Abstract
A myelin basic protein (MBP)-specific BALB/c T helper 1 (Th1) clone was transduced with cDNA for murine latent transforming growth factor-β1 (TGF-β1) by coculture with fibroblasts producing a genetically engineered retrovirus. When SJL x BALB/c F1 mice, immunized 12–15 days earlier with proteolipid protein in complete Freund’s adjuvant, were injected with 3 × 106 cells from MBP-activated untransduced cloned Th1 cells, the severity of experimental allergic encephalomyelitis (EAE) was slightly increased. In contrast, MBP-activated (but not resting) latent TGF-β1-transduced T cells significantly delayed and ameliorated EAE development. This protective effect was negated by simultaneously injected anti-TGF-β1. The transduced cells secreted 2–4 ng/ml of latent TGF-β1 into their culture medium, whereas control cells secreted barely detectable amounts. mRNA profiles for tumor necrosis factor, lymphotoxin, and interferon-γ were similar before and after transduction; interleukin-4 and -10 were absent. TGF-β1-transduced and antigen-activated BALB/c Th1 clones, specific for hemocyanin or ovalbumin, did not ameliorate EAE. Spinal cords from mice, taken 12 days after receiving TGF-β1-transduced, antigen-activated cells, contained detectable amounts of TGF-β1 cDNA. We conclude that latent TGF-β1-transduced, self-reactive T cell clones may be useful in the therapy of autoimmune diseases.
Keywords: autoimmune disease/transduced T cells
Treatment with active transforming growth factor-β (TGF-β) during the latter part of the induction phase of autoimmune diseases, such as acute experimental allergic encephalomyelitis (EAE) and collagen-induced arthritis (CIA), and during relapsing EAE prevents the development and/or exacerbation of disease symptoms (1–6). TGF-β may exert these effects through decreasing tumor necrosis factor (TNF)-α and lymphotoxin (LT) production (5, 7), responses to interleukin (IL)-12 (8), macrophage and microglia activation (9–11), cytokine-enhanced class II major histocompatibility complex expression (12), and migration of cells into the central nervous system (13, 14).
Endogenous TGF-β is one of the factors protecting against autoimmunity, as shown by the adverse effect of neutralizing anti-TGF-β on the course of acute (3, 15, 16) and relapsing EAE (2) as well as of CIA (6). Indeed, TGF-β1−/−, but not TGF-β1−/− severe combined immunodeficient, mice exhibit generalized T lymphocyte infiltrations in various organs (17). Immunoregulatory T cells in humans and animals are thought to exert their inhibitory effects in part through production of TGF-β (5, 18–20).
Most cells, including T cells, produce TGF-β in latent form, attached to a latency associated protein, which needs to be removed to uncover the receptor-binding region (21). Within sites of inflammation this is thought to occur by enzymes and/or acidification in macrophages (22). Immunohistochemical studies to determine the role of TGF-β produced within EAE infiltrates have been inconclusive, because differentiation between active and latent TGF-β was not made in these studies. It is therefore unknown whether the endogenous TGF-β that plays a role in the protection against autoimmunity is primarily functioning locally or systemically. Most treatments with active TGF-β that have shown protection against autoimmune disease have been systemic. Prolonged systemic treatment with active TGF-β, however, is inapplicable to patients, because it induces liver fibrosis and glomerulosclerosis, as also shown in TGF-β transgenic mice (23). Hence, another way in which TGF-β might be of clinical use in autoimmune diseases would be to introduce latent TGF-β directly into the inflammatory infiltrates, expecting that it would get activated by inflammatory cells in sufficient amounts to suppress the local inflammation.
Reasoning that circulating T cells with specificity for the autoantigen would be the most effective way of transporting latent TGF-β1 into the lesions, we have artificially increased the ability of myelin basic protein (MBP)-specific cloned T cells to produce TGF-β1 by infecting them with a recombinant retrovirus producing latent TGF-β1. We will show that these transduced MBP-specific BALB/c T cells lose the capacity to provoke EAE in BALB/c mice and gain, instead, the ability to protect against EAE in (SJL × BALB/c) F1 mice immunized with proteolipid protein (PLP). Thus, the antigen specificity of the cloned T cells that, in the untransduced cells, enabled them to migrate into the central nervous system and cause adoptive EAE has now been co-opted for the site-specific local delivery of therapeutic TGF-β1.
MATERIALS AND METHODS
Mice.
Male BALB/c and female SJL/J mice were purchased from Taconic Farms (Germantown, NY) and The Jackson Laboratory. (SJL × BALB/c) F1 hybrids, bred in our animal facilities, were used at age 6–8 weeks.
Induction of EAE and Cell Transfers.
SJL × BALB/c mice were injected s.c. with 200 μg PLP peptide 139–151 (Molecular Dynamics), emulsified in incomplete Freund’s adjuvant containing 200 μg killed H37RA Mycobacteria tuberculosa (CFA). The mice received 200 ng pertussigen i.v., 24 and 48 h later. EAE was scored (double-blind read) as follows: 1 = limp tail; 2 = partial hind leg paralysis; 3 = total hind leg paralysis; 4 = hind and front limb paralysis; 5 = moribund (3). Transduced and control cloned T cells (3 days after activation with the relevant antigen in vitro, 3 × 106 cells per mouse) were injected i.v. into mice that had been immunized with PLP 12–15 days earlier.
Statistical Significance.
Differences between groups of mice for mean EAE severity were evaluated by Student’s t test; for EAE incidence by χ2 test.
T Cell Clones.
The cloned T cells used, clone 8–4.G6, were derived from BALB/c mice immunized with MBP in CFA (24) and were provided by M. Dorf (Harvard University Medical School). Cells were activated by exposure to MBP peptide 59–76 (10 μg/106 cells, Keck Biotechnical Resource Center, New Haven, CT) in the presence of antigen-presenting cells (5 × 106 γ-irradiated spleen cells). Keyhole limpet hemocyanin (KLH)-specific (D3) and ovalbumin (OVA)-specific (BOT.A3) BALB/c Th1 cell clones were provided by R. H. deKruyff (Stanford University) and grown as described (25). For measurements of TGF-β production, cells were cultured in serum-free medium containing 1% Nutridoma (Boehringer Mannheim). All the T cell clones were stimulated every 2–3 weeks by the corresponding antigens: MBP (10 μg/ml), KLH (1 μg/ml), and OVA (10 μg/ml).
Transduction of T Cell Clones.
The cDNA (base pairs 352-1550) encoding murine TGF-β1 (provided by DNAX) was subcloned into the pMFG retroviral vector as described (26). CRIP-TGF-β packaging cells, producing the replication-defective retrovirus, were generated (27). The titer of the virus was 0.5 copies as determined by Southern analysis. No replication-competent virus was detected when using the his mobilization assay (28). CRIP-TGF-β packaging cells were γ-irradiated (2,800 rad) and plated in a 24-well plate (2 × 105 cells per well). After 4 h, when the CRIP-TGF-β fibroblasts had completely adhered to the well, recently activated cloned T cells (106/well) were added. Cocultures were maintained for 48 h (29). The T cells were then cloned by limiting dilution in 96-well plates, using γ-irradiated spleen (5 × 105/ml) as feeder cells. Clones were expanded until sufficient amounts of DNA could be obtained for PCR analysis.
PCR for Presence of Latent TGF-β cDNA.
DNA was extracted by using the Promega Wizard Genomic DNA purification kit. Primers used for the detection of TGF-β1 cDNA (CLONTECH) were: FP, 5′-GCCCTGGACACCAACTATTGCT-3′; and RP, 5′-AGGCTCCAAATGTAGGGGCAGG-3′. They correspond closely (1 bp difference) to the mouse TGF-β1 sequences, 1187–1208 and 1347–1326. The PCR program was: 95°C for 5 min, 94°C for 30 sec, 55°C for 30 sec, 72°C for 1 min, 40 cycles; 72°C for 10 min, using 1 μg sample DNA/reaction. Approximately 3% of the cloned T cells proved positive for the cDNA of TGF-β1.
Detection of TGF-β cDNA in spinal cord was done similarly. To control for PCR conditions and DNA quality, PCR for mouse mammary tumor virus–long terminal repeat was performed on all spinal cord samples using the FP, 5′-CTACACTTAGGAGAGAAGCAGCCA-3′, and the RP, 5′-CTTACTTAAACCTTGGGAACCGCAAG-3′ (30).
Cytokine Production.
RNA was extracted from 2 × 106 T cells, 2–3 days after stimulation with antigen, using the RNA STAT-60 isolation kit (Tel-Test, Friendswood, TX). Cytokine mRNAs produced by antigen-activated T cells were quantitated by multiprobe ribonuclease protection assays, using two sets of cytokine probes according to the manufacturer’s instructions (PharMingen).
Neutralizing mAb, 4A.11, specific for TGF-β1 (31) was donated by B. M. Fendly (Genentech). The biological activity of TGF-β was assayed in serum-free medium from 24-h cultures of 106 cells/ml by its activating effect on the plasminogen activator inhibitor-1 promoter linked to a luciferase reporter gene in mink lung epithelial cells (32), donated by D. B. Rifkin (New York University Medical Center). Samples were assayed with and without activation of latent TGF-β by treatment with acid (0.1 M HCl at 4°C for 60 min). The protein content of TGF-β1 in these supernatants was assayed by ELISA in Immulon 4 flat-bottom plates, coated with 5 μg/well of an mAb to TGF-β 12H5 (31) using natural TGF-β1 (Genzyme, Cambridge, MA) as a standard, and biotinylated mAb to TGF-β1 (R & D Systems), streptavidin-peroxidase (Zymed), and o-phenylenediamine as developing reagents.
RESULTS
Characterization of Transduced T Cell Clones.
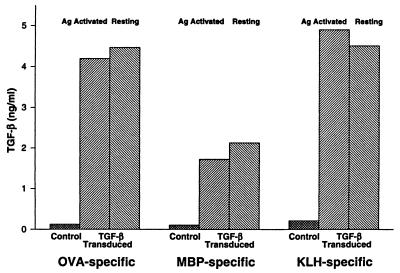
Transduced T cell clones were identified by the presence of cDNA for latent TGF-β1 determined by PCR (Fig. 1). TGF-β1 transduced subclones of the 8–4.G6 T cells were compared with respect to their ability to produce latent and active TGF-β as well as other cytokines. Serum-free culture medium from antigen-activated and resting TGF-β transduced and control cloned T cells (106 cells/ml, 24 h at 37°C) were analyzed for latent and active TGF-β1 contents (Fig. 2). All the TGF-β1 transduced clones, MBP-, OVA-, and KLH-specific T cells, exhibited 1.5–4 ng/ml of latent TGF-β in their supernatants, whether or not they had been activated by antigen. Supernatants from control T cell clones showed barely detectable amounts above the serum-free medium background (<0.2 ng). Results from the assay for biological activity (not shown) agreed well with those obtained with ELISA. No active TGF-β1 was detected by ELISA, but in the biological assay a small amount of active TGF-β above background appeared to be present in the supernatants from the transduced clones activated by antigen and not in medium from resting cells.
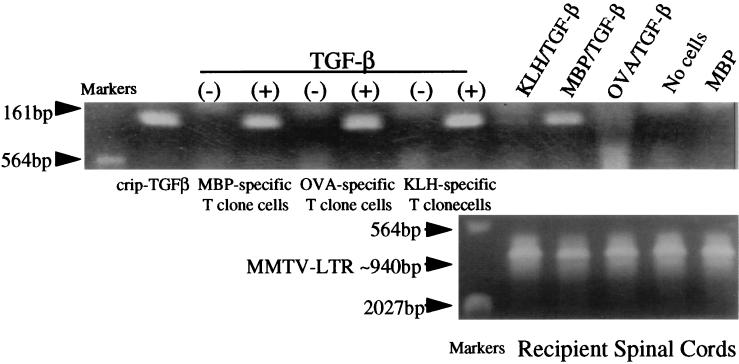
Figure 1.
Detection by PCR of latent TGF-β1 cDNA in DNA from transduced T cell clones and spinal cord of recipients injected 12 days earlier with such clones. The 161-bp PCR product is detected with the DNA from CRIP-TGF-β packaging cells (lane 1), from some of the clones prepared from transduced cloned T cells (lanes 3, 5, and 7), and from spinal cord of mice receiving the antigen-activated, latent TGF-β1 MBP-specific transduced cells, but not with DNA from untransduced T cells or from spinal cord of mice receiving untransduced cells. There is also a little product with the DNA from spinal cord of mice receiving transduced T cells of other specificities. Control PCR [mouse mammary tumor virus–long terminal repeat (MMTV-LTR), ≈940 bp] products for DNA from the spinal cord samples are of similar densities in all the samples.
Figure 2.
Latent TGF-β1 concentrations in the tissue culture supernatants from transduced and untransduced (control) cloned T cells as detected by ELISA. Supernatants were collected from serum-free cultures of 106 cells/ml after 24 h of incubation. Note that the TGF-β1 concentrations were similar in supernatants from antigen-activated and resting cells.
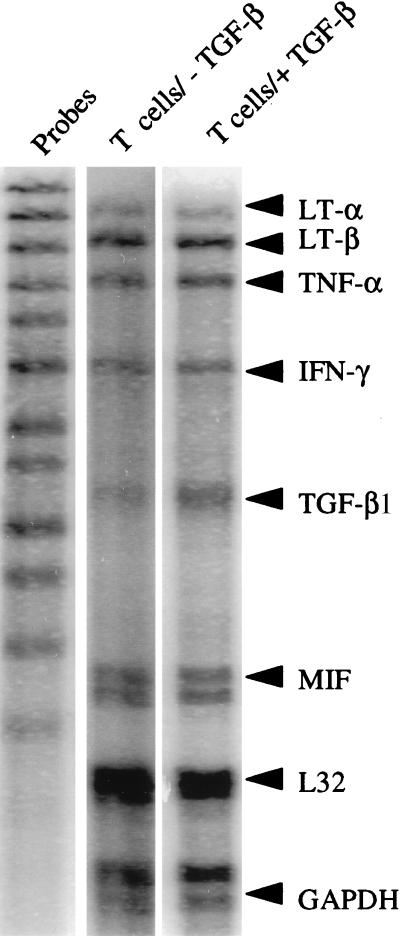
To determine whether the production of latent TGF-β1 caused a change in the cytokine pattern produced by the MBP-specific T cell clone, a ribonuclease protection assay was performed on RNA prepared from the untransduced and the TGF-β1-transduced MBP-specific T cell clone 2–3 days after activation of the cells by antigen, using two sets of cytokine probes. The results obtained for the set containing the probes for LTα, LTβ, TNF-α, interferon-γ (IFN-γ), TGF-β1, TGF-β2, TGF-β3, and migration inhibitory factor are shown in Fig. 3. The mRNA distribution, expressed as a percentage of mRNA for the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase, showed little difference between the untransduced and transduced clones for LTα, LTβ, TNF-α, and IFN-γ. However, transduction caused an increase in TGF-β1 mRNA. The results obtained with the other set of cytokine probes showed an absence of mRNA for IL-4, IL-5, IL-6, or IL-10 before and after transduction (data not shown).
Figure 3.
Multiprobe RNase protection assay for various cytokines on RNA from antigen-activated TGF-β1-transduced and untransduced MBP-specific T cells. Scanning of the band densities, in comparison with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as control, showed a moderate relative increase in TGF-β1 mRNA in the transduced cells. There was also a slight increase in LT-β, and no change in LT-α, TNF-α, IFN-γ, or MIF (migration inhibitory factor).
We confirmed that when 1–8 × 106 untransduced cells from clone 8–4.G6, taken 3 days after activation with MBP in vitro, are injected i.v. into 390 rad γ-irradiated and 200 ng pertussigen-injected BALB/c mice, the recipients developed EAE 7–8 days after transfer with peak disease developing by 9–12 days (24). In contrast, 3 × 106 TGF-β1-transduced MBP-specific T cells did not transfer EAE under these conditions (not shown).
Effect of Latent TGF-β1-Transduced MBP-Specific T Cells on EAE Induced in SJL × BALB/c Mice by Immunization with PLP in CFA.
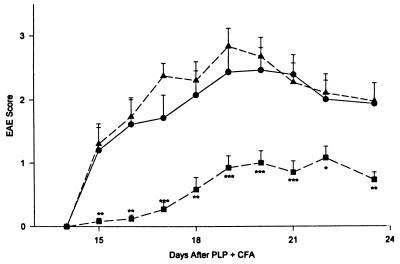
Cells to be assayed for immunomodulating effects were injected at 3 × 106 cells/mouse 12 days after the immunization with PLP in CFA, i.e., 3–4 days before development of the first EAE symptoms. In the experiments illustrated in Fig. 4, MBP-specific T cells were activated by exposure to MBP 3 days before cell transfer. The untransduced activated MBP-specific T cells slightly accelerated the development of EAE in the PLP-immunized SJL × BALB/c recipients. In contrast, the activated MBP-specific T cells that had been transduced with latent-TGF-β1 caused a significant decrease in the EAE score in such recipients. The difference in the EAE score between the group receiving no cells and the group receiving TGF-β-transduced T cells was highly significant throughout the period of observation (P < 0.01 to <0.001). Moreover, there was a significantly lower incidence of EAE in the mice receiving the TGF-β1-transduced cells up to day 19 after immunization with PLP (P < 0.05) (Fig. 4).
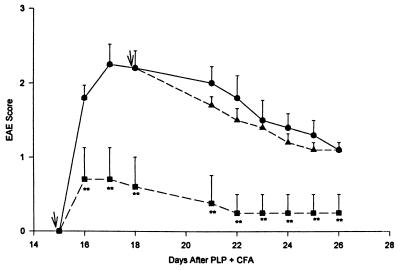
Figure 4.
Influence of MBP-specific latent TGF-β1-transduced and untransduced cloned BALB/c T cells on actively induced EAE in SJL × BALB/c mice. Mice (SJL × BALB/c) were immunized with PLP 139–151 in CFA and developed EAE starting on day 15 if left untreated (n = 15; •). Mice receiving 3 × 106 recently activated MBP-specific, TGF-β1-transduced cloned T cells on day 12 (n = 13; ■) had delayed and significantly less severe EAE throughout the course of the disease than did control mice not receiving cells or mice receiving 3 × 106 MBP-activated, untransduced T cells on day 12 (n = 14; ▴). ∗, P < 0.05; ∗∗, P ≤ 0.01; ∗∗∗, P ≤ 0.001; bars = SE.
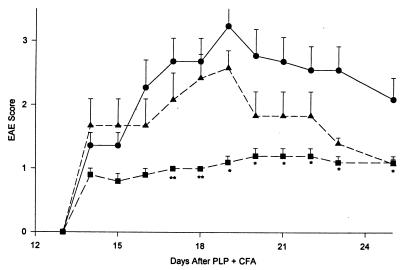
When the transduced cells were injected on the day when EAE symptoms first started to appear, there still was a highly significant protective effect (Fig. 5). However, injection of the transduced cells on day 18 did not significantly influence the course of the EAE. It should be pointed out, however, that the disease in the no-cell control group already started to recover on day 18 (Fig. 5).
Figure 5.
Protective effect of MBP-activated, TGF-β-transduced T cells transferred during development of EAE. Mice were induced to develop EAE as in Fig. 4. MBP-activated, TGF-β-transduced T cells (3 × 106 per recipient) were transferred into groups of five mice each on day 15 (■) or on day 18 (▴). EAE severity was significantly reduced as compared with the mice not receiving cells (•) only in the group receiving cells on day 15. ∗∗, P ≤ 0.01.
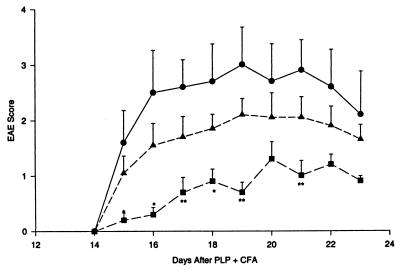
As also needed for the transfer of adoptive EAE with myelin antigen-specific T cells (24), the protective effect of the TGF-β-transduced cells required antigen activation 3 days before transfer into recipient mice. Thus, as shown in Fig. 6, resting TGF-β-transduced cells failed to protect against EAE development, while their antigen-activated counterparts did.
Figure 6.
Protection from EAE by MBP-activated, but not by resting, latent TGF-β1-transduced T cells. Mice were induced to develop EAE as in Fig. 4. Cells (3 × 106) were transferred into these mice on day 12 after PLP + CFA. The recipients of cells that had not been exposed to MBP 3 days before transfer (n = 4; ▴) showed EAE development similar to that in control mice (n = 4, •). In contrast, the mice receiving MBP-activated, untransduced cells (n = 4, ■) exhibited accelerated and more severe disease than the mice receiving no cells (P < 0.05 on day 17), while the mice receiving MBP-activated, TGF-β-transduced T cells had significantly delayed and less disease than the mice receiving no cells (n = 4, ▾). ∗, P < 0.05; ∗∗, P < 0.01.
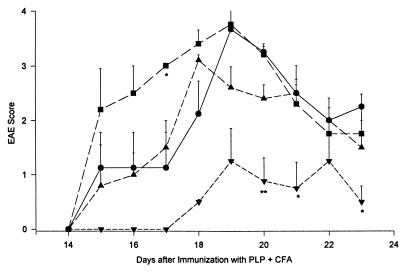
Neutralizing mAb to TGF-β1 Prevents the Effect of TGF-β1-Transduced MBP-Specific T Cells.
An i.p. injection of 0.5 mg mAb 4A.11, which specifically neutralizes active TGF-β1, on the same day as the transduced cells prevented the protective effect against EAE seen with TGF-β1-transduced MBP-specific T cells during the first week after cell transfer (Fig. 8). It is of interest that this effect of the passively transferred mAb wore off toward the end of the experiment, suggesting that the TGF-β1 produced by the transduced T cells was still capable of suppressing the ongoing EAE after the mAb had disappeared. There was no detectable effect of this anti-TGF-β1 on the EAE severity in control mice (not shown).
Figure 8.
Neutralizing monoclonal anti-TGF-β1 (4A11) counteracts the protective effect of latent TGF-β1-transduced MBP-specific T cells. Mice were induced to develop EAE as in Fig. 4. Two groups of mice received 3 × 106 antigen-activated, latent TGF-β1-transduced, MBP-specific T cells on day 13 after immunization with PLP + CFA. One of these groups also received 0.5 mg anti-TGF-β1, 4A11, i.p. on day 13 (n = 6, ▴). This group initially developed EAE similar to the control group with or without anti-TGF-β1 (n = 11; •), significantly more severe (P < 0.05, day 17, and < 0.01, days 18 and 19) than in the group receiving cells alone (n = 5, ■). ∗, P < 0.05; ∗∗, P < 0.01.
Antigen Specificity of the Protective Effect of Latent TGF-β1-Transduced T Cells.
We compared the effect of MBP-specific T cells with those of BALB/c T cell clones of unrelated specificities. TGF-β1-transduced, OVA- or KLH-specific T cells had no significant protective effect against EAE development when injected into recipients on day 12 after immunization with PLP and at a cell dose of 3 × 106, despite the somewhat higher production of latent TGF-β1 by these cells than by the MBP-specific transduced T cells. In contrast, the TGF-β transduced, MBP-specific cells had a significant protective effect as compared with the mice receiving no cells and also as compared with the combined groups of mice receiving KLH- or OVA-specific T cells (Fig. 7).
Figure 7.
Protection from EAE by TGF-β1-transduced antigen-activated T cell clones is exhibited by myelin-specific T cells, but not by T cells specific for unrelated antigens. Mice were induced to develop EAE as in Fig. 4. Cells (3 × 106) were transferred into these mice on day 12 after PLP + CFA. As there was no significant difference between the mice receiving KLH-specific or OVA-specific, TGF-β-transduced T cells, the results for these two groups of mice were taken together (n = 10, ▴). Only the mice receiving MBP-specific, TGF-β-transduced T cells (n = 5, ■) showed significantly less severe EAE than the controls (n = 5, •).∗, P < 0.05; ∗∗, P < 0.01.
At the end of the experiment in Fig. 7, spinal cords from each group of recipient mice were pooled and subjected to DNA extraction. PCR was used to search for the presence of latent TGF-β1 cDNA containing donor T cells in spinal cord. The results in Fig. 1 show that the cDNA with which the cells had been transduced could readily be detected in spinal cord from recipients of transduced MBP-specific donor cells. Under the same PCR conditions, the amount of PCR product was barely detectable with DNA from recipients of OVA- or KLH-specific cells, while a control PCR for genomic DNA, the mammary tumor virus long terminal repeat (30), showed no differences between any of the samples. This suggests that the transduced MBP-specific T cells were much more numerous in the spinal cord 12 days after transfer than the transduced T cells of one of the myelin-unrelated specificities.
DISCUSSION
The present results show that latent TGF-β1-transduced MBP-specific Th1 cells protect against PLP-induced EAE in (SJL × BALB/c) F1 mice, even when given during the development of disease symptoms. When left untransduced, the same Th1 cells slightly increase the severity of actively induced EAE, and induce adoptive EAE in BALB/c mice (24). Clearly, the only difference between the transduced and untransduced cloned T cells is the enhanced production of TGF-β1 by the transduced cells. The cloned cells used are Th1 in that they produce mRNA for TNF, LTα, LTβ, and IFN-γ, but not for IL-4 or IL-10. Even though this Th1 cytokine profile is unaltered after transduction with TGF-β1, the cells lose their capacity to transfer EAE to BALB/c recipients and gain the ability to significantly ameliorate the development of actively induced EAE in (SJL × BALB/c) mice. Therefore, the in vivo properties of these cells are completely changed merely by having been engineered to produce more latent TGF-β1 than they normally would. In addition, the observation that injection of neutralizing anti-TGF-β1 temporarily interferes with the protective effect of the transduced MBP-specific T cells establishes that TGF-β1 mediates their protective effect.
Other cytokine genes also have been used to genetically engineer MBP-specific T cells to protect against EAE induction. In one report, an IL-4-transduced MBP-specific T cell hybridoma was used (33), and in another, IL-10-transduced heterogeneous T cells or a T cell clone derived after transduction was used (34). In the latter report, the transduced T cell clone also showed a high level of endogenous IL-10 production and was not examined for production of other cytokines. It therefore is not certain whether or not the human IL-10 used for transduction was responsible for the protective effect of these cells against EAE. In MBP-specific TCR-transgenic rag−/− mice, the occurrence of spontaneous EAE is prevented by CD4+ T cells of other specificities (35). This is probably not only an effect of Th2 cell activation, as both Th1 and Th2 type T cell clones of the same MBP specificity transfer EAE into rag−/− recipients (36). Thus, although IL-4 given early during induction of EAE (37) and, in some reports, IL-10 (38) may have a protective effect when administered exogenously, IL-4 production by the MBP-specific T cells is not sufficient to prevent disease induction.
TGF-β induces the synthesis of IL-10 by macrophages and inhibits translation of TNF-α and IL-1 (39). Moreover, TGF-β stimulates its own production (40), and, therefore, a few TGF-β-producing T cells retained in an infiltrate on the basis of their specificity for myelin protein may cause oligodendrocytes and macrophages in their vicinity to produce more TGF-β. TGF-β also is reported to promote the development of immunoregulatory CD8+ T cells in vitro (41, 42). Further studies are needed to determine whether latent TGF-β1-transduced MBP-specific Th2 cells would be even more protective than the Th1 cells used in the present study, as synergy between TGF-β, IL-4, and IL-10 is known to occur in the deactivation of macrophages (43).
Since locally injected active TGF-β in normal joints provokes inflammation, probably because of its chemotactic properties (44), it was not clear at the beginning of these experiments whether the local production within EAE lesions of latent and/or active TGF-β would be effective in the combat of autoimmunity. A major advantage of the present approach to control autoimmune disease is that the TGF-β1 constitutively produced in the transferred cells is latent rather than active and therefore is unlikely to have detrimental side effects. Under normal conditions, latent TGF-β is present ubiquitously, in platelet α granules (45), as well as attached to the matrix of connective tissue (46–48) and to γ-globulin in the serum of mice (49) and humans (G.J.T., C.H., and G.M.H., unpublished observations). Moreover, as the mice had been immunized with PLP, which does not cross-react with MBP, it is unlikely that any antigen-specific localization or activation of the TGF-β-producing cells occurs at sites in the body other than those where myelin is present. Apparently, when the MBP-specific T cells are exposed to antigen in the central nervous system lesions, either they are capable of activating some of their own latent TGF-β or else neighboring macrophages in the lesions cause its activation (22). It has been observed that latent TGF-β-transfected epithelial cells transplanted into nu/nu mice as well as peripheral blood cells in vitro can sometimes produce a low amount of active TGF-β (31, 50). In the present study, tissue culture medium from the activated or resting transduced T cells contained high concentrations of latent but little, if any, active TGF-β.
It has been shown that activated T cells that penetrate the blood–brain barrier during EAE have up-regulated adhesion molecules on their surfaces, such as VLA-4 and LFA-1, which influence their capacity to transfer EAE to recipient mice (51, 52). In addition, contact of microvascular endothelial cells with activated T cells causes the enhancement of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression on the endothelial cells (53). Thus, antigen stimulation of MBP-specific T cells is needed before they can either transfer EAE (52) or protect against EAE, when transduced with TGF-β as shown above. It is somewhat surprising that activated T cells of unrelated specificity (KLH or OVA), with the numbers of cells used in the present study, could not protect against EAE, even though they produced large amounts of latent TGF-β in vitro and were detectable in the spinal cord by the presence of latent TGF-β cDNA. The results suggest that, in the absence of specific antigen within the central nervous system, T cell numbers, proliferation, and/or continued localization within infiltrates during the course of the EAE must have been insufficient when compared with those of MBP-specific cells. Further experiments are needed to determine whether additional antigenic stimulation in vivo can render TGF-β-transduced T cells of unrelated specificity protective against EAE.
We conclude from these results that production of TGF-β1 confers immunomodulating properties to autoreactive T cells, allowing them to control the behavior of other inflammatory cells in their immediate vicinity. It is of interest that T cells from multiple sclerosis patients reportedly produce less TGF-β1 in culture than do T cells from normal individuals (54). We propose that the genetic engineering of self-reactive T cells with latent TGF-β may represent a clinically viable approach to the treatment of autoimmune diseases. Alternatively, other means to up-regulate the endogenous TGF-β1 production in self-reactive T cells should be explored.
Acknowledgments
We are greatly indebted to Drs. S. Abromson-Leeman and M. E. Dorf (Harvard Medical School, Boston, MA) for donating the MBP-specific T cell clone to us, and to Drs. R. H. DeKruyff and D. T. Umetsu (Stanford University School of Medicine, Stanford, CA) for donating the KLH- and OVA-specific Th1 clones. The generous gift of anti-TGF-β from Dr. B. M. Fendly (Genentech) is gratefully acknowledged. The help from Dr. D. B. Rifkin (Department of Cell Biology, New York University Medical Center) concerning the biological assay for TGF-β activity is much appreciated. This work was supported by the National Multiple Sclerosis Society (RG-2602A5).
ABBREVIATIONS
- MBP
myelin basic protein
- TGF
transforming growth factor
- PLP
proteolipid protein
- CFA
complete Freund’s adjuvant
- EAE
experimental allergic encephalomyelitis
- TNF
tumor necrosis factor
- LT
lymphotoxin
- LTR
long terminal repeat
- IFN
interferon
- IL
interleukin
- KLH
keyhole limpet hemocyanin
- OVA
ovalbumin
- Th
T helper
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1. Racke M K, Dhib-Jalbut S, Cannella B, Albert P S, Raine C S, McFarlin D E. J Immunol. 1991;146:3012–3017. [PubMed] [Google Scholar]
- 2.Kuruvilla A P, Shah R, Hochwald G M, Liggitt H D, Palladino M A, Thorbecke G J. Proc Natl Acad Sci USA. 1991;88:2918–2921. doi: 10.1073/pnas.88.7.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santambrogio L, Hochwald G M, Saxena B, Leu C H, Martz J E, Carlino J A, Ruddle N H, Palladino M A, Gold L I, Thorbecke G J. J Immunol. 1993;151:1116–1127. [PubMed] [Google Scholar]
- 4.Johns L D, Flanders K C, Ranges G E, Sriram S. J Immunol. 1991;147:1792–1796. [PubMed] [Google Scholar]
- 5.Stevens D B, Gould K E, Swanborg R H. J Neuroimmunol. 1994;51:77–83. doi: 10.1016/0165-5728(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 6.Thorbecke G J, Shah R, Leu C H, Kuruvilla A P, Hardison A M, Palladino M A. Proc Natl Acad Sci USA. 1992;89:7375–7379. doi: 10.1073/pnas.89.16.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espevik T, Figari I S, Shalaby M R, Lackides G A, Lewis G D, Shepard H M, Palladino M A., Jr J Exp Med. 1987;166:571–576. doi: 10.1084/jem.166.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pardoux C, Asselin-Paturel C, Chehimi J, Gay F, Mami-Chouaib F, Chouaib S. J Immunol. 1997;158:136–143. [PubMed] [Google Scholar]
- 9.Nelson B J, Ralph P, Green S J, Nacy C A. J Immunol. 1991;146:1849–1857. [PubMed] [Google Scholar]
- 10.Vodovotz Y, Bogdan C, Paik J, Xie Q W, Nathan C. J Exp Med. 1993;178:605–613. doi: 10.1084/jem.178.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lodge P A, Sriram S. J Leukocyte Biol. 1996;60:502–508. doi: 10.1002/jlb.60.4.502. [DOI] [PubMed] [Google Scholar]
- 12.Epstein S P, Baer R L, Thorbecke G J, Belsito D V. J Invest Dermatol. 1991;96:832–837. doi: 10.1111/1523-1747.ep12474535. [DOI] [PubMed] [Google Scholar]
- 13.Fabry Z, Topham D J, Fee D, Herlein J, Carlino J A, Hart M N, Sriram S. J Immunol. 1995;155:325–332. [PubMed] [Google Scholar]
- 14.Santambrogio L, Hochwald G M, Saxena B, Leu C H, Martz J E, Carlino J A, Ruddle N H, Palladino M A, Gold L I, Thorbecke G J. J Immunol. 1993;151:1116–1127. [PubMed] [Google Scholar]
- 15.Racke M K, Cannella B, Albert P, Sporn M, Raine C S, McFarlin D E. Int Immunol. 1992;4:615–620. doi: 10.1093/intimm/4.5.615. [DOI] [PubMed] [Google Scholar]
- 16.Johns L D, Sriram S. J Neuroimmunol. 1993;47:1–8. doi: 10.1016/0165-5728(93)90278-7. [DOI] [PubMed] [Google Scholar]
- 17.Diebold R J, Eis M J, Yin M, Ormsby I, Boivin G P, Darrow B J, Saffitz J E, Doetschman T. Proc Natl Acad Sci USA. 1995;92:12215–12219. doi: 10.1073/pnas.92.26.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crisi G M, Katz I R, Zucker M B, Thorbecke G J. Cell Immunol. 1996;172:205–216. doi: 10.1006/cimm.1996.0234. [DOI] [PubMed] [Google Scholar]
- 19.Santos L M, al-Sabbagh A, Londono A, Weiner H L. Cell Immunol. 1994;157:439–447. doi: 10.1006/cimm.1994.1240. [DOI] [PubMed] [Google Scholar]
- 20.Powrie F, Carlino J, Leach M W, Mauze S, Coffman R L. J Exp Med. 1996;183:2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakefield L M, Smith D M, Flanders K C, Sporn M B. J Biol Chem. 1988;263:7646–7654. [PubMed] [Google Scholar]
- 22.Nunes I, Shapiro R L, Rifkin D B. J Immunol. 1995;155:1450–1459. [PubMed] [Google Scholar]
- 23.Clouthier D E, Comerford S A, Hammer R E. J Clin Invest. 1997;100:2697–2713. doi: 10.1172/JCI119815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abromson-Leeman S, Bronson R, Dorf M E. J Exp Med. 1995;182:587–592. doi: 10.1084/jem.182.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rizzo L V, DeKruyff R H, Umetsu D T. J Immunol. 1992;148:3733–3739. [PubMed] [Google Scholar]
- 26.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan R C. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danos O, Mulligan R C. Proc Natl Acad Sci USA. 1988;85:6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartman S C, Mulligan R C. Proc Natl Acad Sci USA. 1988;85:8047–8051. doi: 10.1073/pnas.85.21.8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cepko C, Pear W. In: Current Protocols in Molecular Biology. Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Vol. 1. New York: Wiley; 1997. pp. 9.14.11–9.14.16. [Google Scholar]
- 30.Zhang D J, Tsiagbe V K, Huang C, Thorbecke G J. J Immunol. 1996;157:3510–3517. [PubMed] [Google Scholar]
- 31.Lucas C, Bald L N, Fendly B M, Mora-Worms M, Figari I S, Patzer E J, Palladino M A. J Immunol. 1990;145:1415–1422. [PubMed] [Google Scholar]
- 32.Abe M, Harpel J G, Metz C N, Nunes I, Loskutoff D J, Rifkin D B. Anal Biochem. 1994;216:276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- 33.Shaw M K, Lorens J B, Dhawan A, DalCanto R, Tse H Y, Tran A B, Bonpane C, Eswaran S L, Brocke S, Sarvetnick N, et al. J Exp Med. 1997;185:1711–1714. doi: 10.1084/jem.185.9.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathisen P M, Yu M, Johnson J M, Drazba J A, Tuohy V K. J Exp Med. 1997;186:159–164. doi: 10.1084/jem.186.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lafaille J J, Nagashima K, Katsuki M, Tonegawa S. Cell. 1994;78:399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 36.Lafaille J J, Keere F V, Hsu A L, Baron J L, Haas W, Raine C S, Tonegawa S. J Exp Med. 1997;186:307–312. doi: 10.1084/jem.186.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santambrogio L, Crisi G M, Leu J, Hochwald G M, Ryan T, Thorbecke G J. J Neuroimmunol. 1995;58:211–222. doi: 10.1016/0165-5728(95)00022-t. [DOI] [PubMed] [Google Scholar]
- 38.Rott O, Fleischer B, Cash E. Eur J Immunol. 1994;24:1434–1440. doi: 10.1002/eji.1830240629. [DOI] [PubMed] [Google Scholar]
- 39.Maeda K, Kosco-Vilbois M H, Burton G F, Szakal A K, Tew J G. Cell Tissue Res. 1995;279:47–54. doi: 10.1007/BF00300690. [DOI] [PubMed] [Google Scholar]
- 40.Fiorelli G, Ballock R T, Wakefield L M, Sporn M B, Gori F, Masi L, Frediani U, Tanini A, Bernabei P A, Brandi M L. J Cell Physiol. 1994;160:482–490. doi: 10.1002/jcp.1041600311. [DOI] [PubMed] [Google Scholar]
- 41.Rich S, Seelig M, Lee H M, Lin J. J Immunol. 1995;155:609–618. [PubMed] [Google Scholar]
- 42.Quere P, Thorbecke G J. Cell Immunol. 1990;129:468–477. doi: 10.1016/0008-8749(90)90221-c. [DOI] [PubMed] [Google Scholar]
- 43.Oswald I P, Gazzinelli R T, Sher A, James S L. J Immunol. 1992;148:3578–3582. [PubMed] [Google Scholar]
- 44.Wahl S M, Allen J B, Costa G L, Wong H L, Dasch J R. J Exp Med. 1993;177:225–230. doi: 10.1084/jem.177.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fava R A, Casey T T, Wilcox J, Pelton R W, Moses H L, Nanney L B. Blood. 1990;76:1946–1955. [PubMed] [Google Scholar]
- 46.Evanko S P, Raines E W, Ross R, Gold L I, Wight T N. Am J Pathol. 1998;152:533–546. [PMC free article] [PubMed] [Google Scholar]
- 47.Heine U I, Wahl S M, Munoz E F, Allen J B, Ellingsworth L R, Flanders K C, Roberts A B, Sporn M B. Arch Geschwulstforsch. 1990;60:289–294. [PubMed] [Google Scholar]
- 48.Munger J S, Harpel J G, Gleizes P E, Mazzieri R, Nunes I, Rifkin D B. Kidney Int. 1997;51:1376–1382. doi: 10.1038/ki.1997.188. [DOI] [PubMed] [Google Scholar]
- 49.Rowley D A, Becken E T, Stach R M. J Exp Med. 1995;181:1875–1880. doi: 10.1084/jem.181.5.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wallick S C, Figari I S, Morris R E, Levinson A D, Palladino M A. J Exp Med. 1990;172:1777–1784. doi: 10.1084/jem.172.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barten D M, Clark R B, Ruddle N H. J Immunol. 1995;155:5409–5418. [PubMed] [Google Scholar]
- 52.Kuchroo V K, Martin C A, Greer J M, Ju S T, Sobel R A, Dorf M E. J Immunol. 1993;151:4371–4382. [PubMed] [Google Scholar]
- 53.Lou J, Dayer J M, Grau G E, Burger D. Eur J Immunol. 1996;26:3107–3113. doi: 10.1002/eji.1830261242. [DOI] [PubMed] [Google Scholar]
- 54.Mokhtarian F, Shi Y, Shirazian D, Morgante L, Miller A, Grob D. J Immunol. 1994;152:6003–6010. [PubMed] [Google Scholar]