Abstract
By using antisense RNA, Lck-deficient transfectants of a T helper 2 (Th2) clone have been derived and shown to have a qualitative defect in the T cell receptor signaling pathway. A striking feature observed only in Lck-deficient T cells was the presence of a constitutively tyrosine-phosphorylated 32-kDa protein. In the present study, we provide evidence that this aberrantly hyperphosphorylated protein is p34cdc2 (cdc2) a key regulator of cell-cycle progression. Lck-deficient transfectants expressed high levels of cdc2 protein and its regulatory units, cyclins A and B. The majority of cdc2, however, was tyrosine-phosphorylated and therefore enzymatically inactive. The transfectants were significantly larger than the parental cells and contained 4N DNA. These results establish that a deficiency in Lck leads to a cell-cycle arrest in G2. Moreover, transfected cells were hypersusceptible to apoptosis when activated through the T cell receptor. Importantly, however, this hypersusceptibility was largely reversed in the presence of T cell growth factors. These findings provide evidence that, in mature T lymphocytes, cell-cycle progression through the G2–M check point requires expression of the Src-family protein tyrosine kinase, Lck. This requirement is Lck-specific; it is observed under conditions in which the closely related Fyn kinase is expressed normally, evincing against a redundancy of function between these two kinases.
Src-family protein tyrosine kinases (PTKs) play a critical role in signaling via lymphocyte receptors (1). A member of the Src-family PTKs, Lck, is particularly important in T lymphocyte activation and development. Evidence in support of this conclusion was derived from two lines of work. First, genetic manipulation either to overexpress or inhibit the expression of Lck in tumor T cell lines and/or hybridomas has shown that Lck is critical for activation mediated by the T cell receptor (TCR; refs. 2 and 3). Second, mice in which Lck expression is genetically disrupted have a block early in thymic development that leads to >90% reduction in the number of mature T lymphocytes (4). Our laboratory has been interested in studying the function of PTKs in normal, mature T lymphocytes. Given the severe block in T cell development in Lck-deficient mice, a different approach was taken to assess the role of Lck in normal T cell functions. We have reported the derivation, by using antisense-RNA, of several transfectants of a T helper 2 (Th2) clone with decreased levels of Lck (5). Analysis of the transfectants showed a major defect in the TCR signaling pathway that resulted in >90% inhibition of the cells’ proliferative capacity. Further analysis of one of the transfectants, designated D8, showed that the defect manifested in both qualitative as well as quantitative changes in the activation of several signaling-pathway intermediates (5). For example, although some critical proteins, such as TCR ζ and ZAP-70, were not tyrosine-phosphorylated after TCR activation in D8 cells, other unknown substrates were hyperphosphorylated. Thus, there appeared to be a disregulation rather than an absence of TCR-mediated signaling in Lck-deficient cells.
In the present study, we document a novel requirement for Lck in T cell physiology, namely cell-cycle regulation. T cells deficient in Lck were found to have a block in cell cycle that prevents them from entering mitosis. This is caused by a failure to dephosphorylate p34cdc2 (cdc2), a critical regulator of G2–M transition (6). Entry into mitosis is triggered by the activation of a mitotic complex consisting of cdc2 and its regulatory subunit cyclin B. An integral part of the activation process involves the dephosphorylation of cdc2 at Thr-14 and Tyr-15 residues (7). In the phosphorylated form, cdc2 remains in an inactive configuration and thereby prevents the cells from entering mitosis. Our data show the requirement for Lck, a membrane-bound PTK, in regulating the activity of a cell-cycle-associated kinase. The mechanism of the cell-cycle block observed in Lck-deficient T cells is similar to that induced by the vpr gene of HIV 1 after infection of susceptible cells (8). In the latter case, HIV-infected cells also arrest in G2 as a consequence of the failure to dephosphorylate and thereby activate cdc2 (9–11). Whether the two pathways leading to G2 arrest are linked remains to be investigated.
MATERIALS AND METHODS
Cell Lines.
The derivation of Lck-deficient transfectants and maintenance of both the transfectants and the parental D10.G4.1 (D10) Th2 line have been described in detail (5). Briefly, the Lck-deficient D8 line was derived by transfecting a vector containing a 400-bp fragment of murine Lck cloned in the antisense orientation. Expression of the antisense RNA is under the control of the simian virus 40 promoter and the long-terminal repeat enhancer of the type I human T-lymphotrophic virus. The D8 transfectant cells were maintained by weekly stimulation with cognate antigen (conalbumin) plus syngeneic spleen cells in the presence of interleukin 2 (IL-2; 10 units/ml), IL-4 (50 units/ml), and IL-1α (200 pg/ml). Selection for antisense expression is maintained by continuous culture in the presence of G418 (Sigma).
Cell Activation and Immunoblot Analysis.
Cells were activated by antibody cross-linking as described (5) with some modification. Briefly, 5–10 × 106 cells were activated in 250 μl of RPMI medium 1640 containing 20 μg/ml anti-CD3ɛ mAb YCD3–1 (12) at 4°C for 10 min, washed, and cross-linked with 20 μg/ml goat anti-rat IgG (Sigma) at 37°C for the indicated times (Fig. 2A). Incubation was stopped by adding 1 ml of ice-cold medium; cells were spun down and lysed in 1% Nonidet P-40 lysis buffer as described (5). The protein concentration in the cell lysates was determined by the Bradford assay. Routinely, 10% of the lysates was used for immunoblotting and the remainder for immunoprecipitations. For blotting, equal amounts of protein (50–200 μg per lane) were separated on 10% SDS/PAGE and immunoblotted with antibodies specific to the C terminus of cdc2 (Santa Cruz Biotechnology). In some cases, blots were stripped and reprobed with the anti-phosphotyrosine antibody 4G10 (Upstate Biotechnology). Western blotting for cyclin proteins was done by separating 50–100 μg of cell extracts on 10% SDS/PAGE and blotting with either a mAb specific to cyclin B1 (Santa Cruz Biotechnology) or rabbit serum specific to cyclin A (provided by A. Hayday, Yale University, New Haven, CT). Immunoblots were developed by using Enhanced Chemiluminescence (Amersham) or SuperSignal Substrate (Pierce).
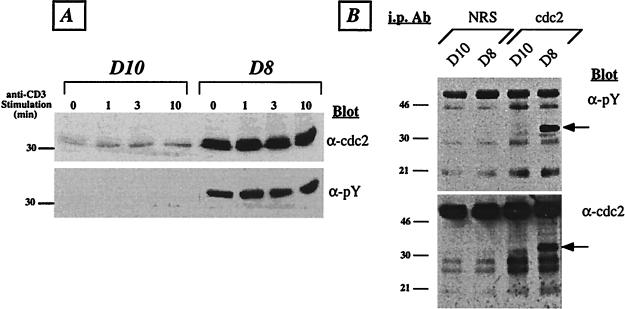
Figure 2.
Lck-deficient T cells express increased levels of predominantly tyrosine-phosphorylated cdc2 kinase. (A) T cells were activated by anti-CD3 cross-linking for the indicated times. Whole-cell lysates were prepared, and ≈50 μg of total protein was run per lane on 9% SDS/PAGE and immunoblotted with anti-cdc2 antibodies (Upper). Subsequently, the membrane was stripped and reprobed with 4G10 (anti-phosphotyrosine) mAb (Lower). The position of the 30-kDa molecular-mass standard is indicated. (B) Resting D10 and D8 cells were lysed in Nonidet P-40 containing lysis buffer, and ≈500 μg of total protein was immunoprecipitated with either normal rabbit serum (NRS) or cdc2-specific serum. The precipitates were resolved on 12.5% SDS/PAGE, transferred to poly(vinylidene difluoride) membrane, and immunoblotted wth 4G10 anti-phosphotyrosine(α-pY) mAb (Upper). Subsequently, the blot was stripped and reprobed with anti-cdc2 antiserum (α-cdc2; Lower). The molecular mass of standards is given in kDa. The cdc2 band is indicated by an arrow. The results are representative of five (A) and two (B) independent experiments.
Immunoprecipitation and Kinase Assay.
Immunoprecipitation of cdc2 was carried out as described (5). Equal amounts of protein (≈500 μg per sample) were immunoprecipitated with either anti-cdc2 rabbit serum or normal serum as a control. The immunoprecipitates were collected on protein A-Sepharose for 1–2 h at 4°C, run on 12.5% SDS/PAGE, and blotted with 4G10 mAb. Subsequently, the blots were stripped and reprobed with anti-cdc2 serum. The cdc2-associated kinase activity was determined as described (13) with some modifications. Immobilized cdc2 precipitates were washed in kinase buffer (25 mM Hepes, pH 7.3/10 mM MgCl2/10 mM MnCl2/100 μM Na3VO4) and used in the kinase reaction in a final volume of 25 μl in the presence of 10 μM ATP, 25 μg/ml histone H1, and 10 μCi of [γ-32P]ATP (3,000 Ci/mmol; 1 Ci = 37 GBq). After incubation at room temperature for 30 min, the reaction was stopped by the addition of an equal volume of 2× SDS-containing sample buffer. Labeled proteins were separated on 15% SDS/PAGE and detected by autoradiography.
In Vivo 35S-Labeling Analysis.
D10 and D8 T cells were activated with IL-2 for 18 h and were then labeled with [35S]methionine (250 μCi/ml) for 6 h in methionine-deficient RPMI medium 1640 (ICN) supplemented with 10% dialyzed fetal bovine serum (GIBCO) and IL-2. The metabolically labeled cells were lysed in a buffer containing 0.5% Nonidet P-40, 150 mM NaCl, 50 mM Tris (pH 7), 100 mM NaF, 1 mM benzamidine, and 10 μg/ml each of the following protease inhibitors: trypsin inhibitor, aprotinin, and leupeptin (Sigma). The cyclin/cyclin-dependent protein kinase (CDK) complexes were isolated with respective antibodies by immunoprecipitation at 4°C for 4 h. The proteins in the immunocomplexes were resolved on SDS/PAGE and visualized by autoradiography. The assignment of each cell-cycle protein in these complexes has been described (14, 15).
Cell-Cycle Analysis.
Cells (1 × 106 per group) were isolated from maintenance cultures a week after the last round of activation and fixed overnight in 0.5 ml of 1% paraformaldehyde/PBS solution at 4°C. After washing, cells were incubated in PBS containing 0.1 M sodium citrate, 0.1% Triton X-100, 200 units/ml RNase A, and 50 μg/ml propidium iodide (PI) for 4–12 h at 4°C (16). The analysis was carried out on 20,000 cells with a FACScan flow cytometer (Becton Dickinson) with FL3 set on linear scale and reflects cell fluorescence after gating out debris and dead cells.
Analysis of Apoptosis by Flow Cytometry.
Cell death was analyzed by annexin V/PI staining with a kit from R & D Systems. Resting T cells were cultured at 1 × 106 cells per ml in 24-well plates with immobilized anti-TCR Cβ mAb H57.597 (17) or with IL-2 (5 units/ml) and IL-4 (50 units/ml) lymphokines. Cells were harvested after 8 or 22 h of culture, washed, and stained with annexin V/fluorescein isothiocyanate and/or PI according to manufacturer’s instructions. The cells were analyzed on a FACScan cytometer, and data from 15,000 cells were collected and analyzed with cellquest software (Becton Dickinson).
RESULTS
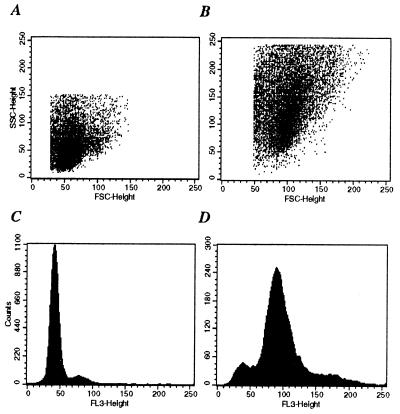
The Lck-deficient D8 T cells were maintained by weekly activation with conalbumin plus syngeneic splenocytes in the presence of IL-1, IL-2, and IL-4 lymphokines. For experimentation, routinely, both the parent D10 clone and D8 transfectant cells were used at least 1–2 weeks after the last round of stimulation. One of the earliest observations concerning D8 cells was their increased cell size. Based on the forward light scatter/side light scatter profile of cells analyzed with flow cytometry, D8 cells were 2–3 times larger than D10 cells (Fig. 1; compare A with B). Analysis of DNA content in resting cells from asynchronous cultures showed that the majority of D10 T cells had a 2N DNA content, whereas over 70% of D8 T cells contained 4N DNA (Fig. 1; compare C with D). This finding suggested that D8 cells may have a block in the cell cycle, most likely at the G2–M phase.
Figure 1.
Lck-deficient T cells have increased size and contain 4N DNA. (A and B) Dot blot diagrams depicting the forward light scatter (FSC) and side light scatter (SSC) profiles of the parental D10 cells (A) and of the Lck-deficient transfectant line D8 (B). (C and D) Representative PI-staining profile of resting D10 (C) and D8 (D) cells. Cells were purified from maintenance cultures 1 week after the last round of stimulation and 1 × 106 cells per group were processed for PI staining as described in Materials and Methods. The results are representative of at least five independent experiments.
Transition from the G2 phase of the cell cycle into mitosis is controlled by the activation of a mitotic complex consisting of cdc2 and its regulatory subunit, cyclin B. There are two events thought to be critical for the activation of this complex: (i) the association of cdc2 with cyclin B and (ii) dephosphorylation of cdc2 at two critical residues, Thr-14 and Tyr-15 (7). One clue to the discovery of the underlying mechanism of the cell-cycle block in D8 cells came from an earlier finding that an ≈32-kDa protein was aberrantly tyrosine-phosphorylated in D8 T cells (5). To ascertain whether the unknown protein band was in fact cdc2, whole-cell extracts were prepared from D10 and D8 T cells (before and after stimulation through the TCR/CD3 complex), separated on SDS/PAGE, and immunoblotted with anti-cdc2 serum. A protein corresponding to cdc2 was highly and constitutively expressed in D8 cell extracts (Fig. 2A). Moreover, there was no alteration in the level of cdc2 protein after activation via the TCR. Reprobing the blot with anti-phosphotyrosine mAb showed that the observed band was indeed highly phosphorylated in D8 cells. In sharp contrast, however, D10 cells expressed a much lower level of cdc2 kinase, which was not tyrosine-phosphorylated (Fig. 2A). To confirm these findings, cdc2 was immunoprecipitated from D10 and D8 cell extracts and blotted with anti-phosphotyrosine mAb. The results clearly showed that the majority of the precipitated cdc2 from D8 cells is tyrosine-phosphorylated (Fig. 2B). Control precipitation with normal serum showed no specific bands. Thus, the level as well as the phosphorylation status of cdc2 correlated with the observed cell-cycle block in D8 cells.
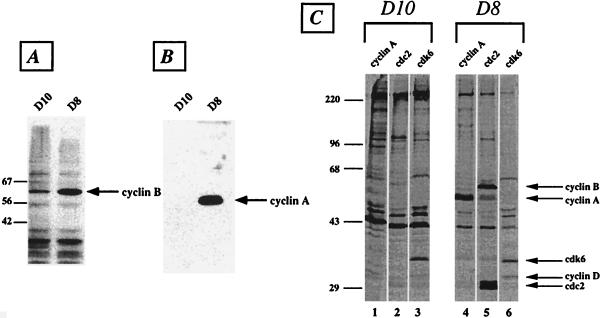
Activation of cdc2 at the G2–M interphase requires association with cyclin B. The level of cyclin B expression is regulated depending on the phase of the cell cycle. Cyclin B levels are very low in G1, begin to increase midway through the S phase, and reach a peak at the G2–M transition. Moreover, cyclin A is another regulatory protein whose level is regulated in a cell-cycle-dependent manner with its maximum expression occurring late in the G2 phase. To examine the D8 phenotype further, the expression of cyclins A and B was analyzed in whole-cell extracts. The results showed that D8 cells express much higher levels of both cyclins in comparison with D10 cells (Fig. 3 A and B).
Figure 3.
Expression of cyclins and cyclin-dependent kinases in D10 and D8 T cells. (A) Whole-cell lysates (50 μg per lane) were resolved on 10% SDS/PAGE, and the blots were probed with cyclin B-specific mAb. (B) Whole-cell extracts were run on 10% SDS/PAGE and blotted with polyclonal rabbit antiserum specific to cyclin A. (C) Lysates of [35S]Met metabolically labeled cells were immunoprecipitated with antiserum specific to cyclin A (lanes 1 and 4), cdc2 (lanes 2 and 5), or cdk6 (lanes 3 and 6). The arrows indicate the positions of the various cell-cycle proteins. The molecular mass of standards is given in kDa. The results are representative of two independent experiments.
To investigate the extent of the defect in D8 cells, extracts from metabolically 35S-labeled cells were prepared and immunoprecipitated with antibodies to various cell-cycle proteins. Immunoprecipitation with an antibody to cdc2 kinase yielded high levels of cdc2 from D8 T cells, confirming the findings of the immunoblotting (Fig. 3C, lanes 2 and 5). Importantly, the anti-cdc2 antibody coprecipitated large amounts of cyclin B, confirming that cdc2 and cyclin B exist as a complex in D8 T cells (Fig. 3C, lane 5). Similarly, immunoprecipitation with anti-cyclin A antibody showed a much higher level of expression of D8 than D10 T cells (Fig. 3C, lanes 1 and 4). A small but significant fraction of cyclin A in D8 cells was also associated with cdc2 (Fig. 3C, lane 5). In contrast, the expression of other cdk/cyclins such as cdk6/cyclin D and cdk2/cyclin E, which are important for progression through the G1 phase, are not altered in D8 cells (Fig. 3C, lanes 3 and 6; data not shown). Taken together, the data suggest that Lck-deficient D8 T cells are blocked in the G2 phase of the cell cycle.
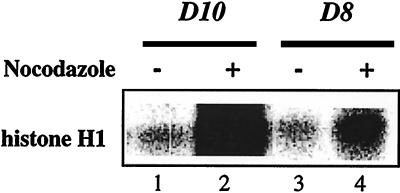
The relationship between the significantly elevated levels of cdc2/cyclin B and the cell-cycle block in D8 T cells was examined further by directly assessing the kinase activity of the complex. The cdc2-associated histone H1 kinase activity was determined in D10 and D8 cells after overnight culture in the presence of IL-2 plus IL-4 only or together with nocodazole, which arrests cells at the G2 phase of the cell cycle. D10 cells grown asynchronously in the presence of lymphokines exhibited a low level of cdc2-associated H1 kinase activity (Fig. 4, lane 1). The kinase activity was increased >10-fold in nocodazole-arrested D10 cells (Fig. 4, lane 2), reflecting the accumulation of cells in G2 phase. In contrast, despite the increased expression of cdc2 protein in D8 cells, the associated kinase activity was very low (Fig. 4, lane 3). This result is consistent with our finding that the majority of cdc2 protein in D8 cells is tyrosine-phosphorylated and therefore inactive (Fig. 2). Moreover, the cdc2-associated kinase activity was increased only by about 2-fold in nocodazole-treated D8 cells (Fig. 4, lane 4), further confirming that the majority of D8 cells are already blocked in the G2 phase. Nevertheless, the finding that nocodazole treatment of D8 cells leads to an increase in kinase activity suggests that a small fraction of cells can pass through G2 arrest, an effect probably mediated by the IL-2 and IL-4 lymphokines present in the culture medium (see below).
Figure 4.
Defective cdc2-associated histone H1 kinase activity in D8 T cells. Resting T cells were grown for 16 h in medium containing IL-2 (20 units/ml) and IL-4 (100 units/ml) lymphokines in the presence (lanes 2 and 4) or absence (lanes 1 and 3) of nocodazole (100 ng/ml). Cell lysates were prepared in Nonidet P-40 lysis buffer, and equal protein amounts (≈500 μg per sample) were immunoprecipitated with antiserum specific to cdc2. Immune complexes were used in a kinase reaction in the presence of histone H1 as the substrate. The results are representative of three independent experiments.
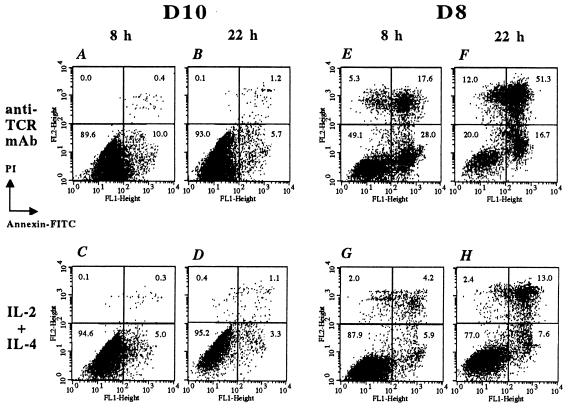
Finally, we analyzed the consequences of the block in cell cycle on the viability of D8 T cells. In preliminary experiments, we determined that D8 cells were more susceptible to apoptosis than D10 cells when cultured in the absence of any stimuli for >6 h (data not shown). We next compared the frequency of apoptotic cells observed after activation of T cells with immobilized anti-TCR mAb. The frequency of apoptotic D10 cells was 10% and ≈7% after activation with anti-TCR mAb for 8 h or 22 h, respectively (Fig. 5 A and B). In contrast, anti-TCR stimulation of D8 cells resulted in a significant degree of apoptosis. After being cultured for 8 h, 28% of cells were in the early phase (annexin V-positive, PI-negative) of apoptosis, and ≈18% were in a late (positive for both annexin V and PI) apoptotic/necrotic state (Fig. 5E). After being cultured for 22 h, ≈17% of cells were in early apoptotic stage, and >51% were already in the late phase of apoptosis/necrosis (Fig. 5F). Thus, D8 cells are hypersusceptible to apoptosis after TCR-triggering. This heightened susceptibility to apoptosis in D8 transfectants was reversed dramatically with culturing in medium containing the T cell growth factors IL-2 and IL-4. In the presence of these lymphokines, the overall frequency of apoptotic cells was reduced to ≈10% and ≈21% after 8 or 22 h of culture, respectively (Fig. 5 G and H). As expected, D10 cells showed a very low level of apoptosis: ≈5% and 4% at 8 or 22 h of culture, respectively (Fig. 5 C and D). These results show that D8 cells have an increased susceptibility to apoptosis after activation of the TCR signaling pathway. Furthermore, the addition of IL-2 and IL-4 lymphokines to D8 cells leads to an increase in cell survival. Whether this is mediated through an inhibition of apoptosis or by an independent effect on other survival-promoting pathways is currently unknown.
Figure 5.
D8 T cells are hypersusceptible to apoptosis. D10 and D8 T cells were stimulated for either 8 h (A, C, E, G) or 22 h (B, D, F, H) with either plate-bound anti-TCR mAb or IL-2 and IL-4 lymphokines, as described in Materials and Methods. The cells were processed for staining with annexin/fluorescein isothiocyanate and PI according to manufacturer’s instructions and then analyzed by flow cytometry. The data are presented in dot blots depicting annexin/fluorescein isothiocyanate (x axis) vs. PI staining (y axis). The percentage of cells in each quadrant is shown. The results are representative of three independent experiments.
DISCUSSION
The present study establishes that Lck, a Src-family PTK, is required for proper cell-cycle progression in T lymphocytes. T cell transfectants in which Lck expression has been reduced by >95% are arrested in the G2 phase of the cell cycle and do not enter M phase. These cells have a significant increase in cell size, a DNA content of 4N, highly elevated protein levels of cyclins A and B, and an increased expression of a predominantly tyrosine-phosphorylated and therefore inactive cdc2 kinase. Our data are consistent with a report that found that Src PTKs are required for the G2–M transition in fibroblasts (18). However, this study found a degree of functional redundancy among the three fibroblast-expressed Src PTKs (Src, Fyn, and Yes) in that any one member was sufficient for cells to exit G2. In contrast, T lymphocytes specifically require Lck to exit G2, because the closely related Src kinase, Fyn, is expressed at normal levels in D8 cells (5). The differences between the two studies are most likely caused by differences in the nature (transformed vs. nontransformed) and type (fibroblast vs. T lymphocytes) of cells used. Our finding further supports the argument against redundant roles for Lck and Fyn PTKs in T cell function and is in agreement with recent studies that show selective roles for these Src PTKs in T lymphocyte activation and development (19–22).
The G2 arrest in Lck deficient cells appears to be caused by an accumulation of tyrosine-phosphorylated cdc2 molecules that, in turn, is associated with a dramatic inhibition of cdc2 kinase activity. The activity of cdc2 is normally regulated by the dynamic balance between protein kinases and phosphatases, the best known examples of which are Wee1 and cdc25C, respectively (7). Increased levels of hyperphosphorylated cdc2 could therefore result from either increased activity of Wee1 or inhibition of cdc25C. We favor the latter possibility, because it has been shown that inhibition of cdc25C activation is associated with inactive cdc2 and a G2 block, consequences of other cellular insults including DNA damage (11, 23).
Despite the clear evidence implicating inactive cdc2 in the cell-cycle block in Lck-deficient T cells, the molecular mechanism, the link between Lck and cdc2, is unknown. Recently, elegant studies have shown that the inactivation of cdc25 phosphatase is mediated through a reversible association with proteins of the 14–3–3 family. Binding to 14–3–3 proteins is facilitated by the specific phosphorylation of cdc25 on Ser-216 residue (24–26). Under certain types of cellular stress, such as DNA damage, the check-point kinase Chk1 is activated, leading to the phosphorylation of cdc25 on Ser-216 residue. This phosphorylation allows 14–3–3 to associate with cdc25, thereby inactivating its function and precipitating an arrest at G2. This model is an attractive one, because it establishes a relationship between a DNA damage-sensitive kinase, Chk1, and important regulators of cell cycle, cdc2 and cdc25. At present, little is known about how Chk1 itself is regulated and whether it is activated in Lck-deficient T cells. However, we have some preliminary evidence suggesting that 14–3–3 is differentially regulated in D8 transfectants compared with the parental D10 T cell clone. (B.K.a.-R., unpublished work). Therefore, it is possible that a similar pathway might underlie the G2 block observed in our system.
The effect of Lck deficiency on cell cycle may help to explain some previously published findings. Several studies have documented that increased Lck expression is associated with neoplasia, both in the whole animal and in cell lines in vitro (27, 28). Conversely, Lck-deficient mice have a block very early in thymic development (4, 29). Moreover, expression of an activated form of Lck in RAG-1 mutant mice, in which thymic development is arrested at the double-negative stage, allows the cells to differentiate to the double-positive stage and triggers a tremendous expansion of double-positive thymocytes (30). This expansion occurs in the absence of TCR expression and does not lead to fully mature, single-positive thymocytes. Although the molecular basis of the Lck effect remains largely unknown, it is clear that it occurs independently of its association with the CD4 and CD8 coreceptors. Our data establishing a role for Lck in the regulation of cell-cycle progression are not only consistent with these studies but may also provide a molecular mechanism for these observations. The characteristics of the cell-cycle block in Lck-deficient T cells are very similar to those induced by the vpr gene of HIV-1 where a G2 block is induced also as a result of deficient cdc25C activation and accumulation of hyperphosphorylated cdc2 (9–11). Expression of the Vpr protein appears to have a predominantly cytostatic effect, because cells can be blocked in G2 for days (31). Nevertheless, Vpr-arrested T cells ultimately undergo apoptosis (8). Thus, Lck-deficient T cells and Vpr-arrested cells seem to share the properties that precipitate the observed G2 block and apoptosis hypersusceptibility. These findings suggest that the effector pathways induced in both cell types may have a common intermediary. Further study is required to investigate this possibility.
In Lck-deficient cells, rescue from apoptosis is achieved in the presence of IL-2 and IL-4, lymphokines that strongly promote T cell growth. These lymphokines rescue cells from apoptosis by a pathway that is not completely understood (32). It is possible that lymphokine-receptor and TCR signaling pathways converge at some point, leading to the activation of an intermediate required for cellular proliferation. In Lck-deficient cells, TCR-mediated signaling is defective, resulting in deficient proliferation, and lymphokine-receptor-mediated activation compensates for this deficiency. A very likely candidate for the signaling intermediate is the serine/threonine kinase Raf-1. Signaling from either IL-2 receptor or TCR can activate the Ras/Raf-1 pathway (33). Recently, Raf-1 was shown to associate with and activate the cdc25 phosphatase (34). Moreover, Raf-1 may also have a role in the apoptotic pathway via its association with Bad, a proapoptotic protein (35). Normally, Bad is bound to Bcl-2 or Bcl-XL, thus preventing them from carrying out their antiapoptotic function (36). Raf-1 may be involved directly or indirectly in the serine phosphorylation of Bad, an event that sequesters Bad away from Bcl-2, allowing the latter to prevent apoptosis (37, 38). Thus, it is reasonable to hypothesize that a critical signaling intermediate, such as Raf-1, may influence both cell-cycle and apoptosis pathways. The two main findings of Lck-deficient T lymphocytes described herein, cell-cycle block at the G2 phase and hypersusceptibility to apoptosis, may therefore be a consequence of a lack of activation of this intermediate.
Acknowledgments
We thank Drs. Marc Solomon, Adrian Hayday, Eric Hoffman, and Sang Kyou Lee for serologic reagents, Drs. Ravi Hingorani and Nick Crispe for advice and help with the apoptosis assay, and Drs. Maria J. Fernandez-Cabezudo and Miodrag Lukic for reviewing the manuscript. This work was supported by National Institutes of Health Grant GM47367 (to A.L.M.B.). B.K.a.-R. was supported in part by a Swebilius Cancer Research Award from Yale Comprehensive Cancer Center.
ABBREVIATIONS
- IL
interleukin
- PI
propidium iodide
- PTK
protein tyrosine kinase
- TCR
T cell receptor
References
- 1. Bolen J B, Brugge J S. Annu Rev Immunol. 1997;15:371–404. doi: 10.1146/annurev.immunol.15.1.371. [DOI] [PubMed] [Google Scholar]
- 2.Straus D B, Weiss A. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 3.Chu K, Littman D R. J Biol Chem. 1994;269:24095–24101. [PubMed] [Google Scholar]
- 4.Molina T J, Kishihara K, Siderovski D P, van Ewijk W, Narendran A, Timms E, Wakeham A, Paige C J, Hartmann K-U, Veillette A, et al. Nature (London) 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 5.al-Ramadi B K, Nakamura T, Leitenberg D, Bothwell A L M. J Immunol. 1996;157:4751–4761. [PubMed] [Google Scholar]
- 6.King R W, Jackson P K, Kirschner M W. Cell. 1994;79:563–571. doi: 10.1016/0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 7.Lew D J, Kornbluth S. Curr Opin Cell Biol. 1996;8:795–804. doi: 10.1016/s0955-0674(96)80080-9. [DOI] [PubMed] [Google Scholar]
- 8.Emerman M. Curr Biol. 1996;6:1096–1103. doi: 10.1016/s0960-9822(02)00676-0. [DOI] [PubMed] [Google Scholar]
- 9.Jowett J B M, Planelles V, Poon B, Shah N P, Chen M-L, Chen I S Y. J Virol. 1995;69:6304–6313. doi: 10.1128/jvi.69.10.6304-6313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He J, Choe S, Walker R, DiMarzio P, Morgan D O, Landau N R. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Re F, Braaten D, Franke E K, Luban J. J Virol. 1995;69:6859–6864. doi: 10.1128/jvi.69.11.6859-6864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Portoles P, Rojo J, Golby A, Bonneville M, Gromkowski S, Greenbaum L, Janeway C A, Murphy D B, Bottomly K. J Immunol. 1989;142:4169–4175. [PubMed] [Google Scholar]
- 13.Xiong Y, Hannon G J, Zhang H, Casso D, Kobayashi R, Beach D. Nature (London) 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Xiong Y, Beach D. Mol Biol Cell. 1993;4:897–906. doi: 10.1091/mbc.4.9.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Hannon G J, Beach D. Genes Dev. 1994;8:1750–1758. doi: 10.1101/gad.8.15.1750. [DOI] [PubMed] [Google Scholar]
- 16.Nicoletti I, Migliorati G, Pagliacci M C, Grignani F, Riccardi C. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 17.Kubo R T, Born W, Kappler J W, Marrack P, Pigeon M. J Immunol. 1989;142:2736–2742. [PubMed] [Google Scholar]
- 18.Roche S, Fumagalli S, Courtneidge S A. Science. 1995;269:1567–1569. doi: 10.1126/science.7545311. [DOI] [PubMed] [Google Scholar]
- 19.Qian D, Lev S, van Oers N S C, Dikic I, Schlessinger J, Weiss A. J Exp Med. 1997;185:1253–1259. doi: 10.1084/jem.185.7.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Da Silva A J, Li Z, de Vera C, Canto E, Findell P, Rudd C E. Proc Natl Acad Sci USA. 1997;94:7493–7498. doi: 10.1073/pnas.94.14.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grovers T, Smiley P, Cooke M P, Forbush K, Perlmutter R M, Guidos C J. Immunity. 1996;5:417–428. doi: 10.1016/s1074-7613(00)80498-7. [DOI] [PubMed] [Google Scholar]
- 22.van Oers N S C, Lowin-Kropf B, Finlay D, Connolly K, Weiss A. Immunity. 1996;5:429–436. doi: 10.1016/s1074-7613(00)80499-9. [DOI] [PubMed] [Google Scholar]
- 23.O’Connor P M, Ferris D K, Hoffmann I, Jackman J, Draetta G, Kohn K W. Proc Natl Acad Sci USA. 1994;91:9480–9484. doi: 10.1073/pnas.91.20.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furnari B, Rhind N, Russell P. Science. 1997;277:1495–1497. doi: 10.1126/science.277.5331.1495. [DOI] [PubMed] [Google Scholar]
- 25.Sanches Y, Wong C, Thoma R S, Richman R, Wu Z, Piwnica-Worms H, Elledge S J. Science. 1997;277:1497–1501. doi: 10.1126/science.277.5331.1497. [DOI] [PubMed] [Google Scholar]
- 26.Peng C-Y, Graves P R, Thoma R S, Wu Z, Shaw A S, Piwnica-Worms H. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- 27.Marth J D, Cooper J A, King C S, Ziegler S F, Tinker D A, Overell R W, Krebs E G, Perlmutter R. Mol Cell Biol. 1988;8:540–550. doi: 10.1128/mcb.8.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abraham K M, Levin S D, Marth J D, Forbush K A, Perlmutter R M. Proc Natl Acad Sci USA. 1991;88:3977–3981. doi: 10.1073/pnas.88.9.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levin S D, Anderson S J, Forbush K A, Perlmutter R M. EMBO J. 1993;12:1671–1680. doi: 10.1002/j.1460-2075.1993.tb05812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mombaerts P, Anderson S J, Perlmutter R M, Mak T W, Tonegawa S. Immunity. 1994;1:261–267. doi: 10.1016/1074-7613(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 31.Bartz S R, Rogel M E, Emerman M. J Virol. 1996;70:2324–2331. doi: 10.1128/jvi.70.4.2324-2331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boise L H, Thompson C B. Science. 1996;274:67–68. doi: 10.1126/science.274.5284.67. [DOI] [PubMed] [Google Scholar]
- 33.Izquierdo M, Reif K, Cantrell D. Immunol Today. 1995;16:159–164. doi: 10.1016/0167-5699(95)80134-0. [DOI] [PubMed] [Google Scholar]
- 34.Galaktionov K, Jessus C, Beach D. Genes Dev. 1995;9:1026–1058. doi: 10.1101/gad.9.9.1046. [DOI] [PubMed] [Google Scholar]
- 35.Gajewski T F, Thompson C B. Cell. 1996;87:589–592. doi: 10.1016/s0092-8674(00)81377-x. [DOI] [PubMed] [Google Scholar]
- 36.Yang E, Zha J, Jockel J, Boise L H, Thompson C B, Korsmeyer S J. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 37.Zha J, Harada H, Yang E, Jockel J, Korsmeyer S J. Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 38.Wang H-G, Rapp U R, Reed J C. Cell. 1996;87:629–638. doi: 10.1016/s0092-8674(00)81383-5. [DOI] [PubMed] [Google Scholar]