Jiv fusion protein of 513 amino acids in addition to a complete set of viral proteins. This protein has the capacity to induce NS2-3 cleavage in trans. Accordingly, CP8 is a representative of a novel type of cp pestivirus with a cp-specific mutation located outside of the NS2-3 gene.
The pestiviral genome is a 12.3-kb single-stranded RNA of positive polarity (5; A. Renard, D. Dino, and J. Martial, 1987, European patent application 86870095.6:publication 02.08672) and encodes the polyprotein NH2-Npro-C-Erns-E1-E2-p7-NS2-3-NS4A-NS4B-NS5A-NS5B-COOH, which is processed by cellular and virus-encoded proteases (14). The autoprotease Npro generates its own C terminus and thereby the N terminus of the capsid protein (C) (25, 29, 33). The three envelope glycoproteins Erns, E1, and E2, as well as p7, are released from the polyprotein by cellular proteases (10, 12, 27). A serine protease located in nonstructural protein 3 (NS3) catalyzes all processing events downstream of NS3 (11, 32, 34).
In the course of lethal mucosal disease, cytopathogenic (cp) bovine viral diarrhea virus (BVDV) strains evolve from noncytopathogenic (noncp) ancestors (14, 18). One striking difference between cp and noncp BVDV concerns the expression of free NS3; free NS3 is expressed only by cp BVDV, while only unprocessed NS2-3 can be detected in noncp BVDV-infected cells (9, 21). Mutations responsible for the expression of NS3 include insertions of cellular protein-coding sequences (14, 18). A cell-derived insertion termed “Jiv” (formerly cIns; see below) was identified in the NS2 genes of several cp pestiviruses, and its presence was linked to NS2-3 cleavage and cytopathogenicity (2, 15, 16, 18, 20, 23). The corresponding cellular chaperone protein Jiv (J-domain protein interacting with viral protein) has the capacity to induce NS2-3 cleavage in trans (24). Here we describe a cp BVDV isolate with a Jiv-containing insertion located outside of NS2-3.
Genome structure of BVDV strain CP8.
Strains CP8 and NCP8 were both isolated from the same animal suffering from mucosal disease and thus represent a so-called virus pair (7). Our first step in the analysis of these viruses was a comparative Northern blot analysis. Five micrograms of total RNA isolated from CP8- and NCP8-infected MDBK cells was separated by denaturing agarose gel electrophoresis and after being blotted onto a membrane was hybridized against different [α-32P]dCTP-labeled cDNA probes. In the RNA of NCP8-infected cells, a BVDV-specific probe detected a band of 12.3 kb, the size expected for a standard pestivirus genome (Fig. 1). In contrast, the genomic RNA of CP8 had a size of about 14 kb, about 1.7 kb larger than that of NCP8.
FIG. 1.
Northern blot analysis. Total RNAs isolated 48 h postinfection from MDBK cells infected with BVDV strains NCP8, CP8, and NADL and from noninfected MDBK cells were blotted after separation by agarose gel electrophoresis and hybridized against [32P]-labeled probes specific for BVDV (left) or Jiv (right). The BVDV-specific probe is 83 or 95% identical to the genomes of BVDV NADL or CP8, respectively; the Jiv probe shows an identity of over 99% to the Jiv insertions in both genomes, explaining the differences in the signal intensities obtained with these probes. Due to its low abundance, the Jiv mRNA could not be detected in total cellular RNA (24). The size standards are shown on the left; the BVDV NADL genome has a length of about 12.6 kb (5) and served as an additional size marker to estimate the length of the CP8 genome.
Hybridization with a Jiv-coding probe revealed that at least part of the additional genomic information present in the CP8 genome is derived from the cellular Jiv mRNA. RNA of BVDV strain NADL-infected cells was used as a control since this strain encompasses a Jiv insertion in its genome (15) (Fig. 1). According to this analysis, the CP8 RNA is the largest pestiviral genome with a Jiv insertion described so far.
Jiv insertions have been detected in the NS2-coding region of several pestiviral genomes (see below). The sequence analysis of the CP8 RNA was therefore started with the NS2 gene. To our surprise, we found neither Jiv-coding sequences nor any other insertion in the NS2 gene (data not shown). A more extended analysis revealed the presence of a unique large insertion in the 5′ region of the CP8 genome (Fig. 2). For this analysis two CP8-specific cDNA clones (Lambda ZAP II system; Stratagene, La Jolla, Calif.) were used. The cDNA of clone CP8/12 has a length of 2,361 nucleotides and starts at position 125 of the viral genome when aligned with the nucleotide sequence of BVDV strain SD-1 (8). The cDNA of clone CP8/22 corresponds to nucleotides 43 to 1893 of clone CP8/12.
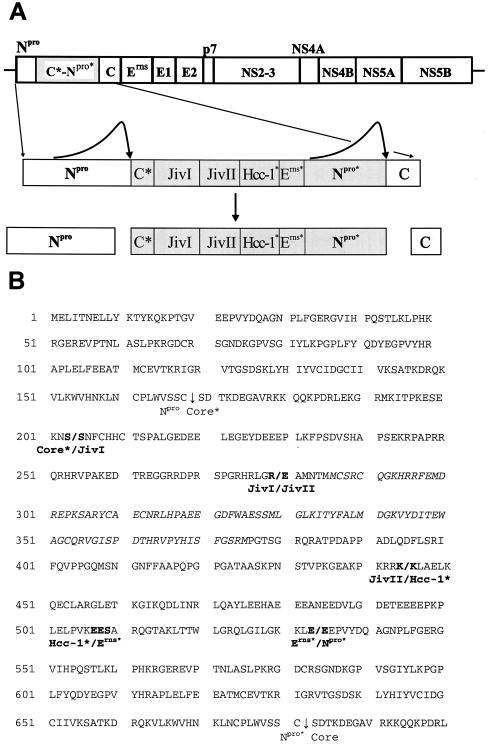
FIG. 2.
(A) The top diagram is the genome organization of BVDV strain CP8 deduced from the analyzed cDNA clones (see also Fig. 3A). The N-terminal region of the CP8 polyprotein and the processing products are depicted below the diagram of the genome. The inserted region is marked by a grey bar. C*, truncated core protein; JivI and JivII, fragments of bovine Jiv; Hcc-1*, fragment of protein Hcc-1; Erns*, Erns-derived peptide; Npro*, truncated Npro. C*-Npro* represents a CP8-specific processing product consisting of 513 amino acids. (B) Amino acid sequence of the N-terminal region of the CP8 polyprotein. Proteolytic cleavage sites are indicated by vertical arrows. Slashes indicate the borders between the rearranged sequence blocks. C*, a 35-amino acid fragment of C (based on the nucleotide sequences, codon S203 is derived from C, while S204 originates from the Jiv mRNA); JivI, amino acids 151 to 226 of bovine Jiv; JivII, amino acids 527 to 692 or 693 of bovine Jiv (since codon K445 matches the corresponding codon of the Jiv mRNA, it was ascribed to JivII; however, to confirm that K445 is not derived from Hcc-1, the nucleotide sequence of the corresponding bovine mRNA would be required); Hcc-1*, amino acids 11 or 12 to 74 or 75 of protein Hcc-1 (to define the border between Hcc-1* and Erns*, the nucleotide sequences of the bovine Hcc-1 mRNA and the Erns gene of CP8 would be required); Erns*, amino acids 170 or 171 to 196 of Erns (see above). The part of Jiv representing Jiv90 is shown in italics.
In the CP8 genome a complete Npro sequence but only 35 amino acids of the core protein are encoded downstream of the 5′ untranslated region (Fig. 2B). This core gene fragment (C*) is followed by two sequence blocks derived from the cellular Jiv mRNA; these sequences represent codons 204 to 445 of the CP8 open reading frame. The inserted Jiv-coding sequences correspond to codons 151 to 226 (JivI) and 527 to 692 (JivII) of the bovine Jiv mRNA. The sequence block downstream of the Jiv-coding sequences encodes a highly acidic peptide; the corresponding peptide shows an amino acid identity of 95% to the N-terminal region of human nuclear protein Hcc-1 (product of the upregulated gene in hepatocellular carcinoma [GenBank accession no. P82979]) (4). The next sequence block in the CP8 open reading frame is a fragment of the pestiviral Erns gene, which is followed by an almost complete standard BVDV genome starting with codon 21 of the Npro gene. The amino acid sequence of the N-terminal region of the CP8 polyprotein is shown in Fig. 2B. Altogether, 513 additional codons were identified in the CP8 RNA when it was compared to a standard BVDV genome. Accordingly, the detected insertion explains the enlarged size of the CP8 RNA and confirms the presence of a Jiv-coding sequence in the CP8 RNA.
Moreover, the high degree of identity observed between the duplicated Npro- and core-coding sequences in the CP8 genome (99.0% at the nucleotide level) (data not shown) suggests that they originate from the same parental RNA.
Processing of the N-terminal region of the CP8 polyprotein.
As described above, the CP8 polyprotein encompasses two copies of Npro in the N-terminal region. Given that both enzymes are proteolytically active, two cis cleavages would be expected, one between the complete Npro and the C-terminally truncated core protein (C*) and a second one between the N-terminally truncated Npro (Npro*) and the complete core protein (C). Npro* was tested for proteolytic activity (Fig. 2A) because of its truncation and its assumed fusion to the upstream peptide. Moreover, additional proteolytic cleavages within the inserted part of the polyprotein could not be excluded.
The processing studies were carried out using the vaccinia virus MVA-T7pol expression system (30), and results were compared to those obtained with cells infected with BVDV CP8 at a multiplicity of infection of 2. The first construct, pC8/Npro-C-His, encompassed at a position downstream of a T7 RNA polymerase promoter a cDNA fragment encoding the entire N-terminal region of the CP8 polyprotein, from the Npro downstream to the complete copy of the C protein fused in frame to a His tag (Fig. 3A). In addition, truncated derivatives of this construct were established. In pC8/C*-C-His, the N-terminal Npro gene was deleted. The polyproteins encoded by pC8/Npro-Erns* and pC8/Npro-Hcc-1* end with Erns* and Hcc-1*, respectively (Fig. 3A). Two micrograms of each plasmid was transfected by the use of Metafectene (Biontex, Munich, Germany). The expressed proteins were metabolically labeled for 4 h with 70 μCi of [35S]methionine-cysteine ([35S]-ProMix; Amersham, Freiburg, Germany) and analyzed by radioimmunoprecipitation (see reference 24). The rabbit antisera used were directed against Jiv90 present in the JivII domain or against Npro (F1) (26); in addition, a monoclonal antibody (MAb) directed against a five-His epitope (Qiagen, Hilden, Germany) was used.
FIG. 3.
(A) (Top) Scheme of the CP8 genome structure; black bars below the genome depict the cDNA clones and reverse transcription-PCR fragments used to analyze the genome structure. (Bottom) Scheme of expression constructs. Numbers below the bars denote the calculated molecular sizes (k, kilodaltons) of the expected processing products; numbers in brackets indicate the amino acids of the CP8 polyprotein which are encoded by their respective constructs. In the polypeptide encoded by pC8/E2-NS4A, S symbolizes the signal peptide preceding E2. (B) Processing of the N-terminal region of the CP8 polyprotein. Metabolically labeled proteins were isolated by radioimmunoprecipitation with anti-Jiv and anti-Npro sera and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on an 8% Tricine gel (28). The strong band at 55 kDa detected by the Jiv serum (lanes 1 to 5) was not recognized by a different Jiv-specific antiserum from another rabbit (data not shown), strongly suggesting that this protein is not related to cellular Jiv or putative Jiv homologues. Constructs used for protein expression are indicated above the lanes. The size standards are shown on the left. The positions of C*-Npro* and Npro are indicated by arrowheads. (C) Detection of C-His. For radioimmunoprecipitation a MAb directed against the His tag was used. Proteins were separated on a 12% Tricine gel, and the position of C-His is indicated by an arrowhead. All lanes were derived from the same gel. (D) Detection of the CP8-specific protein. Constructs used for transient expression in BHK cells are indicated above the lanes. MDBK cells infected with BVDV strain CP8 or NCP8 at a multiplicity of infection of 2 were analyzed at 48 h postinfection. Proteins were separated on an 8% Tricine gel; for Western blot analysis, the Jiv-specific serum was used. All lanes were derived from the same gel.
Upon transfection of pC8/Npro-C-His, the Npro-specific serum detected Npro in the cell lysates with an apparent molecular size of 20 kDa (Fig. 3B, lane 7) as well as a protein of ca. 64 kDa; the latter polypeptide was also recognized by the Jiv-specific serum and thus represents C*-Npro* (Fig. 3B, lane 2). The His tag-specific MAb recognized a 15-kDa cleavage product which corresponds to C-His (Fig. 3C, lane 2); the remarkably low mobility of the C-His peptide may be due to its charged character (isoelectric point, 10.8). In addition, weak bands representing processing intermediates appeared mainly after longer exposure times.
In cells transfected with pC8/Npro-Erns*, the Npro-specific serum detected free Npro (Fig. 3B, lane 10), while the Jiv-specific serum reacted with a 46-kDa protein (Fig. 3B, lane 5) representing the C-terminal cleavage product generated by autocatalytic removal of Npro. Comparable results were obtained with pC8/Npro-Hcc-1*, where free Npro (Fig. 3B, lane 9) and a C-terminal cleavage product with a slightly lower apparent molecular size (44 kDa) (Fig. 3B, lane 4) than the one released from Npro-Erns* were detected. In cells transfected with pC8/C*-C-His, the Jiv-specific serum detected C*-Npro* (64 kDa) (Fig. 3B, lane 3) and the His tag-specific MAb recognized the cleavage product C-His (Fig. 3C, lane 3). These data demonstrate that not only the N-terminal complete Npro protein but also the C*-Npro* fusion protein are enzymatically active. Importantly, we found no indication for additional processing events within polyprotein fragment C*-Npro*.
Based on these findings, we analyzed the proteins from BVDV CP8-infected cells by Western blotting with an antiserum directed against Jiv. In CP8-infected cells a protein of approximately 64 kDa was detected (Fig. 3D); this protein was not present in NCP8-infected cells. The 64-kDa polypeptide comigrated with transiently expressed C*-Npro* but not with any of the processing intermediates derived from the N-terminal region of the polyprotein (Fig. 3D). Accordingly, cp BVDV strain CP8 expresses one additional protein, namely, C*-Npro*.
Generation of NS3.
All cp pestiviruses investigated so far express large amounts of free NS3. In a Western blot analysis (24) performed with lysates of CP8-infected MDBK cells, free NS3 in addition to uncleaved NS2-3 was detected, while after infection with NCP8 only unprocessed NS2-3 was observed (Fig. 4A). The genome organization of CP8 implies that NS3 has to be generated by cleavage of NS2-3. When the NS2 sequences of NCP8 and CP8 were compared, no deletions or insertions but four amino acid exchanges were identified: I1176T, Y1336H, H1552Y, and Q1554E (data not shown) (GenBank accession no. AY182137 and AY182138). To test whether NS2 of CP8 encompasses signals for NS2-3 cleavage, we established pC8/E2-NS4A, a plasmid encoding the entire NS2 and the N-terminal part of NS3 of strain CP8 in the context of the E2-to-NS4A polyprotein fragment of BVDV strain CP7. Plasmid pC8/E2-NS4A was established by replacing an NsiI/AgeI fragment in pC/E2-4A (24) with the corresponding cDNA of BVDV CP8; the CP8 cDNA fragment in pE2-4A encodes amino acids 1118 to 1644 of the BVDV polyprotein. With an analogous approach, it has been demonstrated previously that cp BVDV strains CP7 and Oregon contain signals in NS2 which confer NS2-3 cleavage (13, 31). For protein expression the T7 vaccinia virus system was used, and processing was monitored by Western blotting with the NS3-specific MAb 8.12.7 (6). For the detection of antigen, peroxidase-coupled species-specific secondary antibodies and Renaissance Western Blot Chemiluminescence Reagent Plus (NEN Life Sciences, Boston, Mass.) were applied. Upon transfection of pC8/E2-NS4A, a precursor protein of about 180 kDa as well as NS2-3 but no NS3 was detected, indicating that NS2 of strain CP8 does not contain signals for the induction of NS2-3 cleavage (Fig. 4B).
FIG. 4.
(A) Expression of NS2-3 and NS3 in MDBK cells infected with NCP8 or CP8. Cell lysates prepared 48 h postinfection were separated on an 8% Tricine gel and subjected to Western blot analysis by using a MAb directed against NS3. Noninfected cells served as controls. Positions of NS2-3 and NS3 are marked by arrowheads. (B) Induction of NS2-3 cleavage in trans. Proteins were analyzed by Western blot with a MAb directed against NS3. Constructs used to drive protein expression are indicated above the lanes. Positions of NS2-3 and NS3 are marked by arrowheads. The molecular size standards are shown on the left; k, kilodaltons.
According to this result, it appeared likely that the highly unusual N-terminal polyprotein region of CP8 plays a role in NS2-3 cleavage, especially since cellular Jiv or the derived Jiv90 domain (amino acids 533 to 622 of Jiv) is able to induce the cleavage of NS2-3 in noncp BVDV-infected cells (24). Since Jiv90 is part of C*-Npro* (Fig. 2B), it was reasonable to assume that this protein also has the capacity to induce NS2-3 cleavage in trans.
The induction of NS2-3 cleavage in trans by C*-Npro* was tested by cotransfection of pC8/C*-Npro* and pC8/E2-NS4A. In the corresponding cells, large amounts of NS3 were detected as well as the uncleaved NS2-3 and a larger precursor protein (Fig. 4B, lane 4). This finding demonstrates that the CP8-specific protein C*-Npro* is indeed capable of inducing efficient NS2-3 cleavage. Cotransfection of pGST-Jiv90 (24) and pC8/E2-NS4A, used as a control, resulted in efficient induction of NS2-3 cleavage (Fig. 4B, lane 3).
Jiv90 is present within the JivII domain of C*-Npro*. Our data suggested that despite its unusual context, the Jiv90 peptide is crucial for the NS2-3 cleavage in the CP8 polyprotein. To address the possibility that, besides Jiv90, other parts of C*-Npro* also have the capacity to induce NS2-3 cleavage, two additional expression constructs were established (Fig. 3A). These constructs encode either the N-terminal or the C-terminal part of C*-Npro*. Plasmid pC8/C*-JivII* encodes the CP8 polyprotein from C* to the middle of Jiv90; the protein encoded by pC8/JivII**-Npro* starts in the C-terminal half of Jiv90 and ends with Npro* (constructs shown in Fig. 3A). Both plasmids were cotransfected with pC8/E2-NS4A separately and together. The expression of the CP8-derived proteins was demonstrated by use of the Jiv-specific serum (data not shown). In these three cell lysates, NS2-3 was detected but no NS3 (Fig. 4B, lanes 5 to 7). From these data we conclude that Jiv90, present in the JivII fragment of C*-Npro*, is the only part of the CP8 polyprotein with the capacity to induce NS2-3 cleavage; moreover, the two Jiv fragments do not functionally complement each other.
Studies over the past decade demonstrated that pestiviral cytopathogenicity is correlated with mutations that lead to the expression of NS3 (14, 18). Cell-derived insertions located precisely upstream of NS3 were shown to cause the release of NS3 by cellular proteases (1, 3, 17, 18, 22). In a few cases, e.g., cp BVDV strains Oregon and CP7, point mutations or a small insertion in NS2 is responsible for NS2-3 cleavage by unknown mechanisms (13, 31). In several other cp pestivirus strains, cellular Jiv-coding sequences located within the NS2 gene induce NS2-3 cleavage by a mechanism that is also not yet resolved (2, 15, 18, 20, 23, 24). It is common to all cp pestiviruses mentioned above that the cp-specific mutation concerns the NS2-3-coding region. BVDV strain CP8, analyzed in this study, represents an exception to this scheme since its cp-specific insertion is located in the N-terminal region of the polyprotein. The Jiv fusion protein C*-Npro* encoded by BVDV CP8 induces cleavage of NS2-3 in trans as previously demonstrated for the cellular Jiv protein and its fragment Jiv90 (24). According to our study, the only domain of C*-Npro* capable of inducing NS2-3 cleavage is Jiv90. This peptide interacts with NS2 of BVDV and thereby induces cleavage of NS2-3 by a mechanism which is under investigation (24).
In the final stage of this project, Nagai et al. described another Jiv insertion in the N-terminal region of cp BVDV (19). The polyproteins of cp BVDV viruses NOSE and KS86-1cp encompass a 110-amino-acid Jiv insertion which is located downstream of a C-terminally truncated C and fused to a duplicated Npro which is N-terminally truncated by six amino acids. In that study no data on viral proteins or RNA was included. However, since in the study by Nagai et al., Jiv90 is also part of the insertion, our data strongly suggest that in these viruses the insertion is also causative for NS2-3 cleavage and viral cytopathogenicity. Compared with CP8, the insertion in strains NOSE and KS86-1cp is a simplified version of the same scheme. Accordingly, Jiv insertions in the N-terminal region of the viral polyprotein are obviously not restricted to BVDV CP8 but are characteristic of a novel type of cp BVDV.
Acknowledgments
We thank S. Jacobi for excellent technical assistance. We are grateful to G. Meyers for his contributions in the initial phase of the project.
This study was supported by SFB 535 “Invasionsmechanismen und Replikationsstrategien von Krankheitserregern” (G. Rinck) and by Graduiertenkolleg 455 “Molekulare Veterinärmedizin” (A. Müller) of the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Baroth, M., M. Orlich, H.-J. Thiel, and P. Becher. 2000. Insertion of cellular NEDD8 coding sequences in a pestivirus. Virology 278:456-466. [DOI] [PubMed] [Google Scholar]
- 2.Becher, P., G. Meyers, A. D. Shannon, and H.-J. Thiel. 1996. Cytopathogenicity of border disease virus is correlated with integration of cellular sequences into the viral genome. J. Virol. 70:2992-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becher, P., H.-J. Thiel, M. Collins, J. Brownlie, and M. Orlich. 2002. Cellular sequences in pestivirus genomes encoding gamma-aminobutyric acid (A) receptor-associated protein and Golgi-associated ATPase enhancer of 16 kilodaltons. J. Virol. 76:13069-13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choong, M. L., L. K. Tan, S. L. Lo, E. C. Ren, K. Ou, S. E. Ong, R. C. Liang, T. K. Seow, and M. C. Chung. 2001. An integrated approach in the discovery and characterization of a novel nuclear protein over-expressed in liver and pancreatic tumors. FEBS Lett. 496:109-116. [DOI] [PubMed] [Google Scholar]
- 5.Collett, M. S., R. Larson, C. Gold, D. Strick, D. K. Anderson, and A. F. Purchio. 1988. Molecular cloning and nucleotide sequence of the pestivirus bovine viral diarrhea virus. Virology 165:191-199. [DOI] [PubMed] [Google Scholar]
- 6.Corapi, W. V., R. O. Donis, and E. J. Dubovi. 1990. Characterization of a panel of monoclonal antibodies and their use in the study of the antigenic diversity of bovine viral diarrhea virus. Am. J. Vet. Res. 51:1388-1394. [PubMed] [Google Scholar]
- 7.Corapi, W. V., R. O. Donis, and E. J. Dubovi. 1988. Monoclonal antibody analyses of cytopathic and noncytopathic viruses from fatal bovine viral diarrhea infections. J. Virol. 62:2823-2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng, R., and K. V. Brock. 1992. Molecular cloning and nucleotide sequence of a pestivirus genome, noncytopathogenic bovine viral diarrhea virus strain SD-1. Virology 191:867-879. [DOI] [PubMed] [Google Scholar]
- 9.Donis, R. O., and E. J. Dubovi. 1987. Differences in virus-induced polypeptides in cells infected by cytopathic and noncytopathic biotypes of bovine diarrhoea-mucosal disease virus. Virology 158:168-173. [DOI] [PubMed] [Google Scholar]
- 10.Elbers, K., N. Tautz, P. Becher, D. Stoll, T. Rümenapf, and H.-J. Thiel. 1996. Processing in the pestivirus E2-NS2 region: identification of proteins p7 and E2p7. J. Virol. 70:4131-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grassmann, C. W., O. Isken, and S.-E. Behrens. 1999. Assignment of the multifunctional NS3 protein of bovine viral diarrhea virus during RNA replication: an in vivo and in vitro study. J. Virol. 73:9196-9205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harada, T., N. Tautz, and H.-J. Thiel. 2000. E2-p7 region of the bovine viral diarrhea virus polyprotein: processing and functional studies. J. Virol. 74:9498-9506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kümmerer, B. M., D. Stoll, and G. Meyers. 1998. Bovine viral diarrhea virus strain Oregon: a novel mechanism for processing of NS2-3 based on point mutations. J. Virol. 72:4127-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindenbach, B. D., and C. M. Rice. 2001. Flaviviridae: the viruses and their replication, p. 991-1042. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 1. Lippincott-Raven Publishers, Philadelphia, Pa.
- 15.Mendez, E., N. Ruggli, M. S. Collett, and C. M. Rice. 1998. Infectious bovine viral diarrhea virus (strain NADL) RNA from stable cDNA clones: a cellular insert determines NS3 production and viral cytopathogenicity. J. Virol. 72:4737-4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyers, G., T. Rümenapf, and H.-J. Thiel. 1990. Insertion of ubiquitin-coding sequence identified in the RNA genome of a togavirus, p. 25-29. In M. A. Brinton and F. X. Heinz (ed.), New aspects of positive-strand RNA viruses. American Society for Microbiology, Washington, D.C.
- 17.Meyers, G., D. Stoll, and M. Gunn. 1998. Insertion of a sequence encoding light chain 3 of microtubule-associated proteins 1A and 1B in a pestivirus genome: connection with virus cytopathogenicity and induction of lethal disease in cattle. J. Virol. 72:4139-4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyers, G., and H.-J. Thiel. 1996. Molecular characterization of pestiviruses. Adv. Virus Res. 47:53-118. [DOI] [PubMed] [Google Scholar]
- 19.Nagai, M., Y. Sakoda, M. Mori, M. Hayashi, H. Kida, and H. Akashi. 2003. Insertion of cellular sequence and RNA recombination in the structural protein coding region of cytopathogenic bovine viral diarrhoea virus. J. Gen. Virol. 84:447-452. [DOI] [PubMed] [Google Scholar]
- 20.Neill, J. D., and J. F. Ridpath. 2001. Recombination with a cellular mRNA encoding a novel DnaJ protein results in biotype conversion in genotype 2 bovine viral diarrhea viruses. Virus Res. 79:59-69. [DOI] [PubMed] [Google Scholar]
- 21.Pocock, D. H., C. J. Howard, M. C. Clarke, and J. Brownlie. 1987. Variation in the intracellular polypeptide profiles from different isolates of bovine viral diarrhea virus. Arch. Virol. 94:43-53. [DOI] [PubMed] [Google Scholar]
- 22.Qi, F., J. F. Ridpath, and E. S. Berry. 1998. Insertion of a bovine SMT3B gene in NS4B and duplication of NS3 in a bovine viral diarrhea virus genome correlate with the cytopathogenicity of the virus. Virus Res. 57:1-9. [DOI] [PubMed] [Google Scholar]
- 23.Ridpath, J. F., and J. D. Neill. 2000. Detection and characterization of genetic recombination in cytopathic type 2 bovine viral diarrhea viruses. J. Virol. 74:8771-8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rinck, G., C. Birghan, T. Harada, G. Meyers, H.-J. Thiel, and N. Tautz. 2001. A cellular J-domain protein modulates polyprotein processing and cytopathogenicity of a pestivirus. J. Virol. 75:9470-9482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rümenapf, T., R. Stark, M. Heimann, and H.-J. Thiel. 1998. N-terminal protease of pestiviruses: identification of putative catalytic residues by site-directed mutagenesis. J. Virol. 72:2544-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rümenapf, T., R. Stark, G. Meyers, and H.-J. Thiel. 1991. Structural proteins of hog cholera virus expressed by vaccinia virus: further characterization and induction of protective immunity. J. Virol. 65:589-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rümenapf, T., G. Unger, J. H. Strauss, and H.-J. Thiel. 1993. Processing of the envelope glycoproteins of pestiviruses. J. Virol. 67:3288-3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schägger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 29.Stark, R., G. Meyers, T. Rümenapf, and H.-J. Thiel. 1993. Processing of pestivirus polyprotein: cleavage site between autoprotease and nucleocapsid protein of classical swine fever virus. J. Virol. 67:7088-7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutter, G., M. Ohlmann, and V. Erfle. 1995. Non-replicating vaccinia vector efficiently expresses bacteriophage T7 RNA polymerase. FEBS Lett. 371:9-12. [DOI] [PubMed] [Google Scholar]
- 31.Tautz, N., G. Meyers, R. Stark, E. J. Dubovi, and H.-J. Thiel. 1996. Cytopathogenicity of a pestivirus correlates with a 27-nucleotide insertion. J. Virol. 70:7851-7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warrener, P., and M. S. Collett. 1995. Pestivirus NS3 (p80) protein possesses RNA helicase activity. J. Virol. 69:1720-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiskerchen, M., S. K. Belzer, and M. S. Collett. 1991. Pestivirus gene expression: the first protein product of the bovine viral diarrhea virus large open reading frame, p20, possesses proteolytic activity. J. Virol. 65:4508-4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiskerchen, M., and M. S. Collett. 1991. Pestivirus gene expression: protein p80 of bovine viral diarrhea virus is a proteinase involved in polyprotein processing. Virology 184:341-350. [DOI] [PubMed] [Google Scholar]