Abstract
Distant relatives of major histocompatibility complex (MHC) class I molecules, human MICA and MICB, function as stress-induced antigens that are broadly recognized by intestinal epithelial γδ T cells. They may thus play a central role in the immune surveillance of damaged, infected, or otherwise stressed intestinal epithelial cells. However, the generality of this system in evolution and the mode of recognition of MICA and MICB are undefined. Analysis of cDNA sequences from various primate species defined translation products that are homologous to MICA and MICB. All of the MIC polypeptides have common characteristics, although they are extraordinarily diverse. The most notable alterations are several deletions and frequent amino acid substitutions in the putative α-helical regions of the α1α2 domains. However, the primate MIC molecules were expressed on the surfaces of normal and transfected cells. Moreover, despite their sharing of relatively few identical amino acids in potentially accessible regions of their α1α2 domains, they were recognized by diverse human intestinal epithelial γδ T cells that are restricted by MICA and MICB. Thus, MIC molecules represent a family of MHC proteins that are structurally diverse yet appear to be functionally conserved. The promiscuous mode of γδ T cell recognition of these antigens may be explained by their sharing of a single conserved interaction site.
The extended family of major histocompatibility complex (MHC) class I-related proteins serves a variety of immunological functions, among which those of the polymorphic MHC class I molecules are paramount (1). These heterodimers of a membrane-anchored class I chain and soluble β2-microglobulin (β2m) present intracellularly processed peptide antigens to cytotoxic T cells with αβ T cell receptors (TCRs), thus facilitating the elimination of pathogen-infected cells (2). Class I chains have three extracellular domains, of which the membrane-distal α1α2 domains fold to bind peptide between two α-helices on the platform of a β-pleated sheet (3, 4). Both the α-helices and bound peptide are involved in extensive contacts made by α- and β-chain variable regions of antigen-specific TCRs (5, 6). The membrane-proximal α3 domain includes contact residues for β2m and the main binding site for the T cell coreceptor CD8 (3). These structure–function relationships, however, are greatly modified among molecules that are distantly related to MHC class I (7). Among these are the CD1 molecules, which present mycobacterial lipids and glycolipids to some αβ T cells (8, 9). A more recent example is represented by the human MHC class I-related chains A and B (MICA and MICB), which function as stress antigens that are broadly recognized by intestinal intraepithelial γδ T cells (10).
Although MICA and MICB are encoded in the MHC, they are highly divergent from conventional MHC class I, with an average of only 27% amino acid sequence identity in the extracellular α1α2α3 domains (11). MICA and MICB themselves are closely related, sharing 84% identical amino acids (12). Both are highly glycosylated cell surface proteins that lack the binding site for CD8 (11, 13). As inferred from studies mostly of MICA, they are neither associated with β2m nor with peptides derived from class I antigen processing or other cellular sources (10, 13). The expression of MICA and MICB is regulated similar to heat shock protein 70 (hsp70), with both proteins being strongly induced on proliferating or heat shock-stressed epithelial cells (10). By immunohistochemistry, in vivo expression of MICA has so far been found limited to intestinal epithelial cells (13). The structural and functional characteristics of MICA and MICB are matched by their specific recognition by intestinal epithelial γδ T cells expressing the variable region Vδ1 (10). These are a majority of oligoclonal and mostly CD8 negative γδ T cells in the intestinal mucosa that have been conceptualized to interact with self-antigen instead of foreign peptide or nonpeptide antigens (14, 15). Because stress-induced MICA and MICB are recognized by Vδ1 γδ T cells expressing variable TCRs, they may be at the center of an immune surveillance system that is dedicated to the detection of damaged, infected, or otherwise stressed intestinal epithelial cells (10). However, the broad recognition of MICA and MICB implies an unusual mode of interaction with the Vδ1 γδ T cells, and the generality of this system is not decisively proven.
Sequences homologous to the human genes for MICA and MICB occur in the genomes of diverse mammalian species but have not been characterized (11). We have studied the expression and structural and functional similarities of these sequences obtained as corresponding cDNAs from a number of evolutionarily distant primate species. Although all of the encoded MIC polypeptides share a distinctive array of characteristics, they are extraordinarily diverse, suggesting that unknown selective forces may have driven their rapid evolution or that a lack of functional constraints may have permitted genetic drift. Strikingly, however, all primate MIC molecules expressed on the surfaces of transfected cells were recognized by human Vδ1 γδ T cells specific for MICA and MICB. These results support the model that MICA and MICB are the physiological antigens recognized by intestinal epithelial Vδ1 γδ T cells and suggest the existence of a single conserved recognition site. MICA and MICB and their evolutionarily distant relatives represent components of a recently defined branch of the innate immune system that is likely to be common to most, if not all, mammals.
MATERIALS AND METHODS
Cell Lines, Transfections, and Antibodies.
HCT116 is a human colon carcinoma cell line. The primate kidney epithelial cell lines FRhK-4, COS-1 and CV-1, OMK, and NZP-60 are derived from rhesus monkey (Macaca mulatta), African green monkey (Cercopithecus aethiops), owl monkey (Aotus trivirgatus), and silvery marmoset (Callithrix argentata), respectively. WES is a skin cell line from chimpanzee (Pan troglodytes). All cell lines were from the American Type Culture Collection (Manassas, VA) and were grown in DMEM supplemented with 10% fetal bovine serum, glutamine, and antibiotics. C1R cells were transfected with cDNAs in RSV.5neo and stable transfectants were selected with G418 (1.5 mg/ml), as described (16). C1R-MICB transfectants have been described (10). The mAb 6G6 was generated by immunization of mice with mouse LTK–MICA transfectants and recognizes an epitope on the α3 domain of MICA and MICB; this mAb is of the IgG1 isotype (V.G., unpublished data) (13). The polyclonal antiserum (provided by S. Bauer, Fred Hutchinson Cancer Research Center, Seattle) was raised in rabbits by immunization with soluble MICA protein lacking transmembrane and cytoplasmic tail sequences; the soluble protein was expressed in Drosophila Schneider cells and purified from culture supernatant as described (17). Surface expression of MIC molecules on cell lines and transfectants was detected by indirect immunofluorescence stainings using mAb 6G6 or the polyclonal antiserum, fluorochrome-conjugated goat anti-mouse or anti-rabbit Ig, and a FACScan flow cytometer (Becton Dickinson).
RNA Blot Hybridization, cDNA Cloning, and Sequence Analysis.
For mRNA blot hybridization, total RNA isolated with STAT-60 reagent (Tel-Test, Friendswood, TX) was denatured and fractionated in a 1% agarose-formaldehyde gel (20 μg RNA/lane). After transfer onto nitrocellulose membrane, hybridization was carried out at 65°C with MICA cDNA labeled with [32P]dCTP by random hexamer priming. Washings were in 1× SSC (0.15 M NaCl/0.015 M sodium citrate)/0.1% NaDodSO4, at 65°C. Total cDNA was synthesized using oligo(dT) and avian myeloblastosis virus (AMV) reverse transcriptase (Promega). MIC cDNAs were amplified by PCR in 35–40 cycles of 30 s at 94°C, 20 s at 54°C, and 90 s at 72°C. Patr-MIC1, Mamu-MIC2, Ceae-MIC2, and Aotr-MIC1 cDNAs were obtained with primer sets EXP5 (5′-gcgacgtcgacGCCATGGGGCTGGGC-3′) and EXP3 (5′-gtggatcCTAGGCGCCCTCAGTG-3′) designed for the amplification of MICA cDNA (small letters denote adaptor sequences, including restriction sites for cloning) (11). Mamu-MIC1 and Ceae-MIC1 cDNAs were obtained with primers EXP5B and EXP3B, which are CGGGTCGTGCTA and GAGCCAG 3′-end extensions of EXP5 and EXP3, respectively. The Caar-MIC1 sequence was amplified by using EXP3 and a degenerate primer [5′-ccgtcgacGCTGAGCCCCA(C/T)AG(C/T)(C/T)T(A/C/G/T)CG-3′] derived from the 5′ end of the MICA α1 sequence (11). Amplicons were sequenced directly and after cloning using an automated sequencer (Applied Biosystems). Sequences were aligned with human MICB*01 (12) by using pileup and pretty of the Wisconsin Package version 9.1, Genetics Computer Group (GCG). The GenBank accession numbers of the Patr-MIC1, Ceae-MIC1, Ceae-MIC2, Mamu-MIC1, Mamu-MIC2, Aotr-MIC1, and Caar-MIC1 sequences, in this order, are AF055384–AF055390.
T Cells and Cytotoxicity Assays.
The human Vδ1 γδ T cell clones δ1B-S1, δ1B-S3, and δ1B-S4 have been derived from an intestinal epithelial tumor. Their phenotypical and functional characteristics, specificity for MICA and MICB, and expressed TCR γδ chain sequences have been described (10). The CT Vδ1 γδ T cells isolated from another intestinal epithelial tumor have very similar characteristics (TCRγδ+, Vδ1+, TCRαβ−, CD4−, CD8−, integrin αEβ7+) and are cytolytic against target cells expressing MICA or MICB. Their TCRs are composed of Vγ1.8 and a predominant Vδ1 (NDN) Jδ1 chain (V.G., unpublished data). The sodium [51Cr]chromate release assays were carried out as before, and specific lysis (in percent) was calculated by using the standard formula (10).
RESULTS AND DISCUSSION
Expression of MICA- and MICB-Related Sequences in Nonhuman Primate Cell Lines and Isolation of Corresponding cDNAs.
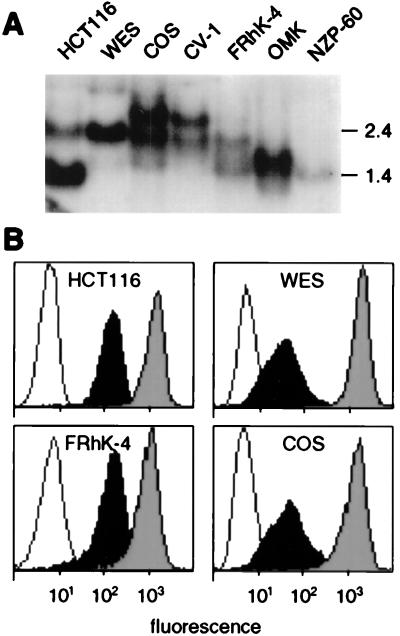
In accord with the lack of expression of MICA and MICB in most human hemopoetic cell lines (11, 13), reverse transcription–PCR (RT-PCR) yielded no homologous cDNA amplicons from various nonhuman primate B cell lines. Epithelial cell lines derived from skin or kidney were available from chimpanzee (Hominidae), African green monkey, and rhesus monkey (Cercopithecidae), owl monkey (Cebidae), and marmoset (Callitrichidae). The family Cercopithecidae represents the Old World monkeys (OWM), whereas Cebidae and Callitrichidae are the two extant families of New World monkeys (NWM), which separated from OWM about 57 million years ago (18). With all of the five epithelial cell lines, blot hybridization of total RNA samples detected one or two MICA- and MICB-related mRNA transcripts that ranged between 1.4 and 3.0 kb in length (Table 1; Fig. 1A). With the chimpanzee and OWM cell lines, substantial amounts of cell surface molecules were detected by flow cytometry with the mAb 6G6, which recognizes an epitope on the α3 domains of human MICA and MICB (Fig. 1B) (V.G., unpublished data). Corresponding cDNAs were obtained from the five primate epithelial cell lines by RT-PCR by using specific oligonucleotide primer pairs and analyzed by sequencing of amplicons directly, and after cloning; because of primer selection, the marmoset sequence was incomplete at the 5′ end (see Materials and Methods) (Fig. 2). The sequences were termed by an abbreviation of the species name (19), followed by MIC1 or MIC2, dependent on the number of gene products found (Table 1). We avoided the MICA and MICB terminology because of the probable recent derivation of these genes by duplication. Two MIC sequences, which by interspecies sequence similarity comparisons were nonallelic and corresponded to distinct genes, were obtained from the rhesus and African green monkey cell lines (Fig. 2). These results provided evidence for expression of MICA- and MICB-related mRNAs and proteins in species other than humans.
Table 1.
Primate species and cell lines referred to in this study, MIC gene designations, and numbers and sizes of mRNA transcripts
| Species | Family | Cell lines | MIC genes | mRNAs, kb |
|---|---|---|---|---|
| Human | Hominidae | HCT116 | MICA | 1.4 |
| MICB | 2.4 | |||
| Chimpanzee | Hominidae | WES | Patr-MIC1 | 2.4 |
| (Pan troglodytes) | ||||
| Rhesus monkey | Cercopithecidae (OWM) | FRhK-4 | Mamu-MIC1 | 1.5, 2.3 |
| (Macacca mulatta) | Mamu-MIC2 | |||
| African green monkey | Cercopithecidae (OWM) | COS-1/CV-1 | Ceae-MIC1 | 2.3, 3.0 |
| (Cercopithecus aethiops) | Ceae-MIC2 | |||
| Owl monkey | Cebidae (NWM) | OMK | Aotr-MIC1 | 1.6 |
| (Aotus trivirgatus) | ||||
| Silvery marmoset | Callitrichidae (NWM) | NZP-60 | Caar-MIC1 | 1.4 |
| (Callithrix argentata) |
Figure 1.
Expression of MICA- and MICB-related mRNAs and proteins by nonhuman primate epithelial cell lines. (A) Hybridization of total RNA samples detected one or two MIC mRNAs in various cell lines derived from chimpanzee (WES), African green monkey (COS and CV-1), rhesus monkey (FRhK-4), owl monkey (OMK), and silvery marmoset (NZP-60). HCT116 is a human colon carcinoma cell line; the lower and upper bands correspond to MICA and MICB mRNA, respectively. Numbers on the right indicate sizes in kilobases. (B) Flow cytometry with mAb 6G6 detected MIC-related molecules on the surfaces of several primate cell lines (filled profiles). Open profiles are IgG1 isotype control stainings. Shaded profiles are stainings with mAb W6/32 (anti-MHC class I HLA-A, -B, and -C) (30).
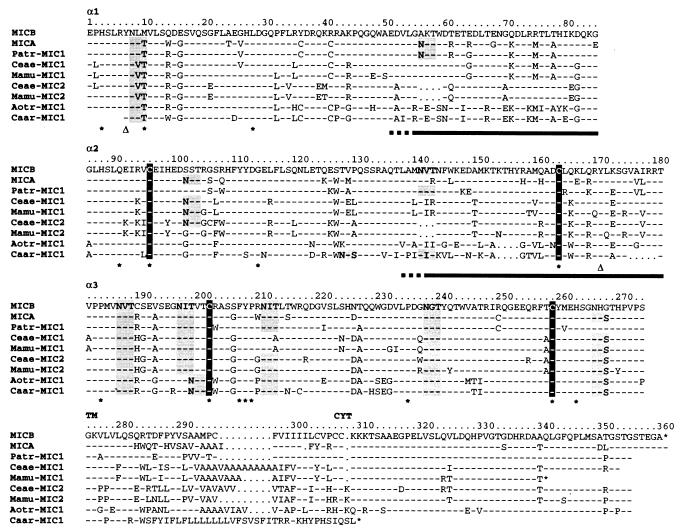
Figure 2.
Diversity of primate MIC molecules. Amino acid sequences deduced from cDNAs are compared with MICA*01 and MICB*01 (12, 23). The α1, α2, α3, and transmembrane (TM) and cytoplasmic tail (CYT) sequences are shown separately. Numbering refers to the MICB*01 sequence. Dashes and dots indicate identical residues or gaps and unaligned sequences, respectively. The positions of the highly conserved cysteines are shown in black. Shaded sequences are potential NXS/T glycosylation sites. Asterisks below sequences identify amino acid residues that are conserved among all vertebrate MHC class I sequences and open triangles indicate the positions of the conserved Tyr-7 and Tyr-171 (4, 20). Bars below the α1 and α2 sequence alignments indicate tentative extensions of α-helical sequences inferred from the previous comparison of MICA with HLA-A2 (11).
Conserved Characteristics of MIC Polypeptides.
All of the cDNA sequences have a single long ORF including the extracellular α1α2α3 domains and a transmembrane region distinctly homologous to human MICA and MICB. Two of the sequences (rhesus Mamu-MIC1 and marmoset Caar-MIC1) have premature stop codons after the transmembrane region and thus lack part or all of a cytoplasmic tail, which may not be essential for function (Fig. 2). Conserved MHC class I signature amino acid residues include the two pairs of cysteines in α2α3 involved in disulfide bonding and a small number of other positions that are common to all vertebrate MHC class I chains (Fig. 2) (11, 20). All of the MIC polypeptide sequences include Tyr-7 and Tyr-171, which interact with the amino terminus of bound peptide in MHC class I molecules (4). Both of these residues are also present in the class I-related human Zn-α2-glycoprotein, which has no function as a peptide binding molecule (21, 22). The presence of these amino acids in MIC molecules may therefore reflect their evolutionary roots rather than an association with unidentified peptidic ligands, although the latter possibility is not entirely ruled out (10).
Characteristic of human MICA and MICB is their extensive N-linked glycosylation at altogether 8 and 5 sites in their α1α2α3 domains, respectively (ref. 13; S. Bauer and T.S., unpublished data). Similar numbers of NXS/T glycosylation acceptor sites are present in the nonhuman primate MIC polypeptides. Although there is some variability in the locations of these sites, none corresponds to the strictly conserved glycosylation site at position 86 in MHC class I chains (Fig. 2) (3). In most of the sequences, potentially glycosylated asparagines at positions 8 in α1 and 238 in α3 replace phenylalanine and aspartic acid at these aligned positions in MHC class I chains, respectively, which are conserved β2m contact residues (Fig. 2) (3). Thus, individual or cumulative steric effects of glycosylation of MIC molecules at single or multiple sites likely contribute to their lack of association with β2m (13). As with human MICA and MICB, the primate sequences lack most of the amino acid residues implicated in binding CD8 (11, 12). Thus, in agreement with the previous sequence, and biochemical and functional analyses of MICA and MICB, a distinctive pattern of family characteristics is shared by all MIC polypeptides.
Extensive Diversity of MIC Polypeptides.
The MIC polypeptides exhibit a high degree of variability, especially in the α1α2 superdomain, where several sequences (Ceae-MIC2, Mamu-MIC2, Aotr-MIC1, and Caar-MIC1) (Table 1) have 3–5 amino acid deletions at various locations within or adjacent to the putative α-helices (Fig. 2). These microdeletions may alter the length of α-helices and affect their positioning relative to the β-sheet platform. No such alterations occur in the α3 domain sequences. As compared with human MICB, the α1α2 sequences display large numbers of amino acid substitutions, of which 80% are nonconservative (Fig. 2). Only 43% of the residues in these domains are identical in all of the primate MIC polypeptides including MICB. According to the previous alignment of MICA with HLA-A2 (11), a significantly lower fraction of 22% of the amino acids in the putative α-helices are identical in all of the primate MIC sequences, whereas 58% of the remainder aligned α1α2 residues (excluding individual sequence gaps) are conserved. The α3 domain sequences exhibit significantly fewer amino acid substitutions, sharing 63% identical residues (Fig. 2). Unusual variability exists among the transmembrane region sequences, because of amino acid replacements, reading frame shifts, and alterations in numbers of GCT/GTT nucleotide triplets (Fig. 2). Because these changes do not alter the overall hydrophobic character of the peptide sequences, and because similar changes occur among human MICA alleles without affecting surface expression (ref. 23; A.S., unpublished data), this variability may be of questionable functional significance.
Recognition by Human Vδ1 γδ T Cells of Diverse Primate MIC Molecules.
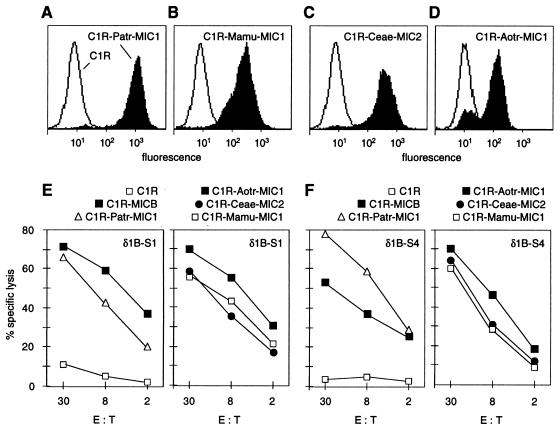
The interaction of human intestinal epithelial Vδ1 γδ T cells with MICA and MICB involves an epitope or epitopes on their α1α2 domains alone, because anti-α1α2 but not anti-α3 domain-specific mAb inhibit recognition, and because expression of mouse H-2Db-MICAα1α2 hybrid molecules is sufficient to sensitize target cells to lysis (10). We explored structure–function relationships by testing whether diverse primate MIC molecules were recognized by Vδ1 γδ T cells specific for MICA and MICB. C1R cells were stably transfected with cDNA constructs directing the expression of all of the primate MIC polypeptides with the exception of Silvery marmoset Caar-MIC1 (Fig. 2). Surface expression of these proteins was confirmed by flow cytometry using mAb 6G6 or a rabbit antiserum raised against soluble MICA (Fig. 3 A–D; data not shown). In chromium release assays with the C1R transfectants as labeled targets, the previously described Vδ1 γδ T cell clones δ1B-S1, δ1B-S3, and δ1B-S4 recognized all of the six primate MIC molecules tested (Fig. 3 E and F; data not shown). The lytic activities were within the same range as those observed with C1R-MICB targets. Similar results were obtained with an intestinal epithelial Vδ1 γδ T cell line (CT) derived from a different individual (data not shown). Thus, MIC molecules from species other than humans had the same function as MICA and MICB. Recognition of these molecules via TCR engagement is supported by anti-Vδ1 mAb-mediated inhibition of target cell lysis (10). The three T cell clones tested have diverse TCRs composed of Vγ1.3, -1.4, or -1.5, and Vδ1/Jδ1 chains with junctional regions differing in amino acid sequence and length (10). A fourth distinct Vδ1/Jδ1 chain paired with Vγ1.8 is predominantly expressed by the CT line (V.G., unpublished data). Hence, the recognition of the various primate MIC molecules by the same human Vδ1 γδ T cells with variable TCRs indicated an unusual mode of interaction.
Figure 3.
Recognition of diverse primate MIC molecules by human Vδ1 γδ T cells specific for MICA and MICB. (A–D) C1R cells transfected with the cDNAs for chimpanzee Patr-MIC1, rhesus monkey Mamu-MIC1, African green monkey Ceae-MIC2, or owl monkey Aotr-MIC1 expressed the encoded MIC proteins on the cell surface (filled profiles), as shown by immunofluorescence stainings and flow cytometry using mAb 6G6 (A–C) and an anti-MICA polyclonal antiserum (D). Open profiles are control stainings of C1R cells mock-transfected with the neomycin selectable marker gene. (E and F) In standard chromium release assays, the various primate MIC transfectants, including C1R-MICB transfectants, were lysed by the human Vδ1 γδ T cell clones δ1B-S1 and δ1B-S4 (10). C1R cells gave negative results. Similar data were obtained in at least one repeat experiment, as well as with C1R transfectants expressing Mamu-MIC2 or Ceae-MIC1. E:T, effector-to-target cell ratio.
The functionally tested primate MIC and human MICA and MICB polypeptides differ extensively in their α1α2 domain α-helical regions, which, by extrapolation from αβ T cell recognition of MHC class I and class II molecules, might be expected to present the main accessible surface that may interact with Vδ1 γδ TCRs (5, 6). Potentially common T cell epitopes in these and adjacent regions may be further limited by the actual orientations of conserved amino acid side chains and the occurrences of three and/or four amino acid deletions in three of the primate MIC molecules (Mamu-MIC2, Ceae-MIC2, and Aotr-MIC1) (Figs. 2 and 4). Thus, unlike the interactions of αβ TCRs with MHC class I peptide complexes, it seems certain that a recognition of the diverse MIC molecules by the same Vδ1 γδ T cells with variable TCRs cannot involve simultaneous engagements of different γ- and δ-chain variable regions. Our results may thus be best explained by the existence of a monomorphic epitope on MIC antigens that may be recognized by similar conformations shared among the different TCRs.
Figure 4.
Hypothetical placement of amino acid residues that are conserved among human MICA*01 and MICB*01, chimpanzee Patr-MIC1, rhesus monkey Mamu-MIC1 and -MIC2, African green monkey Ceae-MIC1 and -MIC2, and owl monkey Aotr-MIC1 on the ribbon diagram of HLA-A2 (adapted from ref. 31); conserved positions are shaded. These molecules were all recognized by the various Vδ1 γδ T cell clones tested. This figure is intended to visualize sequence diversity associated with probable similar secondary structures and folds of MIC molecules, without implying any close similarity to HLA-A2. Numbers are for orientation in the sequence alignment (Fig. 2). Areas marked by broken lines indicate tentative locations of small deletions occurring in the Mamu-MIC2, Ceae-MIC2 and Aotr-MIC1 sequences.
CONCLUSIONS
These results support the model that MICA and MICB represent the natural antigens recognized by human intestinal epithelial Vδ1 γδ T cells by providing indirect evidence that a similar system of immune surveillance may be conserved in other species. The existence of at least one functionally expressed MIC gene in all of the species examined affirms an important beneficial function of this family of genes and molecules. The results further argue against a recognition of these molecules in association with peptide or nonpeptide ligands, because similar binding and presentation of specific ligand by the diverse human and primate MIC molecules would seem implausible. This is in agreement with previous biochemical data and the proposed role of MICA and MICB as self antigens (10). The conservation of Tyr-7 and Tyr-171 may thus be viewed paradoxical. However, mutagenesis of both of these residues to phenylalanine in Patr-MIC1 had no effect on the expression and T cell recognition of the altered molecule (A.S. and V.G., unpublished data).
The apparent conservation of a dominant Vδ1 γδ T cell epitope on all of the MIC molecules tested indicates a mode of MHC ligand–T cell interaction that is incompatible with the principles governing αβ T cell recognition of MHC molecules (5, 6). However, there is some resemblance to the broad recognition of soluble phosphorylated nonpeptide antigens and their derivatives by human Vγ2/Vδ2 T cells (24, 25). Structural and functional evidence has indicated that γδ TCRs may recognize antigens more akin to Igs than to αβ TCRs (26–28). According to this model, human Vδ1 γδ T cells capable of recognizing MICA and MICB may have TCRs with conformationally similar antigen combining sites. This finding would fit with the recently found conservation of similar conformations by different γδ TCRs expressed on dendritic epidermal cells from normal and TCR-γ chain mutant mice (29). The remarkable diversity displayed by the primate MIC molecules implies that unknown selective forces may have driven their rapid evolution or that a relative lack of functional constraints may have permitted genetic drift. In this regard, our functional results are not informative. This lack of knowledge highlights the importance of gaining precise understanding of the molecular interactions involved in γδ T cell recognition of MIC antigens. Finally, these molecules could be adapted to a biological context including yet undefined cellular and immunological functions.
Acknowledgments
We thank anonymous reviewers for their helpful comments; S. Bauer for the rabbit antiserum; and D. Watkins for cell lines. A.S. was supported by a fellowship from the Deutsche Forschungsgemeinschaft. This work was supported by National Institutes of Health Grants RO1 AI30581 and PO1 CA18221.
ABBREVIATIONS
- MHC
major histocompatibility complex
- MIC
MHC class I-related chain
- β2m
β2-microglobulin
- TCR
T cell receptor
Footnotes
References
- 1. Beckman E M, Brenner M B. Immunol Today. 1995;16:349–352. doi: 10.1016/0167-5699(95)80154-5. [DOI] [PubMed] [Google Scholar]
- 2.Germain R N, Margulies D H. Annu Rev Immunol. 1993;11:403–450. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- 3.Bjorkman P J, Parham P. Annu Rev Biochem. 1990;59:253–288. doi: 10.1146/annurev.bi.59.070190.001345. [DOI] [PubMed] [Google Scholar]
- 4.Madden D R. Annu Rev Immunol. 1995;13:587–622. doi: 10.1146/annurev.iy.13.040195.003103. [DOI] [PubMed] [Google Scholar]
- 5.Garboczi D N, Ghosh P, Utz U, Fan Q R, Biddison W E, Wiley D C. Nature (London) 1996;384:134–141. doi: 10.1038/384134a0. [DOI] [PubMed] [Google Scholar]
- 6.Garcia K C, Degano M, Pease L R, Huang M, Peterson P A, Teyton L, Wilson I A. Science. 1998;279:1166–1172. doi: 10.1126/science.279.5354.1166. [DOI] [PubMed] [Google Scholar]
- 7.Wilson I A, Bjorkman P J. Curr Opin Immunol. 1998;10:67–73. doi: 10.1016/s0952-7915(98)80034-4. [DOI] [PubMed] [Google Scholar]
- 8.Beckman E M, Porcelli S A, Morita C T, Behar S M, Furlong S T, Brenner M B. Nature (London) 1994;372:691–694. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- 9.Sieling P A, Chatterjee D, Porcelli S A, Prigozy T I, Mazzaccaro R J, Soriano T, Bloom B R, Brenner M B, Kronenberg M, Brennan P J, Modlin R L. Science. 1995;269:227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- 10.Groh V, Steinle A, Bauer S, Spies T. Science. 1998;279:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 11.Bahram S, Bresnahan M, Geraghty D E, Spies T. Proc Natl Acad Sci USA. 1994;91:6259–6263. doi: 10.1073/pnas.91.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bahram S, Spies T. Immunogenetics. 1996;43:230–233. doi: 10.1007/BF00587305. [DOI] [PubMed] [Google Scholar]
- 13.Groh V, Bahram S, Bauer S, Herman A, Beauchamp M, Spies T. Proc Natl Acad Sci USA. 1996;93:12445–12450. doi: 10.1073/pnas.93.22.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chowers Y, Holtmeier W, Harwood J, Morzycka-Wroblewska E, Kagnoff M F. J Exp Med. 1994;180:183–190. doi: 10.1084/jem.180.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holtmeier W, Witthoft T, Hennemann A, Winter H S, Kagnoff M F. J Immunol. 1997;158:5632–5641. [PubMed] [Google Scholar]
- 16.Grandea A G, III, Androlewicz M J, Athwal R S, Geraghty D E, Spies T. Science. 1995;270:105–108. doi: 10.1126/science.270.5233.105. [DOI] [PubMed] [Google Scholar]
- 17.Bauer S, Willie S T, Spies T, Strong R K. Acta Crystallogr D. 1998;54:451–453. doi: 10.1107/s0907444997015229. [DOI] [PubMed] [Google Scholar]
- 18.Takahata N, Satta Y. Proc Natl Acad Sci USA. 1997;94:4811–4815. doi: 10.1073/pnas.94.9.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein J, Bontrop R E, Dawkins R L, Erlich H A, Gyllensten U B, Heise E R, Jones P P, Parham P, Wakeland E K, Watkins D I. Immunogenetics. 1990;31:217–219. doi: 10.1007/BF00204890. [DOI] [PubMed] [Google Scholar]
- 20.Grossberger D, Parham P. Immunogenetics. 1992;36:166–174. doi: 10.1007/BF00661093. [DOI] [PubMed] [Google Scholar]
- 21.Freije J P, Fueyo A, Uria J, Lopez-Otin C. FEBS Lett. 1991;290:247–249. doi: 10.1016/0014-5793(91)81271-9. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez L M, Lopez-Otin C, Bjorkman P J. Proc Natl Acad Sci USA. 1997;94:4626–4630. doi: 10.1073/pnas.94.9.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fodil N, Laloux L, Wanner V, Pellet P, Hauptmann G, Mizuki N, Inoko H, Spies T, Theodorou I, Bahram S. Immunogenetics. 1996;44:351–357. doi: 10.1007/BF02602779. [DOI] [PubMed] [Google Scholar]
- 24.Constant P, Davodeau F, Peyrat M A, Poquet Y, Puzo G, Bonneville M, Fournie J J. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka Y, Morita C T, Tanaka Y, Nieves E, Brenner M B, Bloom B R. Nature (London) 1995;375:155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 26.Rock E P, Sibbald P R, Davis M M, Chien Y-h. J Exp Med. 1994;179:323–328. doi: 10.1084/jem.179.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schild H, Mavaddat N, Litzenberger C, Ehrich E W, Davis M M, Bluestone J A, Matis L, Draper R K, Chien Y H. Cell. 1994;76:29–37. doi: 10.1016/0092-8674(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Lebedeva M I, Llera A S, Fields B A, Brenner M B, Mariuzza R A. Nature (London) 1998;391:502–506. doi: 10.1038/35172. [DOI] [PubMed] [Google Scholar]
- 29.Mallick-Wood C A, Lewis J M, Richie L I, Owen M J, Tigelaar R E, Hayday A C. Science. 1998;279:1729–1733. doi: 10.1126/science.279.5357.1729. [DOI] [PubMed] [Google Scholar]
- 30.Parham P, Barnstable C J, Bodmer W F. J Immunol. 1979;123:342–349. [PubMed] [Google Scholar]
- 31.Bjorkman P J, Saper M A, Samraoui B, Bennett W S, Strominger J L, Wiley D C. Nature (London) 1987;329:506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]