Abstract
A rapid and sensitive neutralization assay is required to evaluate alternative smallpox vaccines. Here we describe the development and use of a 96-well plate, semi-automated, flow cytometric assay that uses a recombinant vaccinia virus expressing enhanced green fluorescent protein and which would be applicable to other viruses.
Routine smallpox vaccination with live vaccinia virus ended after the eradication of the disease more than 30 years ago, and most people are now unprotected or incompletely protected (7). Recent world events, however, have mandated an evaluation of plans for mass immunization as well as the development of safer vaccines (11). Although development of a lesion at the site of scarification with vaccinia virus is sufficient to confirm the potency of the present vaccine, a battery of immunological assays are necessary to evaluate new ones. The plaque reduction assay, the classical method of determining neutralizing antibody titers, is cumbersome, labor-intensive, and impractical for large numbers of samples. A high-throughput assay is needed to support preclinical and clinical investigations.
Infectious virus particles are typically enumerated by a plaque or end-point assay on cell monolayers. In the case of vaccinia virus, plaques can be recognized after 48 h by staining with crystal violet or neutral red (5). The detection of infected cells can be simplified by using recombinant viruses that express reporter genes such as β-galactosidase, β-glucuronidase, and enhanced green fluorescent protein (GFP) (2-4). Nevertheless, the many steps required to carry out a plaque assay as well as the counting of individual virus plaques in cell monolayers remains tedious and relatively inaccurate. The enumeration of infected cells by flow cytometry offers considerable advantages in speed and accuracy. Here we describe a flow cytometric assay that utilizes expression of GFP as a readout.
Enumeration of vaccinia virus-infected cells by flow cytometry.
HeLa S3 cells (ATCC CCL2.2) were grown in suspension culture in MEM Spinner (Quality Biologicals, Inc., Gaithersburg, Md.) containing 5% equine serum (HyClone, Logan, Utah) and were diluted every 1 to 2 days to maintain a density of 2 × 105 to 5 × 105 cells per ml (5). The recombinant vaccinia virus VV.NP-S-EGFP was constructed for immunological studies (1, 10) and contains a chimeric gene encoding the influenza virus nucleoprotein, the ovalbumin SIINFEKL peptide, and enhanced GFP regulated by the P7.5 early-late promoter (9). As an indication that the recombinant vaccinia virus was derived from the Western Reserve strain, we refer to it as WR-GFP. WR-GFP was propagated in HeLa cells and purified by sedimentation through a sucrose cushion and sucrose gradient centrifugation (5, 6). The virus titer was determined by a standard plaque assay on BS-C-1 cell monolayers (described below), and the virus was stored in single-use aliquots at −80°C. In initial experiments, we verified that GFP expression could be detected by flow cytometry within several hours after infecting HeLa cells with vaccinia virus WR-GFP. Fluorescent cells were analyzed with a FACSCaliber flow cytometer coupled to a multiwell autosampler (Becton Dickinson, San Jose, Calif.). Data analysis was performed with FlowJo software (Tree Star, Inc., San Carlos, Calif.). Live cells were gated for forward and side scatter and subsequently for GFP expression. The number of GFP-expressing cells was determined by using a bivariate plot of fluorescence versus side scatter, with the gate set with uninfected cells.
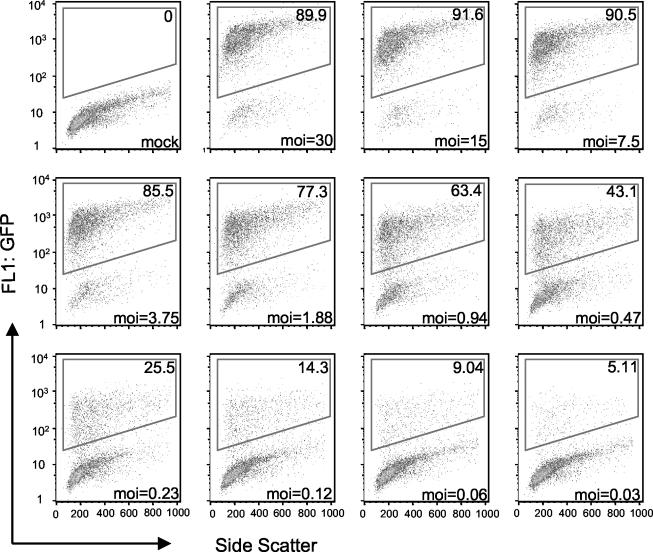
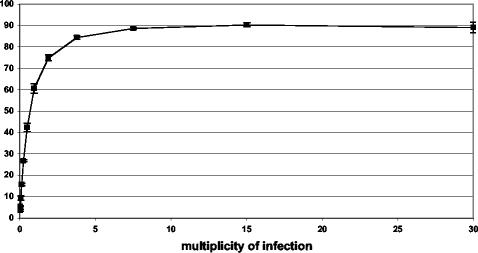
For the experiment depicted in Fig. 1, HeLa cell suspensions were infected with WR-GFP for 17 h in a 96-well plate in the presence of the DNA replication inhibitor cytosine arabinoside to prevent virus replication and spread. The percentage of fluorescent cells was determined as a function of virus input and is represented as a density plot. A dose-response curve from a similar experiment carried out in triplicate is shown in Fig. 2. At a multiplicity of infection of 0.5 PFU per cell, approximately half the cells expressed GFP. With increasing virus input, a plateau occurred, with 90% of cells being fluorescent. For subsequent virus neutralization experiments, we used a virus dilution that caused 20 to 25% of the cells to express GFP in the absence of antibody.
FIG. 1.
Detection of GFP-expressing cells by flow cytometry. HeLa cells were mock infected or infected with dilutions of purified WR-GFP in a 96-well plate. After 17 h at 37°C, the cells were analyzed by flow cytometry. Live cells were gated for forward and side scatter and subsequently for GFP expression. The number of GFP-expressing cells (upper right corner of each box) was determined by using a bivariate plot of fluorescence versus side scatter, with the gate set by using uninfected cells. Virus titers, used to indicate the multiplicity of infection (MOI) at the bottom of each panel, were determined by plaque assay.
FIG. 2.
Dose-response curve. HeLa cells were infected with WR-GFP in triplicate as described for Fig. 1. Bars indicate standard deviations.
Determination of virus neutralization titers by flow cytometry.
After preliminary studies, the following protocol was developed for routine use. (i) Eight twofold serial dilutions of heat-inactivated serum were prepared in a 96-well U-bottomed polypropylene plate (Corning, Acton, Mass.), with 50 μl of Spinner-2 (MEM Spinner from Quality Biologicals, Inc., supplemented with 2% fetal bovine serum) per well. WR-GFP was diluted to 2.5 × 106 PFU per ml in Spinner-2, and 10 μl of the suspension containing 2.5 × 104 PFU was added to each well. The plate was rotated at 900 rpm for 5 min on an orbital mixer and then placed in a 37°C CO2 incubator for 1 h. (ii) Approximately 30 min after the start of the virus-antibody incubation, HeLa cells were centrifuged at 2,500 rpm in an Eppendorf 5403 centrifuge and the pellet was resuspended to a final concentration of 2.5 × 106 cells per ml of Spinner-2 containing 44 μg of cytosine arabinoside per ml. The cells were placed in a 37°C CO2 incubator until the 1-h incubation of virus and antibody had elapsed. At that time, 40 μl of the cell suspension were added to each well to achieve a virus PFU-to-cell ratio of approximately 0.25 and the plate was returned to the incubator. For each 96-well plate, 4 to 8 wells contained uninfected cells and 4 to 8 wells contained infected cells without antibody for gating purposes and to determine the maximum percentage of GFP-expressing cells, respectively. After 16 to 18 h at 37°C, the plate was rotated at 1,000 rpm for 10 to 15 min, 0.1 ml of 2% paraformaldehyde was added, and the plate was rotated again for 10 to 15 min. (iii) After fixation, the 96-well plates were analyzed within 45 days with a multiwell autosampler attached to a FACSCaliber cytofluorometer. Typically, 5,000 events were collected per well. The serum dilution that reduced the percentage of GFP-expressing cells by 50% (IC50) was determined by nonlinear regression using the PRISM software package (GraphPad Software, Inc., San Diego, Calif.).
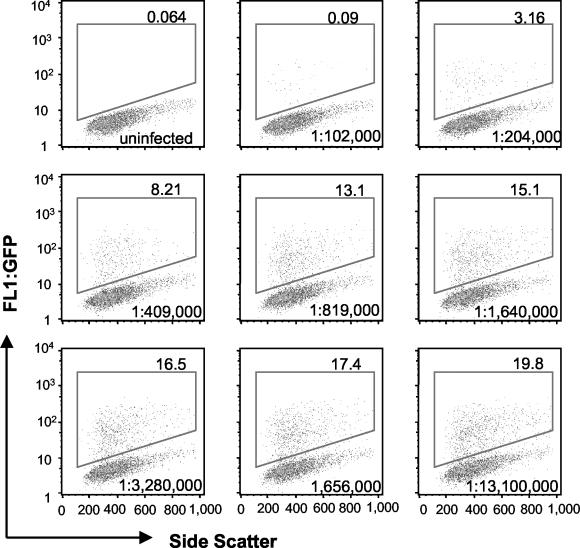
The neutralization assay described above was developed by using serum from a hyperimmunized rabbit. Hyperimmune serum 8191 was produced by intradermal immunization of a rabbit with 2 × 109 PFU of purified vaccinia virus strain WR at 2 sites and boosting subcutaneously twice with 2 × 109 PFU of purified virus at each of 10 sites. The first and second boosters were complete and incomplete Freund's adjuvant, respectively. Immunizations were done at monthly intervals. The neutralization of WR-GFP by the rabbit hyperimmune serum is shown as dot plots in Fig. 3.
FIG. 3.
Neutralization of vaccinia virus by hyperimmune rabbit serum. Purified WR-GFP was incubated with dilutions of hyperimmune rabbit serum in a 96-well plate. After 1 h at 37°C, HeLa cells were added and the incubation was continued for 17 h. The numbers of GFP-expressing cells (upper right corner of each box) were enumerated as for Fig. 1; the serum dilution is shown in the lower right corner of each box.
The GFP flow cytometric analysis was compared with a traditional plaque reduction assay, which was carried out as follows. A 96-well polypropylene U-bottomed plate was coated overnight at 37°C with phosphate-buffered saline containing 3% fetal bovine serum. The plate was then used to make eight twofold serial dilutions of heat-inactivated antiserum with phosphate-buffered saline containing 0.5% bovine serum albumin. Approximately 2.3 × 103 PFU of vaccinia virus strain WR was added to each well, and the 200-μl mixture was incubated at 37°C for 1 h. BS-C-1 cell monolayers in 6-well tissue culture plates were infected with 60-μl aliquots of the virus-antibody mixtures in 1 ml of Eagle minimum essential medium (E-MEM) containing 2% fetal bovine serum. After 2 h, the medium was replaced with E-MEM containing 2% fetal bovine serum and 0.5% methylcellulose. Two days later, the methylcellulose was removed, the cell monolayers were stained with crystal violet, and the plaques were counted.
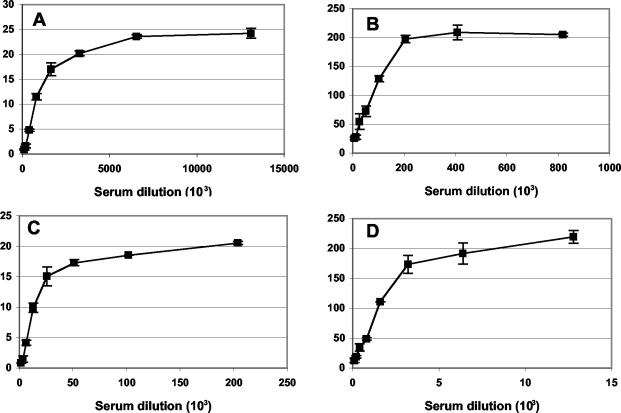
The IC50 values for the rabbit hyperimmune serum were determined to be 1,008,000 and 80,340 in the flow cytometric and plaque reduction assays, respectively (Fig. 4A and B). The flow cytometric assay was also determined to be 10- to 20-fold more sensitive than the plaque reduction assay when tested with samples of pooled vaccinia virus human immune globulin (VIG) (Fig. 4C and D) provided by Hana Golding (Center for Biologics Evaluation and Research, Food and Drug Administration).
FIG. 4.
Comparison of the flow cytometric and plaque reduction neutralization assays. Purified WR-GFP was incubated with dilutions of hyperimmune rabbit serum (A and B) or human VIG (C and D) in duplicate as for Fig. 3 for the flow cytometric assay (A and C) or as described in the text for the plaque reduction assay (B and D). Experiments were done in duplicate and bars indicate standard deviations. The percentage of neutralized virus was determined by comparing the numbers of GFP-expressing cells or plaques in the presence and absence of serum.
Long-term stability of GFP-expressing cells.
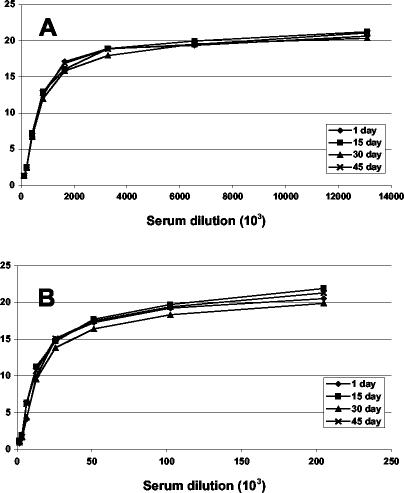
Because it is not always possible or practical to perform flow cytometry immediately after the infection step, the stability of fixed cells was tested over a period of 45 days. Replicate 96-well neutralization assays were performed with either rabbit hyperimmune serum or human VIG. Seventeen hours after infection, the cells were fixed with paraformaldehyde. One plate was analyzed immediately, while others were kept at 4°C and analyzed after 15, 30, or 45 days (Fig. 5). The neutralization curves could be superimposed, demonstrating the flexibility and reproducibility of the assay.
FIG. 5.
Stability of cells expressing GFP. Flow cytometric neutralization assays were carried using hyperimmune rabbit serum 8191 (A) or human VIG (B). After the incubation period, the cells were fixed with paraformaldehyde and stored at 4°C for up to 45 days before flow cytometric analysis. The number of days of storage of fixed cells is indicated in each inset.
Analysis of sera from unvaccinated and vaccinated individuals.
We tested serum samples from individuals who stated that they had not previously been vaccinated and from others who had been vaccinated more than 10 years ago. The neutralization titers, determined by the GFP assay, are shown in Table 1. All of the sera from purportedly naïve individuals, except one, exhibited less than 50% neutralization at the lowest dilution tested (1:50). The titers of sera from individuals immunized more than 10 years previously ranged from 474 to 1,278. Vaccinia virus enzyme-linked immunosorbent assay (ELISA) titers for these individuals ranged from 1:25,000 to 1:100,000. The “naïve” individual with the neutralization titer of 497 also had a positive ELISA titer (1:50,000) and thus was probably vaccinated at some time in the past.
TABLE 1.
Neutralization titers of human sera
| Type of patient | Sample code | IC50 |
|---|---|---|
| Naïvea | B-1 | <50 |
| B-2 | <50 | |
| Dc | 497 | |
| F | <50 | |
| L | <50 | |
| W | <50 | |
| Vaccinatedb | U | 714 |
| V | 474 | |
| W | 622 | |
| X | 1,278 |
No history of vaccination.
Vaccination more than 10 years previously.
Sample was positive by ELISA.
Conclusions.
A flow cytometric assay that is more rapid, reproducible, and sensitive than the traditional plaque reduction test was developed. The many advantages of the new assay include the ability to (i) carry out all procedures for multiple samples in a single 96-well plate, (ii) conveniently perform single-round infections overnight, (iii) store plates up to 45 days after fixation, (iv) transfer plates to the flow cytometer and enumerate GFP-expressing cells automatically, and (v) output data in a biologically meaningful form as reductions in the percentage of cells infected. High throughput may be achieved with a new automated sample changer and stacker for flow cytometry (model HTS from Becton Dickinson) which allows for analysis of a 96-well plate in only 12 min.
The higher neutralization titers obtained with the GFP assay compared to those from the plaque reduction test are advantageous, as they increase the sensitivity of the antibody determinations. We believe that the enhanced sensitivity is due to differences in the protocols. With both assays, the virus was incubated with antibody for 1 h. However, in the GFP assay the virus-antibody suspension was diluted only twofold when the cells were added and was not removed from the overnight incubation. In contrast, the antibody was diluted more than 15-fold when the virus-antibody suspension was added to the cell monolayer and then was removed after 2 h, when the liquid medium was replaced with a semisolid overlay in order to obtain discrete plaques. Neutralization of vaccinia virus can occur even after the virus has attached to cells (8).
One use of the new assay will be to compare attenuated vaccines with the currently licensed smallpox vaccine in clinical trials. However, because of its simplicity, the GFP flow cytometric assay has replaced the plaque reduction assay for all purposes in our laboratory. For example, we are using it to study the immune response in mice and monkeys to attenuated vaccinia viruses and to individual viral proteins as well as to demonstrate vaccinia virus neutralization by mouse monoclonal antibodies (unpublished data). The assay could be particularly useful for screening hybridoma supernatants for neutralizing monoclonal antibodies, which might eventually replace human VIG. Although developed specifically for vaccinia virus, a similar assay could be developed for any virus capable of expressing or inducing expression of GFP.
Acknowledgments
We thank Norman Cooper for maintenance of cells and production of virus, Jack Bennink and Jonathan Yewdell for WR-GFP and helpful discussions, Hana Golding for samples of VIG, and Cliff Lane for human sera.
REFERENCES
- 1.Anton, L. C., U. Schubert, I. Bacik, M. F. Princiotta, P. A. Wearsch, J. Gibbs, P. M. Day, C. Realini, M. C. Rechsteiner, J. R. Bennink, and J. W. Yewdell. 1999. Intracellular localization of proteasomal degradation of a viral antigen. J. Cell Biol. 146:113-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carroll, M. W., and B. Moss. 1995. E. coli β-glucuronidase (GUS) as a marker for recombinant vaccinia viruses. BioTechniques 19:352-355. [PubMed] [Google Scholar]
- 3.Chakrabarti, S., K. Brechling, and B. Moss. 1985. Vaccinia virus expression vector: coexpression of β-galactosidase provides visual screening of recombinant virus plaques. Mol. Cell. Biol. 5:3403-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dominguez, J., M. D. Lorenzo, and R. Blasco. 1998. Green fluorescent protein expressed by a recombinant vaccinia virus permits early detection of infected cells by flow cytometry. J. Immunol. Methods 220:115-121. [DOI] [PubMed] [Google Scholar]
- 5.Earl, P. L., N. Cooper, S. Wyatt, B. Moss, and M. W. Carroll. 1998. Preparation of cell cultures and vaccinia virus stocks, p. 16.16.1-16.16.3. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 2. John Wiley and Sons, New York, N.Y.
- 6.Earl, P. L., B. Moss, L. S. Wyatt, and M. W. Carroll. 1998. Generation of recombinant vaccinia viruses, p. 16.17.1-16.17.19. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 2. Greene Publishing Associates & Wiley Interscience, New York, N.Y.
- 7.Fenner, F., D. A. Henderson, I. Arita, Z. Jezek, and I. D. Ladnyi. 1988. Smallpox and its eradication, 1st ed. World Health Organization, Geneva, Switzerland.
- 8.Ichihashi, Y., and M. Oie. 1996. Neutralizing epitopes on penetration protein of vaccinia virus. Virology 220:491-494. [DOI] [PubMed] [Google Scholar]
- 9.Mackett, M., G. L. Smith, and B. Moss. 1984. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J. Virol. 49:857-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norbury, C. C., D. Malide, J. S. Gibbs, J. R. Bennink, and J. W. Yewdell. 2002. Visualizing priming of virus-specific CD8(+) T cells by infected dendritic cells in vivo. Nat. Immunol. 3:265-271. [DOI] [PubMed] [Google Scholar]
- 11.Whitley, R. J. 2003. Smallpox: a potential agent of bioterrorism. Antivir. Res. 57:7-12. [DOI] [PubMed] [Google Scholar]