Abstract
Bordetella pertussis secretes a calmodulin-activated adenylate cyclase toxin, CyaA, that is able to deliver its N-terminal catalytic domain (400-aa residues) into the cytosol of eukaryotic target cells, directly through the cytoplasmic membrane. We have previously shown that CyaA can be used as a vehicle to deliver T cell epitopes, inserted within the catalytic domain of the toxin, into antigen-presenting cells and can trigger specific class I-restricted CD8+ cytotoxic T cell responses in vivo. Here, we constructed a series of recombinant toxins harboring at the same insertion site various peptide sequences of 11–25 amino acids, corresponding to defined CD8+ T cell epitopes and differing in the charge of the inserted sequence. We show that inserted peptide sequences containing net negative charges (−1 or −2) decreased or completely blocked (charge of −4) the internalization of the toxin into target cells in vitro and abolished the induction of cytotoxic T cell responses in vivo. The blocking of translocation due to the inserted acidic sequences can be relieved by appropriate mutations in the flanking region of CyaA that counterbalance the inserted charges. Our data indicate that (i) the electrostatic charge of the peptides inserted within the catalytic domain of CyaA is critical for its translocation into eukaryotic cells and (ii) the delivery of T cell epitopes into the cytosol of antigen-presenting cells by recombinant CyaA toxins is essential for the in vivo stimulation of specific cytotoxic T cells. These findings will help to engineer improved recombinant CyaA vectors able to stimulate more efficiently cellular immunity.
Bordetella pertussis, the causative agent of whooping cough, secretes a calmodulin-stimulated adenylate cyclase toxin (CyaA) that is one of the major virulence factors of this organism (1–3). This toxin is a 1,706-residue long protein, encoded by the cyaA gene, that is able to enter a large variety of eukaryotic cells where, upon activation by calmodulin, it synthesizes high levels of cAMP that disrupt cell metabolism (4–6). The calmodulin-dependent catalytic domain is located in the first 400-aa residues (5, 7). The 1,306 C-terminal residues display substantial sequence similarity with the RTX (Repeat in ToXin) toxin family (8): this part of the molecule is endowed with an intrinsic, albeit low, hemolytic activity that results from its ability to form cation-selective channels in membranes (9–13). More importantly, the C-terminal domain mediates the binding and internalization of the toxin into eukaryotic cells (6, 10). The CyaA toxin has a remarkable and unique mechanism of penetration into eukaryotic cells by which the catalytic domain is translocated directly across the plasma membrane of the target cells into the cytosol, where it associates with calmodulin. Hence, its entry is independent of receptor-mediated endocytosis, and it can invade cells that lack membrane traffic, like mammalian erythrocytes (10, 14–16).
The mechanism of toxin internalization into target cells is largely unknown, but it seems to occur in two steps (16). In a first step, CyaA binds to the cell surface, possibly to gangliosides. This binding requires both the integrity of the C-terminal part of CyaA (residues 400–1,706) and a post-translational palmitoylation of the lysine residue 983, elicited by the B. pertussis cyaC gene product (17–20). Binding of toxin to erythrocytes will eventually lead to cell lysis caused by the pore-forming capacity of CyaA. The second step is the actual translocation of the catalytic domain of bound CyaA through the plasma membrane of the cells and depends on the temperature, the membrane potential of the target cells, and the presence of calcium ions in the millimolar range (6, 14, 21).
Recently, we found that exogenous peptides could be inserted into defined permissive sites within the catalytic domain of CyaA without altering the biological activities of the protein, suggesting that the toxin could be used as a vehicle to deliver polypeptides into eukaryotic cells (22). In particular, we used CyaA as a means to deliver major histocompatibility complex (MHC) class I-associated T cell epitopes into antigen-presenting cells (APCs) because it is well established that efficient antigen presentation by MHC class I molecules requires the presence of the antigen in the cytosolic compartment of the presenting cells (23). We showed previously that a recombinant CyaA toxin carrying a CD8+ T cell epitope from the nucleoprotein of the lymphocytic choriomeningitis virus inserted within the catalytic domain was able to induce specific MHC class I-restricted CD8+ cytotoxic T cell (CTL) responses in vivo (24) and protected mice against infection with lethal doses of the lymphocytic choriomeningitis virus (25).
In the present work, we have characterized various recombinant toxins harboring, within the catalytic domain, different T cell epitopes derived from ovalbumin or from tumor-specific antigens. We found that the electrostatic charge of the inserted peptide was critical for the entry of the toxin into target cells. We further demonstrated that the active CyaA-mediated delivery of the inserted T cell epitope into the cytosol of APCs is essential for the in vivo induction of CTL responses.
MATERIALS AND METHODS
Bacterial Strains, Plasmids, and Growth Media.
The Escherichia coli strain XL1-Blue (Stratagene) was used for DNA manipulation and for production of CyaA toxins. The E. coli strain BLR (Novagen) was used for production of some recombinant toxins. The plasmids pACM224 and pCACT3 were described already (22, 26). Transformants were selected on Luria–Bertani medium agar media containing 100 mg/liter ampicillin. All bacteria were grown in Luria–Bertani medium containing 100 mg/liter ampicillin.
Constructions of Recombinant CyaA Toxins.
All in vitro DNA manipulations were performed according to standard protocols (27). The plasmid pACM224 (22) was cut at its unique PstI site, blunted with T4-polymerase treatment in the presence of the four dNTPs, and ligated with the oligonucleotide P815A1 (and its complementary strand), which codes for the amino acid sequence ASLPYLGWLVFGT. The underlined segment corresponds to the sequence of a CD8+ T cell epitope identified in the tumor cell line P815 by T. Boon and coworkers (28). The AS- and GT-flanking sequences are encoded by DNA with two unique restriction sites, NheI and KpnI, that were generated upon ligation of the oligonucleotide into the blunted PstI site of pACM224. Correct insertion of the oligonucleotide was verified by local DNA sequencing. Then, a 1.3-kb PvuII–BstBI DNA fragment, encoding the modified catalytic domain with the P815 epitope, was subcloned into plasmid pCACT3 (digested with BamHI, blunt-ended by T4 DNA polymerase plus all four dNTPs, and further digested with BstBI, after heat inactivation of the polymerase), which carries both the cyaC and the cyaA genes, under the transcriptional control of the lac UV5 promoter (23). The resulting plasmid pCACT–P815A1, which encodes a recombinant CyaA toxin with the P815A epitope inserted between amino acids 224 and 225, was used as a general vector to construct all other plasmids encoding the different recombinant toxins harboring peptide inserts between amino acids 224 and 225 by simple exchange of the DNA sequence between the two unique restriction sites NheI and KpnI and by the desired synthetic oligonucleotides (sequences available on request). All insertions into recombinant plasmids, pCACT-tum, pCACT-mut11, pCACT-ova11, pCACT-ova21, pCACT-4R, pCACT-4E, pCACT-4D, and pCACT-2D, were verified by local DNA sequencing. Plasmids pCACT-4D-M1 and pCACT-4D-M2 were generated by PCR mutagenesis with appropriate primers (sequences available on request). Plasmid pCACT-ova21-4E was constructed by inserting an oligonucleotide coding for four glutamic acid residues into the KpnI site of pCACT-ova21.
Purification of Wild-Type and Recombinant CyaA Toxins.
The bacterial cultures and extraction of toxins from inclusion bodies with 8 M urea were performed as described (29). The toxins were purified by chromatography on DEAE-Sepharose (Pharmacia Biotech, Uppsala) under denaturing conditions. The urea extracts, supplemented with 0.15 M NaCl, were applied to DEAE-Sepharose columns (1 vol. of packed gel/vol. of urea extract) equilibrated with 8 M urea and 0.15 M NaCl in 20 mM Hepes-Na, pH 7.5, and the columns were washed with 10-column volumes of the equilibration buffer. The bound toxin was eluted with 8 M urea and 0.5 M NaCl in 20 mM Hepes-Na, pH 7.5. The eluted proteins were diluted five times with 20 mM Hepes-Na and 1 M NaCl, pH 7.5 and applied onto a Phenyl-Sepharose column equilibrated with the same buffer. After washing with 20 mM Hepes-Na, pH 7.5, the toxin was eluted with 8 M urea in 20 mM Hepes-Na. All toxins purified by this method, were >90% pure as judged by SDS-gel analysis (not shown). The toxin concentrations were determined spectophotometrically from the absorption at 280 nm by using a molecular extinction coefficient of 142,000 M−1 cm−1 (calculated from the content in amino acid Trp, Tyr, and Phe). Alternatively, protein concentrations were measured with the Bio-Rad Bradford assay reagent by using purified wild-type CyaA as a standard. The isolated catalytic domain and the unmodified pro-CyaA toxin, which were used as controls in internalization assays, were purified as described (22, 29).
CyaA-Binding and Translocation Assays.
Adenylate cyclase activity was measured as described (30): 1 unit of adenylate cyclase activity corresponds to 1 μmol of cAMP formed in 1 min at 30°C and pH 8.0. Toxin binding and translocation into sheep erythrocytes were assayed essentially as described (31). Toxins in 8 M urea and 20 mM Hepes-Na were diluted directly (≈200-fold) into suspensions of sheep erythrocytes (2% of dry pellet) in buffer A (10 mM Tris⋅HCl, pH 8.0/150 mM NaCl/2 mM CaCl2) and incubated at 37°C for 15–30 min. An aliquot was removed to determine the total adenylate cyclase activity added to each sample (in the range of 0.8–1 unit/ml of cell suspension, corresponding to ≈2 μg/ml of CyaA protein). The cell suspensions were chilled on ice and centrifuged at 4°C, and the pelleted cells were resuspended in buffer A and separated into two batches. One batch was centrifuged again and the pelleted cells were lysed with 0.1% Triton X-100. The enzymatic activity measured in this extract corresponded to that of the toxin bound to the cells and was expressed as a percentage of total activity added to the cells. To the second batch, 20 μg of TPCK(L-1-(tosylamino)-2-phenylethyl chloromethyl ketone)-treated trypsin (Sigma) were added and the mixture was incubated for 10 min at room temperature to digest the adenylate cyclase that remained at the external surface of the erythrocytes. After addition of soybean trypsin inhibitor (5-fold excess), the erythrocytes were washed and lysed with 0.1% Triton X-100. The internalized adenylate cyclase activity protected from trypsin digestion was then measured and expressed as a percentage of total activity added.
Cell Lines.
EL4 is a C57BL/6 thymoma and E.G7 is a chicken egg ovalbumin (OVA)-transfected subclone of EL4 (32). P815 (H-2d) and RDM4 (H-2k) cells were used to assay the MHC class I restriction of effector cells. Cells were maintained in RPMI 1640 supplemented with 10% heat inactivated fetal calf serum, 2 mM glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 5.10−5 M of 2-mercaptoethanol. E.G7 cells were maintained in medium containing G418 at 0.4 mg/ml.
In Vivo Induction of CTL Responses and Cytotoxicity Assays.
Female C57BL/6 mice, aged 8–14 weeks (obtained from Iffa Credo, L’Arbresle, France) were immunized i.p. on days 0 and 21 with 50 μg of purified CyaA, CyaA-ova21, or CyaA-ova21–4E, mixed with 1 mg of aluminum hydroxide in PBS. Control mice were injected i.p with PBS. Seven days after the last injection, responder spleen cells (2.5 × 107 cells per 10 ml) from in vivo-primed mice (spleens from three mice in each group were pooled) were cocultured with 106-irradiated E.G7-ova cells (32). Cytotoxicity assays were then performed by incubating effector cells with EL4 cells radiolabeled with 51Cr and coated with 50 μg/ml of the synthetic OVA (257–264) peptide (SIINFEKL, which corresponds to the H-2b CD8+ T cell epitope of ovalbumin; synthesized by Neosystem, Strasbourg, France). Nonspecific killing was evaluated on noncoated target cells. The percentage of specific lysis was calculated as follows: (experimental cpm − spontaneous cpm release)/(total cpm − spontaneous cpm release) × 100, where spontaneous release was obtained from target cells incubated with medium alone, and total cpm was obtained from cells incubated in 1 M HCl. Background (target cells in medium) release was always <25% of maximal release. The SD of duplicate wells was always <5% of the specific 51Cr release. In some cases, effector cells were depleted of CD4+ or CD8+ T cells by incubation with anti-CD4 or anti-CD8 mAb, followed by separation with immunomagnetic beads, before the CTL assay (24). To determine the Kb specificity of the cytotoxic response, the effector cells were tested for CTL activity on 51Cr-labeled P815 (H-2d) or RDM4 (H-2k) cells incubated either with medium alone or with the OVA p257–264 peptide.
RESULTS
Recombinant CyaA Toxins Carrying Exogenous Peptides Are Delivered into Target Cells with Different Efficacies, Depending on Their Electrostatic Charge.
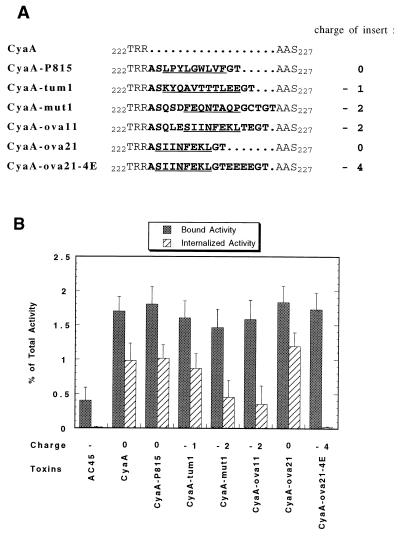
To examine the tolerance of CyaA to insertion of foreign epitopes, we constructed a set of recombinant toxins harboring different peptide sequences of 11–18 amino acids (Fig. 1A) at the same insertion point, between Arg224 and Ala225. These sequences correspond to CD8+ T cell epitopes that were identified in different tumor cell lines by the groups of T. Boon (ref. 28; epitopes P815A and tum) and L. Eisenbach (ref. 33; epitope mut1). The epitope OVA was identified by Bevan and coworkers (32) as the main H-2b CD8+ T cell epitope derived from ovalbumin when this protein was artificially introduced into, or expressed by mammalian cells.
Figure 1.
Invasive activity of recombinant CyaA toxins carrying various T cell epitopes. (A) Amino acid sequences of recombinant CyaA toxins around the region of insertion. The sequences of the exogenous peptides inserted between amino acid 224 and 225 of wild-type CyaA are indicated in bold letters (one letter code for amino acids), and the precise sequences corresponding to the characterized T-epitopes are underlined. The Ala-Ser and Gly-Thr dipeptides flanking the inserts are encoded by DNA corresponding to the two unique restriction sites NheI and KpnI. The electrostatic charge, indicated on the right part, is calculated on the peptide segment from amino acid Thr222 to Ser227 of wild-type CyaA. (B) For each recombinant toxin, adenylate cyclase activities bound to and internalized into erythrocytes were determined by a trypsin protection assay as described in Materials and Methods and expressed as percentage of total input. AC45, the noninvasive catalytic domain of CyaA (amino acid 1–399), was used as a control. The results represent the means of three independent experiments.
Biochemical characterization of these toxins indicated that the insertions affected neither the stability nor the catalytic activity of the recombinant adenylate cyclases (data not shown). However, as shown in Fig. 1B, their ability to translocate their catalytic domain into eukaryotic cells (i.e., sheep erythrocytes), as determined by a trypsin-protection assay, differed significantly. At a first sight, the translocation efficiency of the recombinant toxins appeared to correlate with the overall electrostatic charge of the inserted peptide sequences. The translocation efficiency of the recombinant toxins harboring negatively charged inserts, was significantly reduced (up to 50%) compared with wild-type CyaA. In contrast, the internalization of recombinant toxins carrying uncharged inserts did not differ from that of the wild-type toxin. The correlation between the electrostatic charge of the insert and translocation is even more conspicuous in the case of the two recombinant toxins CyaA-ova11 and CyaA-ova21, for which the inserted epitopes differ in only six amino acid residues (SQLE and TE, flanking the common OVA epitope, SIINFEKL), including two negatively charged glutamic acids (Fig. 1).
To examine the role of charges in CyaA translocation further, we constructed and analyzed a set of recombinant toxins that harbor short peptide inserts with different charges (Fig. 2A). As shown in Fig. 2B, insertion of four glutamic acids (inserted sequence: ASEEEEGT) or four aspartic acids (inserted sequence: ASDADGDPDGT) completely blocked or severely reduced the internalization of the toxin into the target cells. Insertion of two aspartic acids (inserted sequence: ASDADGT) reduced it to approximately one-half of that of wild-type CyaA, in good agreement with the results presented in Fig. 1. In contrast, insertion of four arginine residues did not affect the internalization of the recombinant toxin. It is important to note that all of these recombinant toxins bound with the same efficiency to the target cells as did the wild-type CyaA. This result indicates that the negative charges of the peptide sequences inserted in CyaA only affect the translocation of the catalytic domain through the cell membrane without interfering with the binding of the toxin to the target cells.
Figure 2.
Cell binding and internalization of recombinant CyaA toxins carrying peptides with different electrostatic charges. (A) The amino acid sequences of the peptides inserted in CyaA are indicated in bold letters. Dash marks in the sequences of CyaA-4DM1 and CyaA-4DM2 correspond to the deleted CyaA amino acids. The additional amino acid changes in CyaA-4DM2 are underlined. The electrostatic charges shown on the right were calculated for each toxin, from Thr222 to Trp242 (numbering of wild-type CyaA). (B) For each recombinant toxin, the adenylate cyclase activities bound to and internalized into erythrocytes were determined and expressed as percentage of total input. The noninvasive AC45 was used as a control. The results represent the means of four independent experiments.
Mutations in the Flanking Regions of Recombinant CyaA Toxins that Neutralize the Inserted Acidic Charges Restore Translocation.
Next, we examined whether the negatively charged sequences introduced within the catalytic domain might act as stop-transfer signals, preventing the passage of the polypeptide through the lipid bilayer due to the cluster of charged amino acid residues. To address this question, we constructed two recombinant toxins CyaA-4DM1 and CyaA-4DM2 that, in addition to the four-Asp sequence inserted in CyaA-4D, contained changes in the immediate downstream region of CyaA that counterbalance the inserted charges (Fig. 2A). As shown in Fig. 2B, both CyaA-4DM1 and CyaA-4DM2 toxins were translocated more efficiently into erythrocytes than was CyaA-4D. This result demonstrates that the inserted sequence of four Asp residues did not have an intrinsic blocking effect on the translocation of the catalytic domain of CyaA but rather, that the local electrostatic charge around amino acids 220–240 is critical for the internalization of the toxin into eukaryotic cells.
Active Delivery of CD8+ T cell Epitopes into the Cytosol of APCs by Recombinant CyaA Is a Prerequisite for Induction of CTL Responses in Vivo.
In previous studies, we reported that a recombinant CyaA toxin harboring a T cell epitope derived from the nucleoprotein of lymphocytic choriomeningitis virus was able to stimulate specific cytotoxic activity against lymphocytic choriomeningitis virus-infected target cells (24). The present demonstration that insertion of negatively charged residues specifically blocks the translocation of the catalytic domain of CyaA across the membrane of target cells allowed us to investigate the role of active transport of T cell epitopes into the APCs cytosol in the in vivo induction of CTL responses by recombinant CyaA toxins.
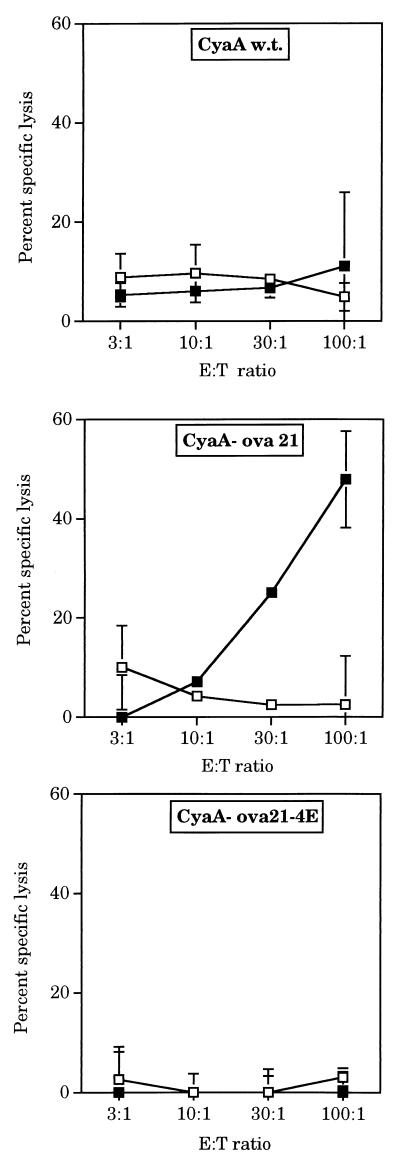
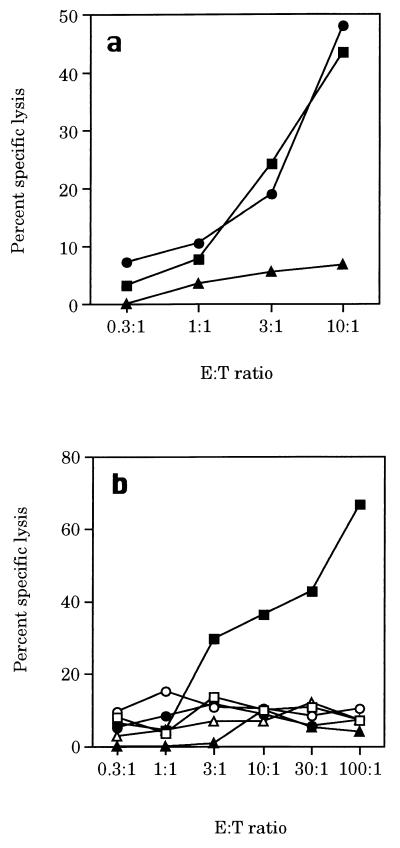
To address this question, we used the CyaA-ova21 toxin as a model antigen. As shown in Fig. 3, mice immunized with the CyaA-ova21 toxin, carrying the ovalbumin epitope SIINFEKL, displayed potent cytotoxic activity toward target cells coated with the OVA peptide. This cytotoxic activity was mediated by CD8+ T cells and was restricted by H-2b MHC class I molecules (Fig. 4). Furthermore, preliminary data indicate that immunization of mice with CyaA-ova21 induces a protective immunity against the growth of melanoma cells transfected with the ovalbumin gene (C.F., D.L., G.K., A.U., and C.L., unpublished data). We then constructed and produced a CyaA-ova21 derivative that, in addition to the OVA epitope, harbors a four-glutamic acid insert (Fig. 1A). As expected, this toxin, CyaA-ova21-4E bound efficiently to the erythrocytes but did not translocate its catalytic domain through the plasma membrane of target cells (Fig. 1B). When injected into mice, CyaA-ova21-4E did not trigger detectable CTL responses against target cells coated with the OVA peptide, in contrast to CyaA-ova21 (Fig. 3). These results show that the ability of the recombinant CyaA toxin to transport the OVA CD8+ T cell epitope to the cytosol of APCs is absolutely essential for the in vivo induction of cytolytic T cell responses. In addition, a mutant toxin carrying the OVA CD8+ epitope but devoid of adenylate cyclase activity was constructed by insertion of a dipeptide into the catalytic domain of the molecule (24). This genetically detoxified molecule fully retained its capacity to activate OVA-specific CTL responses showing that invasivity but not catalytic activity is required for CTL priming by these recombinant toxins (C.F., D.L., G.K., A.U., and C.L., unpublished data).
Figure 3.
Active delivery of the OVA CD8+ T cell epitope into the cytosol of APCs by recombinant CyaA is a prerequisite for induction of CTL responses in vivo. Assay of OVA-specific CTL responses of mice immunized with wild-type CyaA, CyaA-ova21, or CyaA-ova21–4E: on days 0 and 21, mice were injected with 50 μg of immunogens, and cytotoxicity assays were performed as described in Materials and Methods on EL4 cells (□) and EL4 cells coated with 50 μg/ml of OVA peptide (■). Results are expressed as the mean percentage of specific lysis of duplicate cultures.
Figure 4.
CTL responses induced by CyaA-ova21 are mediated by CD8+ cytotoxic T cells. C57BL/6 were immunized i.p. on days 0 and 21 with 50 μg of CyaA-ova21 mixed with 1 mg of aluminum hydroxide. (a) After in vitro stimulation of spleen cells with E.G7, the effector cells were left untreated (■) or were depleted of CD4+ (•) or CD8+ (▴) T cells as described in Materials and Methods and then tested for CTL activity on 51Cr-labeled E.G7 targets. Lysis of EL4 cells was <10% (not shown). The SEM of the duplicate was <15% of the mean. (b) After in vitro stimulation of spleen cells with E.G7, the effector cells were tested for CTL activity on 51Cr-labeled EL4 (■, □), P815 (•, ○) or RDM4 (▴, ▵) cells incubated with medium alone (open symbols) or with the OVA p257–264 peptide (closed symbols). The SEM of the duplicate was <15% of the mean.
DISCUSSION
The adenylate cyclase toxin from B. pertussis is endowed with the unique capability of translocating its N-terminal catalytic domain directly across the plasma membrane of eukaryotic cells. Besides, it was shown to be an efficient vehicle to deliver foreign epitopes, inserted in its catalytic domain, into APCs in vivo. In this study, we show that (i) the electrostatic charge of the epitopes inserted within the catalytic domain of recombinant CyaA is critical for its translocation into eukaryotic cells, and (ii) the CyaA-mediated transport of foreign epitopes into the cytosol of APCs is essential for the in vivo induction of CTL responses.
Our present results clearly demonstrate that insertions of negatively charged peptides in the catalytic domain of CyaA inhibit its translocation, whereas insertions of neutral or positively charged peptides have no effect (Fig. 5). Importantly, none of these insertions alter the binding of the toxin to the cells. The insertions of sequences containing four Asp or four Glu are therefore the first identified changes that specifically block the process of translocation of the catalytic domain of the toxin across the plasma membrane of target cells. Two interpretations could account for the blocking effects of negatively charged peptides on CyaA translocation: one structural and one thermodynamic. From a structural point of view, the negatively charged inserts in the catalytic domain could disrupt essential contacts between this region and the C-terminal part of the toxin, contacts that would be a prerequisite for translocation. Alternatively, the charged inserts could “freeze” the catalytic domain and/or the full toxin molecule in a translocation-incompetent form. However, we consider it unlikely that such structural effects would be responsible for the inhibition of translocation, for two reasons: (i) as we demonstrate here, the blocking effect of a four-aspartate residue insert can be relieved by additional local changes that neutralize the negative charges of this insert; (ii) we have been able to insert various peptides at the same position without affecting the translocation capability of the toxin (22, 24). From a thermodynamic point of view, our results can be easily rationalized by considering the results of Otero et al. (21) who have shown that translocation of CyaA into frog atrial myocytes is dependent on the membrane potential of these cells. The membrane electrical potential would be expected to oppose the translocation of negatively charged residues across the plasma membrane of target cells, as it is indeed observed here. Taken together, these results suggest that CyaA uses the electrical field across the plasma membrane as a driving force to enter into target cells.
Figure 5.
Correlation between the translocation efficiency of recombinant CyaA toxins and the local electrostatic charge of the polypeptide segment 222–242. The translocation efficiency for each recombinant toxin was calculated as the ratio of internalized activity to the bound activity and expressed as percentage of that of wild-type CyaA, taken as 100% (□). It was then plotted as a function of the global electrostatic charge of the segment spanning from Thr222 to Trp242 (numbered according to wild-type CyaA). Each of the 12 data points (•) corresponds to a different recombinant toxin.
The identification of a modification that blocks the translocation of the catalytic domain of CyaA through the plasma membrane without affecting its binding to these target cells, prompted us to examine a key issue regarding the ability of the recombinant CyaA toxin to induce in vivo CTL responses directed against a CD8+ T cell epitope inserted within the catalytic domain. Our previous results demonstrated that CyaA was an efficient nonreplicating vector to stimulate specific CTL responses in vivo against inserted CD8+ T cell epitopes (24, 25). We hypothesized that the ability of the toxin to deliver inserted CTL epitopes into the cytosol of APCs is essential for the triggering of CTL responses. Indeed, a noninvasive, truncated derivative of this toxin lacking the cell-binding domain was not able to activate CD8+ T cell responses (24).
Our present data provide clear evidence that, in vivo, the CyaA-mediated direct delivery of the T cell epitope into the cytosol of APCs is crucial for triggering specific CTL responses. Two recombinant toxins, CyaA-ova21 and CyaA-ova21-4E, that harbor the same CD8+ T cell epitope bound equally well to eukaryotic cells. However, CyaA-ova21-4E, containing an additional insert of four glutamic acid residues, did not internalize into target cells. This noninvasive toxin was unable to trigger cytotoxic T cell responses specific for the OVA epitope, whereas CyaA-ova21 did. These results suggest that the processing of the inserted epitope occurs mainly in the cytosol of the APCs, once the catalytic domain has crossed the plasma membrane. Apparently, endocytic degradation of the CyaA-ova21-4E bound to the membrane of the APCs did not result in significant delivery of the OVA epitope to MHC class I molecules. Recent in vitro results indicate that presentation of the OVA epitope by target cells exposed to CyaA-ova21 is critically dependent on the proteasome activity, further supporting a cytosolic processing of this antigen (P. Guermonprez, D.L., G.K., A.U., and C.L., unpublished results).
Recently, two groups have reported the use of another bacterial toxin, the anthrax toxin, to deliver proteins and/or peptides into APC. The first study showed that, in vivo, a recombinant anthrax toxin in which the lethal factor component was fused to a listeriolysin epitope, conferred protective immunity against Listeria monocytogenes (34). The second study demonstrated that, in vitro, the translocation of a passenger antigen fused to lethal factor into the cytosol of the target cells was required for specific CD8+ T cell lysis (35). In addition, the proteasome activity of the target cells was shown to be critical (35). Hence, it seems that the delivery of antigens or epitopes into the cytosol of APC is a prerequiste for efficient priming of specific CTL responses by bacterial toxins. Delineating the molecular mechanisms of toxin translocation into eukaryotic cells, as illustrated here for the CyaA toxin, will help to design more effective vectors to stimulate cellular, and in particular, anti-tumor immunity.
Acknowledgments
We thank J. Pidoux and Y. Le Metayer for construction and expression of some of recombinant toxins described here. We are grateful to Anthony Pugsley for critical reading of the manuscript. Financial support came from the Institut Pasteur, the Centre National de la Recherche Scientifique (URA 1129), the Association pour la Recherche sur le Cancer (ARC), and a Pasteur-Weizmann grant to C.L.
ABBREVIATIONS
- CyaA
calmodulin-activated adenylate cyclase toxin
- MHC
major histocompatibility complex
- CTL
cytotoxic T cell
- APCs
antigen-presenting cells
- OVA
ovalbumin
References
- 1. Weiss A A, Hewlett E L. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]
- 2.Hanski E. Trends Biochem Sci. 1989;14:459–463. doi: 10.1016/0968-0004(89)90106-0. [DOI] [PubMed] [Google Scholar]
- 3.Mock M, Ullmann A. Trends Microbiol. 1993;1:187–192. doi: 10.1016/0966-842x(93)90089-a. [DOI] [PubMed] [Google Scholar]
- 4.Confer D L, Eaton J W. Science. 1982;217:948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- 5.Glaser P, Ladant D, Sezer O, Pichot F, Ullmann A, Danchin A. Mol Microbiol. 1988;2:19–30. doi: 10.1111/j.1365-2958.1988.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 6.Rogel A, Schultz J E, Brownlie R M, Coote J G, Parton R, Hanski E. EMBO J. 1989;8:2755–2760. doi: 10.1002/j.1460-2075.1989.tb08417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ladant D, Michelson S, Sarfati R, Gilles A M, Predeleanu R, Barzu O. J Biol Chem. 1989;264:4015–4020. [PubMed] [Google Scholar]
- 8.Coote J G. FEMS Microbiol Rev. 1992;8:137–161. doi: 10.1111/j.1574-6968.1992.tb04961.x. [DOI] [PubMed] [Google Scholar]
- 9.Glaser P, Sakamoto H, Bellalou J, Ullmann A, Danchin A. EMBO J. 1988;7:3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellalou J, Sakamoto H, Ladant D, Geoffroy C, Ullmann A. Infect Immun. 1990;58:3242–3247. doi: 10.1128/iai.58.10.3242-3247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehrmann I E, Gray M C, Gordon V M, Gray L S, Hewlett E L. FEBS Lett. 1991;278:79–83. doi: 10.1016/0014-5793(91)80088-k. [DOI] [PubMed] [Google Scholar]
- 12.Benz R, Maier E, Ladant D, Ullmann A, Sebo P. J Biol Chem. 1994;269:27231–27239. [PubMed] [Google Scholar]
- 13.Szabo G, Gray M C, Hewlett E L. J Biol Chem. 1994;269:22496–22499. [PubMed] [Google Scholar]
- 14.Hanski E, Farfel Z. J Biol Chem. 1985;260:5526–5532. [PubMed] [Google Scholar]
- 15.Gordon V M, Young W J, Lechler S M, Gray M C, Leppla S H, Hewlett E L. J Biol Chem. 1989;264:14792–14796. [PubMed] [Google Scholar]
- 16.Rogel A, Hanski E. J Biol Chem. 1992;267:22599–22605. [PubMed] [Google Scholar]
- 17.Barry E M, Weiss A A, Ehrmann I E, Gray M C, Hewlett E L, Goodwin M S. J Bacteriol. 1991;173:720–726. doi: 10.1128/jb.173.2.720-726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hewlett E L, Gray M C, Ehrmann I E, Maloney N J, Otero A S, Gray L, Allietta M, Szabo G, Weiss A A, Barry E M. J Biol Chem. 1993;268:7842–7848. [PubMed] [Google Scholar]
- 19.Hackett M, Guo L, Shabanowitz J, Hunt D F, Hewlett E L. Science. 1994;266:433–435. doi: 10.1126/science.7939682. [DOI] [PubMed] [Google Scholar]
- 20.Westrop G D, Hormozi E K, Da C N, Parton R, Coote J G. Gene. 1996;180:91–99. doi: 10.1016/s0378-1119(96)00412-x. [DOI] [PubMed] [Google Scholar]
- 21.Otero A S, Yi X B, Gray M C, Szabo G, Hewlett E L. J Biol Chem. 1995;270:9695–9697. doi: 10.1074/jbc.270.17.9695. [DOI] [PubMed] [Google Scholar]
- 22.Ladant D, Glaser P, Ullmann A. J Biol Chem. 1992;267:2244–2250. [PubMed] [Google Scholar]
- 23.Sebo P, Fayolle C, d’Andria O, Ladant D, Leclerc C, Ullmann A. Infect Immun. 1995;63:3851–3857. doi: 10.1128/iai.63.10.3851-3857.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fayolle C, Sebo P, Ladant D, Ullmann A, Leclerc C. J Immunol. 1996;156:4697–4706. [PubMed] [Google Scholar]
- 25.Saron M F, Fayolle C, Sebo P, Ladant D, Ullmann A, Leclerc C. Proc Natl Acad Sci USA. 1997;94:3314–3319. doi: 10.1073/pnas.94.7.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Betsou F, Sebo P, Guiso N. Infect Immun. 1993;61:3583–3589. doi: 10.1128/iai.61.9.3583-3589.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 28.Van den Eynde B, Lethe B, Van Pel A, De Plaen E, Boon T. J Exp Med. 1991;173:1373–1384. doi: 10.1084/jem.173.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sebo P, Glaser P, Sakamoto H, Ullmann A. Gene. 1991;104:19–24. doi: 10.1016/0378-1119(91)90459-o. [DOI] [PubMed] [Google Scholar]
- 30.Ladant D. J Biol Chem. 1988;263:2612–2618. [PubMed] [Google Scholar]
- 31.Heveker N, Ladant D. Eur J Biochem. 1997;243:643–649. doi: 10.1111/j.1432-1033.1997.00643.x. [DOI] [PubMed] [Google Scholar]
- 32.Moore M W, Carbone F R, Bevan M J. Cell. 1988;54:777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- 33.Mandelboim O, Berke G, Fridkin M, Feldman M, Eisenstein M, Eisenbach L. Nature (London) 1994;369:67–71. doi: 10.1038/369067a0. [DOI] [PubMed] [Google Scholar]
- 34.Ballard J D, Collier R J, Starnbach M N. Proc Natl Acad Sci USA. 1996;93:12531–12534. doi: 10.1073/pnas.93.22.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goletz T J, Klimpel K R, Arora N, Leppla S H, Keith J M, Berzofsky J A. Proc Natl Acad Sci USA. 1997;94:12059–12064. doi: 10.1073/pnas.94.22.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]