Abstract
We performed a comprehensive analysis of T cell receptor (TCR) γ rearrangements in T cell precursors of the mouse adult thymus. Using a sensitive quantitative PCR method, we show that TCRγ rearrangements are present in CD44+CD25+ Pro-T thymocytes much earlier than expected. TCRγ rearrangements increase significantly from the Pro-T to the CD44−CD25+ Pre-T cell transition, and follow different patterns depending on each Vγ gene segment, suggesting that ordered waves of TCRγ rearrangement exist in the adult mouse thymus as has been described in the fetal mouse thymus. Recombinations of TCRγ genes occur concurrently with TCRδ and D-Jβ rearrangements, but before Vβ gene assembly. Productive TCRγ rearrangements do not increase significantly before the Pre-T cell stage and are depleted in CD4+CD8+ double-positive cells from normal mice. In contrast, double-positive thymocytes from TCRδ−/− mice display random proportions of TCRγ rearranged alleles, supporting a role for functional TCRγ/δ rearrangements in the γδ divergence process.
Most αβ and γδ T cells develop in the thymus through a complex developmental program leading to the generation of a specific T cell receptor (TCR) repertoire. The diversity of this repertoire is insured by the expression of distinct TCR α/β or γ/δ chains and by their variable region encoded by several variable (V), joining (J), and diversity (D) gene segments (1). The diversity at the TCRγ locus (limited by no D, and only a few V/J segments) is increased by the presence of three functional C regions. Relative to the expressed Vγ-Jγ chain, γδ T cells have been subdivided into distinct subsets. Overall, cells of the γδ lineage (which predominate during the fetal life) represent only ≈1% of the thymic population in the adult mouse. Adult γδ thymocytes mostly express Vγ2/4-Jγ1, Vγ1.1/1-Jγ4, and Vγ1.2/2-Jγ2 chains, also found in peripheral lymphoid organs (for nomenclature see Fig. 1). The Vγ5/7 subset (whose origin is still obscure) is preferentially found in intestinal intraepithelial lymphocytes (for reviews, see refs. 2 and 3).
Figure 1.
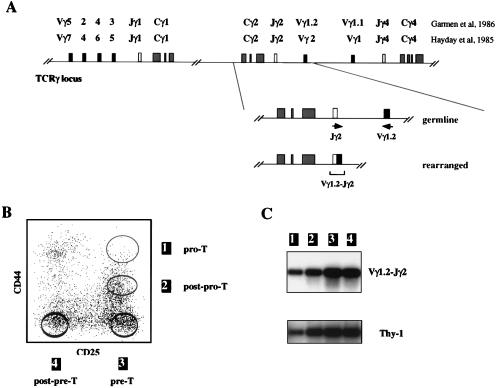
Analysis of Vγ1.2-Jγ2 rearrangements in early thymic progenitors. (A) Schematic representation of the TCRγ locus and of the Vγ1.2-Jγ2 PCR assay. The name of the Vγ, Jγ segments according to the two different nomenclatures are indicated, as is the position of the oligonucleotide primers utilized for the Vγ1.2-Jγ2 amplification reaction. (B) Sort scheme used for obtaining the four CD44+CD25+ Pro T, CD44+/−CD25+ post-Pre, CD44−CD25+ Pre-T, and CD44−CD25− post-Pre-T thymocyte subsets. The latter were purified from TN thymocytes after staining with anti-CD44 and anti-CD25 antibodies, as described in Materials and Methods. Sort purities were at least 99.9% upon reanalysis (>99.95% for CD44+CD25+ thymocytes). The corresponding Pro-T, post-Pro-T, Pre-T, and post-Pre-T nomenclature used in the text is shown. (C) PCR analyses of Vγ1.2-Jγ2 rearrangements were performed for the four populations indicated in B. The amount of amplified Thy-1 product was representative of the quantity of input DNA.
The different subtypes of γδ cells as well as the low frequency of adult γδ thymocytes represent obstacles in our understanding of γδ T cell development. Although it is known that γδ lineage divergence is an early event, it remains to be defined at which cell stage γδ cells leave the triple negative (TN) step of the adult αβ differentiation pathway. TN thymocytes have been subdivided into four subpopulations according to the expression of the CD25 and CD44 antigens, which progress from the CD44+CD25+ (Pro-T) to the CD44−CD25+ (Pre-T) cell stage, prior to becoming CD44−CD25− post-Pre T thymocytes (reviewed in refs. 4 and 5). Our initial study culturing CD25+ TN thymocytes in vitro (which include both the Pro-T and Pre-T subsets) suggested that most γδ T cells split before or at the pre-T cell stage (6). In contrast, Petrie et al. described αβ/γδ divergence as a relative late event (i.e., at the CD25− TN cell stage) (7). An alternate way to study this may be to follow progenitor cells through analysis of their TCR variable gene segments, which conserve their configuration once assembled.
TCR gene rearrangements occur at the beginning of the differentiation pathway, in a highly ordered fashion. Recombinations of TCR genes are sequential between the different loci as well as within a particular locus. Thus, recombination of TCRβ, γ, and δ genes precede TCRα rearrangements. At the TCRγ locus, previous studies in the embryo have shown that within the Vγ/Jγ1/Cγ1 cluster, Vγ segments are assembled from the proximal Vγ3/5 to the distal Vγ2/4 segments, following a pattern which mirrors the waves of appearance of the different subtypes of γδ cells (2, 8–10). The existence of such regulation is unknown in the adult thymus, and the exact order of TCRγ, δ, and β rearrangements remains to be established. Since the latter events happen in the same time frame as lineage commitment, it has been postulated that the outcome of the recombination step plays a pivotal role in the γδ/αβ lineage decision. However, the attempts to determine the proportion of in-frame/out-of-frame TCRδ rearrangements in adult αβ lineage cells yielded controversial results, either in support or against a hierarchical scheme of successive gene rearrangements for γδ/αβ divergence (11, 16).
In an effort to complete some of the missing steps of murine γδ T cell development, we performed a comprehensive (qualitative and quantitative) PCR analysis of TCRγ recombination in adult thymic progenitors. Our results highlight the tight regulation of TCRγ recombination/expression in the life of developing T cells and draw parallels between these events in the fetal and adult thymus which, in retrospect, appear logical. Our observations have important implications for the understanding of the γδ versus αβ lineage decision.
MATERIALS AND METHODS
Mice.
C57BL/6 normal, TCRδ−/−, and RAG-1−/− mice were obtained from The Jackson Laboratory. All animals were maintained in germ-free condition and analyzed at 3–5 wk of age.
Cell Isolation, Staining, and Sorting.
Total thymocytes were prepared from single-cell thymus suspensions. For isolation of double negative cells, total thymocytes were depleted of CD4+ and CD8+ cells by incubation with anti-CD4 (clone RL172) and anti-CD8 (clone AD4, Cedarlane Laboratories, Hornby, Ontario), followed by treatment with low-tox M rabbit complement (Cedarlane) and 20 mg/ml DNase I (Sigma). Viable cells were isolated with Histopaque 1083 (Sigma). Pro-T, post-Pro-T, Pre-T, and post-Pre-T subsets were sorted from double negative thymocytes through a lineage-negative gate after staining with a panel of biotinylated lineage antibodies directed against CD4, CD8, B220, Mac-1, Gr-1, Ter-119 and CD3, anti-CD25 FITC, and anti-CD44 PE. γδ cells were sorted from double negative cells through a Lin− gate after staining with biotinylated anti-CD4, -CD8, -B220, -Mac-1, -Gr-1, -Ter-119, and -γδ fluorescein isothiocyanate. Double positive (DP) cells were sorted from total thymocytes through a CD3low gate, after staining with biotinylated anti-CD3, anti-CD4 phycoerithrin, and anti-CD8 fluorescein isothiocyanate. After washing, cells were incubated with streptavidin-Tri-Color (Caltag, South San Francisco, CA). The preceding antibodies were obtained from PharMingen. Sorts were performed using a FACStar Plus or a FACS Vantage Flow Cytometer, yielding a purity >99.9% (Becton Dickinson). Only highly purified (>99.95%) Pro-T cells were used, often achieved after a second round of purification.
Qualitative Analyses of TCR Rearrangements.
Pellets of 20,000–50,000 thymocytes were resuspended in 20–50 μl of lysis buffer [10 mM Tris, pH 8.4, 2.5 mM MgCl2, 50 mM KCl, 200 mg/ml gelatin, 0.45% Nonidet P-40, and 0.45% Tween 20] and incubated for 5 min at 95°C and for 30 min at 56°C in the presence of proteinase K (60 μg/ml). After inactivation of the latter enzyme, 2 μl of the preceding suspension were amplified with a Perkin–Elmer/Cetus DNA thermal cycler for 30–35 cycles (1 min at 94°C, 0.5 min at 55°C, and 2 min at 72°C) in a volume of 50 μl containing 0.25 mM of the following primers pairs: Vγ1.2: 5′-GTGCAAATATCCTGTATAGTT-3′ and Jγ2: 5′-ACAGTAGTAGGTGGCTTCAC-3′; Vγ1.1: 5′-GAGAGTGCGCAAATATCCTGTATA-3′ and Jγ4: 5′-TGGGGGAATTACTACGAGCT-3′; Vγ2/4: 5-TATGTCCTTGCAACCCCTAC-3 and Jγ1: ATGAGCTTAGTTCCTTCTGC-3′; Vγ5/7: 5′-GTTTATGAAGGCCCGGACA-3′ and Jγ1; Vγ4/6: 5′-ACAAGTGTTCAGAAGCCCGA-3′ and Jγ1; Vγ3/5:5′-TGGATATCTCAGGATCAGCT-3′ and Jγ1; Vδ5: 5′-CAGATCCTTCCAGTTCATCC-3′ and Jδ1: 5′-CAGTCACTTGGGTTCCTTGTCC-3′; Vδ4: 5′-CCGCTTCTCTGTGAACTTCC-3′ and Jδ1; Dβ2.1: 5′-AGGACATCTCCAAGCTC-3′ and Jβ2.3: 5′-TCCTGAGCCAAAATACAGCGA-3′; Vβ3: 5′-GCTACAAGGCTCCTCTG-3′ and Jβ2.3; Vβ8: 5′-GGTGACATTGAGCTGT-3′ and Jβ2.3. After amplification, the identity of the PCR products was confirmed by sequencing. Vγ1.1-Jγ4 rearrangements were not investigated further, since sequencing of Vγ-Jγ fragments amplified with two different primer pairs [up above, or based on published sequences (14)] revealed simultaneous amplification of TCRγ rearrangements using other Vγ gene segments, with predominance of Vγ2/4 (not shown).
For PCR Southern blot analyses, the amplified fragments were separated on 1.2% agarose. Relative amounts of input DNA were visualized by amplification of the Thy-1 gene using the 5′ Thy-1: 5′-CCATCCAGCATGAGTTCAGC-3′ and 3′ Thy-1: 5′-CTTGACCAGCTTGTCTGTGG-3 primers. TCRγ/δ rearrangements were detected with the following 32P primer probes: Jγ1.2: 5′-GCAGAAGGAACAAAGCTCATAGTAATTCCCTC-3′; Jγ1: 5′-AGCTCAGGTTTTCACAAGGTATTT-3′; Jδ1: 5′-GTTCCTTGTCCAAAGACGAGTT-3′. TCRβ rearrangements and Thy-1 amplified fragments were analyzed with 32P DNA probes prepared using the Dβ2.1/Jβ2.3 primers and the 5′ Thy-1/3′Thy-1 primers, respectively.
For cloning and sequencing, fragments amplified with the preceding primers were purified using QIAEX extraction (Qiagen, Chatsworth, CA), cloned using the TA cloning kit (Invitrogen), and sequenced using either T7 or specific Vγ primers and automatic sequencing.
Quantitative Analyses of TCR Rearrangements.
The numbers of Vγ-Jγ/Vδ-Jδ/Dβ-Jβ rearranged alleles in the different thymic subsets were measured by a modification of the enzyme immunoassay procedure utilized earlier by Hockett et al. (15) to quantitate cytokine mRNAs. New competitors were constructed by cloning appropriate DNA sequences (i.e., homologous to the TCR primer pairs) into pQPCRMCC7, instead of the cytokine primers. The IL10-specific sequences were maintained to control the numbers of alleles within each preparation and the length of the “stuffer” chosen so that amplified fragments were about the same size (300–500 bp) for both analyte and competitor.
The proportion of Pro-T cells carrying TCR rearranged alleles was estimated based on the numbers of TCR D-Jβ/Vγ-Jγ/Vδ-Jδ amplified fragments present in these cells. The latter were about 50 per 50,000 IL10 alleles, i.e., 50 per 25,000 cells (Table 1). Since isotypic exclusion does not occur at the TCRγ locus (16), one Pro-T cell could bear each of the five alleles (Vδ5-Jδ1/Dβ2.1-Jβ2.3/Vγ2/4-Jγ1/Vγ1.2-Jγ2/Vγ1.1-Jγ4) found rearranged in Pro-T cells; thus, the proportion of cells carrying TCR rearranged genes is ≈250 (5 × 50) per 25000 Pro-T cells, i.e., 1/100. Although the preceding calculations do not consider other Vδ segments, we estimate that their participation is limited since Vδ5 is the most utilized by adult thymocytes.
Table 1.
Quantitation of TCR rearrangements in sorted Pro-T and Pre-T cell populations
| Rearrangements | Populations
|
|
|---|---|---|
| CD44+CD25+(Pro-T) | CD44−CD25+(Pre-T) | |
| Number of rearranged alleles (per 50,000 IL10 alleles) | ||
| Vγ1.2-Jγ2 | 59 | 1,200 |
| Vγ5/7-Jγ1 | 4 | 430 |
| Vγ2/4-Jγ1 | 44 | 15,000 |
| Vδ5-Jδ1 | 70 | 73 |
| Dβ2.1-Jβ2.3 | 50 | 522 |
For our calculation of the alleles 1 and 2, the interassay variability (variability of the PCR assay) was measured in six of the Pro-T and Pre-T samples and was found to be in the range of 5–10% as described (15). The data in Tables 1 and 2 represent the mean number of rearrangements from different sorts of Pro-T and Pre-T thymocytes.
Table 2.
Quantitation of Vγ-Jγ1 rearrangements in sorted T cell progenitors
| Population | Rearrangements
|
|
|---|---|---|
| Vγ5/7-Jγ1 | Vγ2/4-Jγ1 | |
| Number of rearranged alleles (per 50,000 IL10 alleles) | ||
| CD44+CD25+ (Pro-T) | 4 | 44 |
| CD44−CD25+ (Pre-T) | 430 | 15,000 |
| CD44−CD25− (post-Pre-T) | 510 | 12,000 |
| DPCD3low | 435 | 6,900 |
RESULTS
TCRγ Rearrangements Occur at the Earliest Stages of Adult Thymic Development.
We have shown by Southern blot analyses that murine CD44+CD25+ Pro-T thymocytes have their TCRγ locus in germ-line configuration, while CD44−CD25+ Pre-T cells already display significant proportions of Vγ-Jγ rearranged alleles (17). To define more precisely at which moment TCRγ rearrangements commence during thymic development in the adult mouse, various subsets of T cell progenitors were tested by PCR for the presence of Vγ-Jγ rearranged fragments. As shown in Fig. 1A, the Vγ1.2-Jγ2-Cγ2 cluster was chosen for initial studies. The previously defined Pro-T, Pre-T, and post-Pre-T cells were FACS sorted after CD25/CD44 staining of TN thymocytes (Fig. 1B). To avoid the presence of more mature contaminants, Pro-T cells were purified through a CD44hiCD25hi gate (Fig. 1B, population 1); only cells from sorts yielding a purity >99.95% were used for the PCR reaction. Finally, we purified a subpopulation of CD25+ thymocytes expressing intermediate levels of CD44, hereafter referred to as post-Pro-T cells (Fig. 1B, population 2). Representative data are shown in Fig. 1C. Vγ1.2-Jγ2 rearranged alleles were detected in all of the populations tested including post-Pro-T cells and, surprisingly, the Pro-T cell subset.
Kinetics of TCRγ/δ Gene Rearrangements During Adult Thymic Development.
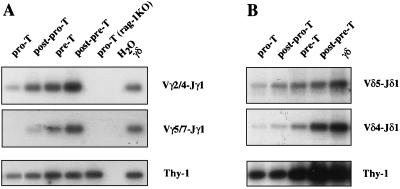
The presence of TCRγ rearrangements before the CD44−CD25+ Pre-T cell stage is an unexpected finding, and there is a possibility that it could be restricted to the Vγ1.2-Jγ2-Cγ2 cluster. We therefore extended our PCR analyses to other loci, beginning with the Vγ-Jγ1-Cγ1 cluster. As expected from their predominance during fetal life (1), recombinations of the more 3′ (Vγ4/6 and Vγ3/5, Fig. 1A) genes were low in adult triple negative (TN) thymocytes (not shown). In contrast, the genes with a more 5′ location (Vγ2/4 and Vγ5/7) were extensively rearranged, but the profile observed was different depending on the Vγ segment used (Fig. 2A). Although Vγ2/4-Jγ1 rearrangements were detected even in Pro-T cells, Vγ5/7-Jγ1 rearrangements were rare in post-Pro-T cells and not detectable at all in Pro-T cells. In Pre-T cells, the intensity of the Vγ-Jγ amplified fragments was within the same range (for Vγ2/4) but lower (for Vγ5/7) than the one found in γδ thymocytes. In subsequent cell stages, our analyses confirmed the presence of significant numbers of rearranged TCRγ loci in αβTCR-committed thymocytes (Fig. 2A). The recombination kinetics of the Vγ1.1-Jγ4-Cγ4 cluster followed the kinetics of Vγ2/4-Jγ1 rearrangements (data not shown), but were not examined further for technical reasons (see Materials and Methods).
Figure 2.
Sequential rearrangements of TCRγ (A) and TCRδ (B) genes during early thymic development. The various V-J PCR assays were performed using different aliquots of the same DNA suspension prepared from each of the four early thymic populations described, as well as for DP cells for TCRδ rearrangements. Southern blots of the products were probed with labeled primer probes specific to each Vγ/δ gene segment.
Considering that mature γδ TCRs frequently express Vγ2/4-Jγ1/Vδ5-Jδ1 and Vγ5/7-Jγ1/Vδ4-Jδ1 dimers (18), we next studied the kinetics of Vδ5- and Vδ4-Jδ1 rearrangements during adult thymic development. We included the CD4+CD8+ DP subset in our study, since most TCRδ loci are retained in αβ T cells and contain complete V-D-Jδ1 rearrangements involving Vδ5 and Vδ4 genes (11). Accordingly, Vδ5- and Vδ4-Jδ1 rearrangements were abundant in post-Pre-T and DP thymocytes (Fig. 2B). As in the case of TCRγ genes, the intensity of the Vδ-Jδ1 rearranged fragments within the TN subsets varied depending on the Vδ segment (Vδ4 or Vδ5) used; however, TCRδ rearrangements (for either Vδ4 or Vδ5) were detected at the Pro-T cell stage (Fig. 2B).
Kinetics of D-Jβ Gene Rearrangements During Adult Thymic Development.
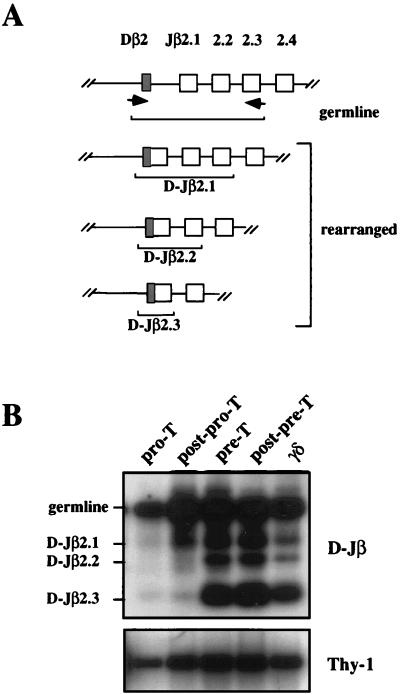
The presence of TCRγ rearrangements in CD44+CD25+ thymocytes suggested that TCRγ/δ rearrangements may precede recombinations of TCRβ genes, an important observation with implications in the developmental fate of αβ/γδ uncommitted progenitors. To address this issue, thymocytes were tested for the presence of TCRβ rearranged alleles. In the adult thymus, TCRβ genes are rearranged in the sequential order D-Jβ, followed by Vβ-DJβ (19, 35). Accordingly, recombinations of currently used Vβ genes (Vβ3, Vβ8) were not detectable in Pro-T cells (not shown). We then investigated the D-Jβ recombination step. Our analysis at the Cβ2 cluster is shown as an example. The PCR assay used allows the simultaneous amplification of both unrearranged and partially (D-J) rearranged TCRβ alleles (Fig. 3A) before assembly of Vβ genes (which would delete the upstream primer). While the strong intensity of the unrearranged fragment confirmed that the vast majority of TCRβ2 variable genes are still in germ-line configuration in Pro-T cells (17), a long exposure of our “PCR blot” revealed that some D-Jβ2 rearrangements had indeed occurred at this early cell stage (Fig. 3B). Moreover, the relative proportion of rearranged D-Jβ2 versus germ-line–amplified fragments increased drastically as cells proceed from the Pro-T to the Pre-T cell stage, but only slightly from the Pre-T to the post-Pre-T cell stage transition [when significant amounts of Vβ-DJβ rearrangements occur (17)]. Finally, the pattern of rearranged versus germ-line D-Jβ2 alleles in sorted γδ thymocytes was similar (although slightly higher) than the one found in Pre-T cells (Fig. 3B and lower exposure of the same blot).
Figure 3.
Analysis of TCR D-Jβ rearrangements during early thymic development. (A) Schematic representation of the PCR assay. The position of the primers as well as the different fragments amplified are shown. (B) Represents a long exposure of the blot obtained as described in experimental procedures after applying the assay described in A on DNA purified from the different early thymic subsets.
Quantitation of TCR Gene Rearrangements in Early Thymic Progenitors.
To further evaluate Vγ gene assembly in T cell progenitors, Vγ-Jγ rearranged alleles were quantitated in different samples of highly purified thymocytes using a modification of an enzyme immunoassay-based method (15, 20). Different aliquots of extracted DNA were incubated with increasing concentrations of a DNA competitor, which contained sequences homologous to specific Vγ/Jγ primer pairs; the number of alleles in each preparation was assessed by simultaneous amplification with 5′/3′ IL10 oligonucleotides. As shown in Table 1, low but significant proportions of Vγ1.2-Jγ2- and Vγ2/4-Jγ1 rearranged alleles were present in Pro-T cells, whereas Vγ5/7-Jγ1 rearranged alleles were barely detectable at this early cell stage. We then quantitated the number of Vδ5-Jδ1 rearranged alleles in Pro-T cells, which are likely to be associated with Vγ1.2-Jγ2 and Vγ2/4-Jγ1 proteins (18, 21). These results confirmed that, as is the case for TCRγ genes, assembly of TCRδ variable segments occurs at a significant frequency in CD44+CD25+ thymocytes.
Consecutive analyses in Pre-T cells revealed that the number of Vγ-Jγ amplified fragments increased in intensity relative to the locus considered, leading to an overrepresentation of Vγ2/4-Jγ1 rearranged alleles (Table 1). In particular, the high frequency of Vγ2/4-Jγ1 (15,000), compared with the low proportion (430) of Vγ5/7-Jγ1 amplified fragments in Pre-T cells (Table 1), suggests that at the Vγ-Jγ1-Cγ1 cluster, Vγ2/4 rearrangements are favored. To confirm this, we performed quantitative analyses in subsequent cell stages. As shown in Table 2, the numbers of Vγ2/4-Jγ1 rearranged alleles decreased after the Pre-T cell stage, but still remained very abundant compared with Vγ5/7-Jγ1 rearrangements. Taken together, these observations as well as previous data (22) suggest that most γδ T cells have diverged from the main αβ pathway by the Pre-T cell stage.
Productively Rearranged Vγ2/4 Genes and Developmental Fate of γδ T Cells.
Given their predominance during adult thymic development, we investigated the influence of productive TCRγ2/4 rearrangements in the fate of γδ progenitors. To this end, we cloned and sequenced Vγ2/4-Jγ1 amplified alleles from different subsets of adult thymocytes. The latter method was chosen instead of the PCR-restriction fragment-length polymorphism technique (23) because of the presence of a stop codon near the 3′ end of the Vγ2/4 gene (24).
We first questioned why TCRγ2/4 rearrangements decreased after the Pre-T cell stage, by sequencing some Vγ2/4-Jγ1 alleles amplified from “early” DP thymocytes (those expressing low levels of the CD3). The results are summarized in Table 3. From a total of 50 clones sequenced, 10 of the 12 (i.e., 83%) in-frame rearrangements were unproductive. This implies a dramatically low proportion (4%) of productive Vγ2/4-Jγ1 alleles in DP thymocytes from normal mice; in contrast, DP thymocytes from TCRδ−/− mice displayed a much higher percentage (16%) of Vγ2/4-Jγ1 rearranged alleles (Table 3). These results indicate that productive γδ rearrangements lead to a “split” of γδ T cells from the main αβTCR pathway.
Table 3.
Sequence analysis of Vγ2/4-Jγ1 rearrangements in sorted T cell progenitors
| Mice | Population | In-frame rearrangements
|
|||||
|---|---|---|---|---|---|---|---|
| Total
|
With stop codons
|
Productive rearrangements
|
|||||
| Clones | % | Clones | % | Clones | % | ||
| CONTROL | post-Pro-T | 14/15 | 25 | 7/13 | 54 | 6/56 | 11 |
| Pre-T | 13/55 | 24 | 5/13 | 38 | 8/55 | 15 | |
| DPCD3low | 12/50 | 24 | 10/12 | 83 | 2/50 | 4 | |
| TCRδKO | Pre-T | 16/57 | 28 | 10/16 | 62 | 6/57 | 11 |
| DPCD3low | 16/49 | 33 | 8/16 | 50 | 8/49 | 16 | |
We next considered the potential existence of a “TCRγ selection step” during early thymic development. We hypothesized that if the latter exists, it might happen during the time frame when TCRγ rearrangements increase extensively, that is, between the Pro- to the Pre-T cell stages for thymocytes expressing Vγ2/4 genes. We cloned and sequenced Vγ2/4-Jγ1 fragments amplified from Pre-T cells as well as their immediate post-Pro-T precursors. As shown in Table 3, the percentage of Vγ2/4-Jγ1 productive sequences in Pre-T cells (≈15%) was not significantly different from the one found in post-Pro-T cells (≈11%). Finally, Pre-T cells from TCRδ−/− mice also displayed a similar amount (≈11%) of productive TCRγ2/4 rearrangements. These results do not support the existence of a TCRγ-dependent selection event at this point.
DISCUSSION
The experiments described here were undertaken to explore the maturation pathway of adult γδ lineage cells and to clarify their developmental relationship with αβ T cells. To our knowledge, this is the first study exploring the kinetics of TCRγ, δ, and β gene assembly during early thymic development in the adult mouse. Using a sensitive enzyme immunoassay-based PCR methodology for our study, we were able to detect some TCRγ/δ rearranged alleles as early as the CD44+CD25+ Pro-T cell stage, indicating a primary developmental event in favor of γδ T cells. In addition, we showed that Vγ/δ gene assembly is highly regulated at both the qualitative and quantitative levels and that TCRγ/δ gene rearrangements follow different patterns depending on each particular Vγ/δ gene segment. These findings support a model with multiple points of γδ T cell divergence during early T cell development, a conclusion that makes it difficult to detect the potential existence of a TCRγ selection event or to pinpoint a particular step for γδ lineage divergence.
Our study demonstrates that TCRγ rearrangements occur much earlier than expected, at the CD44+CD25+ Pro-T cell stage. Earlier Southern blot analyses did not detect TCR gene rearrangements in adult mouse CD44+CD25+ Pro-T thymocytes (4). Although the use of PCR increases the probability of detecting false positives, the detection of Vγ1.2-Jγ2 rearranged alleles in Pro-T cells (Fig. 1C) cannot be explained by contamination by more mature cells. Given the purity of the sort (>99.95%), the number of contaminants would be limited to 1 cell per 2000 Pro-T cells sorted. This contaminant would likely be a CD44−CD25+ Pre-T cell given our sort conditions, which would represent 0.05 (1,200/25,000) Vγ1.2-Jγ2 rearranged fragments (Table 1). This small number of events lies under the limit of detection of our PCR-blotting assays, since the theoretical number of 0.3 (8,000/25,000) Vγ5/7-Jγ1 rearranged alleles present in 2,000 Pro-T cells was not detected (Table 1 and Fig. 2A). In subsequent studies, we showed that the presence of TCR rearrangements in Pro-T cells is not restricted to TCRγ genes. Moreover, these cells display similar frequencies of TCRδ and D-Jβ rearranged alleles (Table 1). Taken together, our results show the presence of TCR rearrangements in early CD44+CD25+ckit++ Pro-T cells.
The presence of some Vγ-Jγ/Vδ-Jδ rearranged alleles in CD44+CD25+ckit++ Pro-T thymocytes has important implications for early T cell differentiation. In contrast to the multipotent CD4low cells, Pro-T cells can no longer generate B cells in vivo, indicating that a further differentiation step has occurred (25, 26). As a consequence, the progeny of Pro-T cells in fetal thymus organ cultures belongs mostly to the T cell lineage (25). Based on the number of TCR-rearranged alleles in Pro-T cells (50/25,000), we estimate that the proportion of cells carrying TCR-rearranged genes is ≈1/100 Pro-T cells or 10/1,000 Pro-T cells used for seeding the fetal thymus organ culture (25). Since as few as 10–100 cells are sufficient to efficiently repopulate fetal thymus organ culture (25), we conclude that the repopulation observed is mediated by Pro-T cells that, while having their TCR genes in germ-line configuration, have already undergone at least partial commitment to the T cell lineage. Thus, the presence of TCR rearrangements in Pro-T cells is more likely a consequence of T cell lineage commitment rather than its cause. The latter conclusion is supported by the observation that in genetically manipulated mice, T cells can follow developmental programs different from the one expected from the expressed TCR (27, 28). In support of this conclusion, recent evidence suggests that the functional and phenotypic characteristics of T cells are determined by the activation of TCR-independent signals (29).
Our study also addressed the regulation of TCRγ/δ gene assembly during adult thymic development. Our results indicate that Vγ5/7-Jγ1 rearrangements are delayed when compared with Vγ2/4-Jγ1 rearrangements. The number of Vγ-Jγ1 amplified fragments reaches a maximum at the Pre-T cell stage for Vγ2/4 genes (Table 2), when they reached an intensity comparable to the one observed in sorted γδ thymocytes (Fig. 2A). In contrast, Vγ5/7-Jγ1 rearrangements increase as thymocytes progress from Pre- to post-Pre-T cells (Table 2). Indeed, assembly of Vγ5/7 genes is likely not complete at the Pre-T cell stage, since the intensity of Vγ5/7-Jγ1 amplified fragments in the latter cells was lower than the one found in mature γδ thymic cells (Fig. 2A). These patterns of TCRγ rearrangement suggest the presence of waves of TCRγ rearrangement in the adult thymus similar to those observed during fetal γδ T cell development (30). Our quantitative analyses also provide point to a γδ developmental program which favors the production of the Vγ2/4 subset, which is common among peripheral T cells (31). An extrathymic differentiation program may have evolved to overcome the deficiency in the production of the thymic Vγ5/7 subset.
A corollary of the existence of waves of TCRγ rearrangements is that γδ T cells may split from the main αβ pathway at different points during T cell development. Accordingly, rearrangements of the more common Vγ2/4 genes decrease during the Pre-T/DP transition, while successfully rearranged Vγ2/4 sequences are used (Tables 2 and 3). In contrast, the lower proportion of Vγ5/7-Jγ1 rearranged alleles in Pre-T cells (compared with post-Pre-T and γδ thymocytes) suggests a delayed branch point for this particular subset (Fig. 2A and Table 2). Taken together, these results strongly suggest that most γδ lineage divergence has occurred by the Pre-T cell stage, i.e., in the same time frame as the β selection step, but before the differentiation to DP.
The existence of TCRβ selection was revealed by an increase in in-frame Vβ-DJβ sequences during early thymic development (14). In contrast, the potential existence of a TCRγ selection mechanism remains obscure. We did not detect any significant increase in productive TCRγ sequences in the major subsets of TN thymocytes. Another study has attempted to define a TCRγ selection step, but in adult TCRβ−/− mice (32). While its physiological relevance remains to be proven, the latter study described an increase in in-frame TCRγ/δ sequences during early thymic development. However, analyses of Vγ2/4-Jγ1 rearrangements were performed by the PCR-restriction fragment-length polymorphism technique, which cannot detect the existence of stop codons. Moreover, the increase in in-frame sequences was observed in different cell types relative to the Vγ/δ segment analyzed. Our results pointing to a sequential program for Vγ/δ genes assembly provide an alternate explanation for these findings. In any case, if thymocytes are somehow selected by productively rearranged TCRγ genes in TCRβ−/− mice, it is likely to be in conjunction with functional TCRδ genes, as we showed by analyzing the CD4+CD8+ thymocytes of normal and TCR δ−/− mice. Thus, the increase in in-frame TCRγ/δ sequences during adult thymic development, if real, might simply reflect enhanced survival of cells expressing functional γδ TCR.
We also found evidence for an active role of the productively rearranged γδ chains in γδ T cell divergence from the main αβ T cell pathway. The proportion of productive TCRγ2/4 rearrangements in DP cells from normal mice was only 4%, in agreement with a previous study performed in mature αβ T cells (24). In contrast, DP from TCRδ−/− mice displayed 16% of productively rearranged Vγ2/4-Jγ1 alleles (Table 3), a proportion equivalent to the 18% defined previously for randomness (33). Taken together, these results support a model where successfully rearranged TCRγ and δ genes are required to divert precursors from the αβ toward the γδ lineage (11, 12). Conversely, productive TCRβ rearrangements favor the differentiation of αβ lineage cells (23, 34), leading to a competitive/sequential model where TCRγ/δ versus β rearrangements influence γδ versus αβ lineage divergence. Our observation that Pro-T cells exhibit D-Jβ but not V-D-Jβ rearranged alleles (Table 1) suggests that Vβ rearrangements occur after Vγ/δ assembly during thymic development and, consequently, that γδ lineage divergence may be favored over the αβ lineage in early thymic development.
In conclusion, we have shown that TCRβ, γ, and δ rearrangements are concurrently increased before the Pre-T cell stage in a progressive and sequential fashion. The fact that TCR gene assembly is not a linear process suggests that immature T cells may have a certain degree of freedom when choosing the αβ versus γδ lineages. Previous reports (27, 29) have documented that TCR rearrangements do not, by themselves, determine the developmental fate of immature T cells. Our findings support this notion, suggesting instead the existence of specific signals triggering different TCR rearrangements in immature T cells that may or may not be part of the commitment to a given T cell lineage.
Acknowledgments
The authors thank Alain Vicari, Tom Moore, and John Ford for helpful discussions. We also thank Jackie Kennedy and Devora Rossi for help and support, James Cupp, Eleni Callas, and Josephine Polakoff for help with FACS sorting, and Daniel Gorman and his staff for sequencing help. DNAX Research Institute is supported by Schering-Plough Corporation.
ABBREVIATIONS
- TCR
T cell receptor
- DP
double positive
- TN
triple negative
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1. Pardoll D M, Fowlkes B J, Bluestone J A, Kruisbeek A, Maloy W L, Coligan J E, Schwartz R H. Nature (London) 1987;326:79–81. doi: 10.1038/326079a0. [DOI] [PubMed] [Google Scholar]
- 2.Raulet D H, Spencer D M, Hsiang Y H, Goldman J P, Bix M, Liao N S, Zijstra M, Jaenisch R, Correa I. Immunol Rev. 1991;120:185–204. doi: 10.1111/j.1600-065x.1991.tb00592.x. [DOI] [PubMed] [Google Scholar]
- 3.Haas W, Pereira P, Tonegawa S. Annu Rev Immunol. 1993;11:637–685. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- 4.Godfrey D I, Zlotnik A. Immunol Today. 1993;14:547–553. doi: 10.1016/0167-5699(93)90186-O. [DOI] [PubMed] [Google Scholar]
- 5.Shortman K, Wu L. Annu Rev Immunol. 1996;14:29–47. doi: 10.1146/annurev.immunol.14.1.29. [DOI] [PubMed] [Google Scholar]
- 6.Suda T, Zlotnik A. J Immunol. 1993;150:447–555. [PubMed] [Google Scholar]
- 7.Petrie H T, Scollay R, Shortman K. Eur J Immunol. 1992;22:2185–2188. doi: 10.1002/eji.1830220836. [DOI] [PubMed] [Google Scholar]
- 8.Garman R D, Doherty P J, Raulet D H. Cell. 1986;45:733–742. doi: 10.1016/0092-8674(86)90787-7. [DOI] [PubMed] [Google Scholar]
- 9.Raulet D H. Annu Rev Immunol. 1989;7:175–207. doi: 10.1146/annurev.iy.07.040189.001135. [DOI] [PubMed] [Google Scholar]
- 10.Goldman J P, Spencer D M, Raulet D H. J Exp Med. 1993;177:729–739. doi: 10.1084/jem.177.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livak F, Petrie H T, Crispe I N, Schatz D G. Immunity. 1995;2:617–627. doi: 10.1016/1074-7613(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 12.Dudley E C, Girardi M, Owen M J, Hayday A C. Curr Biol. 1995;5:659–669. doi: 10.1016/s0960-9822(95)00131-x. [DOI] [PubMed] [Google Scholar]
- 13.Wilson A, de Villartay J P, MacDonald H R. Immunity. 1996;4:37–45. doi: 10.1016/s1074-7613(00)80296-4. [DOI] [PubMed] [Google Scholar]
- 14.Dudley E C, Petrie H T, Shah L M, Owen M J, Hayday A C. Immunity. 1994;1:83–93. doi: 10.1016/1074-7613(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 15.Hockett R D, Jr, Janowski K M, Bucy R P. J Immunol Methods. 1995;187:273–285. doi: 10.1016/0022-1759(95)00195-5. [DOI] [PubMed] [Google Scholar]
- 16.Heilig J S, Tonegawa S. Proc Natl Acad Sci USA. 1987;84:8070–8074. doi: 10.1073/pnas.84.22.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godfrey D I, Kennedy J, Mombaerts P, Tonegawa S, Zlotnik A. J Immunol. 1994;152:4783–4792. [PubMed] [Google Scholar]
- 18.Bluestone J A, Cron R Q, Barrett T A, Houlden B, Sperling A I, Dent A, Hedrick S, Rellahan B, Matis L A. Immunol Rev. 1991;120:5–33. doi: 10.1111/j.1600-065x.1991.tb00585.x. [DOI] [PubMed] [Google Scholar]
- 19.Capone M, Watrin F, Fernex C, Horvat B, Krippl B, Wu L, Scollay R, Ferrier P. EMBO J. 1993;12:4335–4346. doi: 10.1002/j.1460-2075.1993.tb06118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shutter J, Cain J A, Ledbetter S, Rogers M D, Hockett R D., Jr Mol Cell Biol. 1995;15:7022–7031. doi: 10.1128/mcb.15.12.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korman A J, Marusic-Galesic S, Spencer D, Kruisbeek A M, Raulet D H. J Exp Med. 1988;168:1021–1040. doi: 10.1084/jem.168.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burtrum D B, Kim S, Dudley E C, Hayday A C, Petrie H T. J Immunol. 1996;157:4293–4296. [PubMed] [Google Scholar]
- 23.Mallick C A, Dudley E C, Viney J L, Owen M J, Hayday A C. Cell. 1993;73:513–519. doi: 10.1016/0092-8674(93)90138-g. [DOI] [PubMed] [Google Scholar]
- 24.Kang J, Baker J, Raulet D H. Eur J Immunol. 1995;25:2706–2709. doi: 10.1002/eji.1830250946. [DOI] [PubMed] [Google Scholar]
- 25.Moore T A, Zlotnik A. Blood. 1995;86:1850–1860. [PubMed] [Google Scholar]
- 26.Wu L, Li C L, Shortman K. J Exp Med. 1996;184:903–911. doi: 10.1084/jem.184.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruno L, Fehling H J, von Boehmer H. Immunity. 1996;5:343–352. doi: 10.1016/s1074-7613(00)80260-5. [DOI] [PubMed] [Google Scholar]
- 28.Vicari A P, Mocci S, Openshaw A, O’Garra A, Zlotnik A. Eur J Immunol. 1996;26:1424–1429. doi: 10.1002/eji.1830260704. [DOI] [PubMed] [Google Scholar]
- 29.Washburn T, Schweighoffer E, Gridley T, Chang D, Fowlkes B J, Cado D, Robey E. Cell. 1997;88:833–843. doi: 10.1016/s0092-8674(00)81929-7. [DOI] [PubMed] [Google Scholar]
- 30.Havran W L, Allison J P. Nature (London) 1988;335:443–445. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- 31.Zorbas M, Scollay R. Eur J Immunol. 1993;23:1655–1660. doi: 10.1002/eji.1830230739. [DOI] [PubMed] [Google Scholar]
- 32.Passoni L, Hoffman E S, Kim S, Crompton T, Pao W, Dong M Q, Owen M J, Hayday A C. Immunity. 1997;7:83–95. doi: 10.1016/s1074-7613(00)80512-9. [DOI] [PubMed] [Google Scholar]
- 33.Kang J, Raulet D H. Semin Immunol. 1997;9:171–179. doi: 10.1006/smim.1997.0069. [DOI] [PubMed] [Google Scholar]
- 34.von Boehmer H, Fehling H J. Annu Rev Immunol. 1997;15:433–452. doi: 10.1146/annurev.immunol.15.1.433. [DOI] [PubMed] [Google Scholar]
- 35.Tourigni M R, Mazel S, Burtrum D B, Petrie H. J Exp Med. 1997;185:1549–1556. doi: 10.1084/jem.185.9.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]