Abstract
Synaptotagmin I has two tandem Ca2+-binding C2 domains, which are essential for fast synchronous synaptic transmission in the central nervous system. We have solved four crystal structures of the C2B domain, one of them in the cation-free form at 1.50 Å resolution, two in the Ca2+-bound form at 1.04 Å (two bound Ca2+ ions) and 1.65 Å (three bound Ca2+ ions) resolution and one in the Sr2+-bound form at 1.18 Å (one bound Sr2+ ion) resolution. The side chains of four highly conserved aspartic acids (D303, D309, D363, and D365) and two main chain oxygens (M302:O and Y364:O), together with water molecules, are in direct contact with two bound Ca2+ ions (sites 1 and 2). At higher Ca2+ concentrations, the side chain of N333 rotates and cooperates with D309 to generate a third Ca2+ coordination site (site 3). Divalent cation binding sites 1 and 2 in the C2B domain were previously identified from NMR NOE patterns and titration studies, supplemented by site-directed mutation analysis. One difference between the crystal and NMR studies involves D371, which is not involved in coordination with any of the identified Ca2+ sites in the crystal structures, while it is coordinated to Ca2+ in site 2 in the NMR structure. In the presence of Sr2+, which is also capable of triggering exocytosis, but with lower efficiency, only one cation binding site (site 1) was occupied in the crystallographic structure.
Keywords: Synaptotagmin I, C2B domain, X-ray crystallography, calcium binding, strontium binding
Synaptotagmins (Syt) are membrane trafficking proteins characterized by an N-terminal transmembrane domain and tandem cytoplasmic C2 domains (C2A and C2B) that bind Ca2+ with different affinities (Sudhof 2002; Tucker and Chapman 2002; Koh and Bellen 2003; Yoshihara et al. 2003). The calcium binding properties of the C2 domains are thought to determine whether kiss-and-run or full-fusion will occur at the plasma membrane (Wang et al. 2001, 2003). Furthermore, the involvement of distinct synaptotagmins with unique Ca2+-binding signatures in diverse types of regulated exocytosis may explain how fusion of distinct secretory vesicles is differentially triggered at select calcium concentrations. Synaptotagmin I (Syt I), located on the synaptic vesicle membrane, is most likely the Ca2+-sensor controlling regulated exocytosis at the brain synapse (Geppert et al. 1994; Fernandez-Chacon et al. 2001). Biochemical and genetic studies suggest that the C2B domain is essential for triggering Ca2+-dependent fast exocytosis. In Drosophila, mutation of Ca2+-binding aspartate residues within the C2B domain abolish exocytosis (Mackler et al. 2002).
Sr2+, an analog of Ca2+ in the alkaline earth series, is known to also trigger fast neurotransmitter release, but less efficiently (Goda and Stevens 1994; Shin et al. 2003). Although both the C2A and the C2B domain of Syt I bind Ca2+ in phospholipid complexes, only the C2B domain forms Sr2+/phospholipid complexes. Unlike Ca2+, Sr2+ is unable to induce binding of Syt I to the SNARE complex (Shin et al. 2003). Thus, it has been proposed that Sr2+ binding to the Syt I C2B domain is sufficient to trigger fast exocytosis (Shin et al. 2003).
NMR spectral analysis has outlined the structure of the Ca2+-bound C2B domain of Syt I and proposed the coordination geometries of two adjacent Ca2+-binding sites (Fernandez et al. 2001). Two Ca2+ ions were built into the final NMR structure based on NOE patterns and titration-based chemical shift changes, supplemented by the results of site-directed mutagenesis experiments. As a result, a two-site Ca2+ binding model was proposed, in which the side chains of five aspartic acid residues from two loops form a pair of adjacent Ca2+ binding sites. In addition, similar to what has been reported for the C2A domain (Shao et al. 1998), Ca2+ binding does not introduce a conformational change in the C2B domain (Fernandez et al. 2001), but rather most likely modifies the surface potential, thereby regulating lipid interactions.
We have initiated a research effort aimed at understanding the structural basis of calcium-triggered membrane fusion at the neuronal synapse. The eventual goal is to determine the structure of the synaptotagmin C2 domains in complex with SNAREs and other regulatory components. As a first step, we report the crystal structures of the C2B domain of Syt I in divalent cation-free form, in the presence of two and three bound Ca2+ ions and in the presence of a single bound Sr2+ ion. Our high resolution crystal structures of the C2B domain have identified the coordination geometries at the three divalent cation-binding sites, as well as the protein side chains and water molecules involved in the coordination. This allows a direct comparison to be made between the Ca2+ coordination sites determined from crystallography with those proposed by NMR, which were supplemented by site-directed mutagenesis experiments (Fernandez et al. 2001). It also allows a comparison of divalent cation coordination geometries between Ca2+ and Sr2+-bound C2B domains.
Results
Crystallization conditions
Our efforts at growing diffraction quality crystals of the C2B domain have been undertaken in the absence and presence of divalent cations. We first crystallized C2B from a 1.8 M ammonium sulfate solution. These crystals, designated C2B-A, diffracted on our in-house X-ray diffractometer up to 1.5 Å resolution. Following structure refinement, no visible electron density was detectable for loop 1, whose residues are known to participate in the Ca2+ coordination site(s). We next turned to crystallization in the presence of Mg2+ ions, which are known to stabilize the local structure of Syt III C2A-C2B (Sutton et al. 1999). However, loop 1 could not be traced in the Syt I C2B crystals grown from 1.0 M magnesium sulfate and 0.4 M ammonium sulfate.
Previous studies have demonstrated that crystals of Syt I C2A domain can be grown in Li2SO4 solution, and Ca2+ could be subsequently introduced into these crystals following soaking with low concentrations (0.1 mM) of CaCl2 solution for 24 h (Sutton et al. 1995). It is likely that Ca2+ stabilizes the local structure of the Ca2+-binding loops of synaptotagmin (Shao et al. 1998). We succeeded in growing diffraction-quality crystals of the complex of C2B domain and Ca2+ using a similar approach. To avoid the precipitation of CaSO4 (caused by CaCl2 addition to crystals in ammonium sulfate buffer), C2B crystals were carefully washed before transfer to CaCl2-containing soaking solutions, which included cryoprotectant. Further, we found that C2B crystals can be kept in cryoprotectant solution for 5 min with only minor changes of crystallographic parameters and mosaicity. We obtained a crystal, designated C2B-B, following 1 min soaking in cryoprotectant solution containing 200 mM CaCl2, and another crystal, designated C2B-C, following 1 min soaking in cryoprotectant solution containing 600 mM CaCl2. We also obtained a crystal, designated C2B-D, following 1 min soaking in cryoprotectant solution containing 200 mM SrCl2.
Structure refinement
The crystal structure of C2B-A in the absence of divalent cations was solved by molecular replacement against the published NMR-based solution structure of the Ca2+-bound C2B (Fernandez et al. 2001). Five contiguous residues within a loop segment (D303V304G305G306L307), together with the last three residues (VKK) are disordered in the crystal structure of C2B-A. In turn, the divalent-cation bound C2B-B, C2B-C and C2B-D crystal structures were solved by molecular replacement against the C2B-A crystal structure. The D303V304G305G306L307 segment could be traced in all three structures stabilized by bound divalent cations. In addition, a residue tag (SGGGGGIL) from the fusion protein cleavage site, was also observed in the 1.04 Å C2B-B and 1.18 Å C2B-D structures. Further, double conformations were assigned to the side chains of E295, M302, I314, N336, S344, V376, S391, and V409 in these two-high resolution structures. The final structural statistics data for C2B-A (no divalent ions bound), C2B-B (two bound Ca2+ ions), C2B-C (three bound Ca2+ ions) and C2B-D (one bound Sr2+ ion) are listed in Table 1. Ramachandran analysis of the main-chain conformations showed that for each structure, ~90% of the residues lay in the most-favored regions, while none fell in disallowed regions (data not shown). The C2B-B structure contained one bound acetate molecule from the crystallization buffer, while all the crystal structures contained one bound glycerol molecule from the soaking buffers. None of the crystal structures contained bound sulfate.
Table 1.
Summary of crystallographic data collection and refinement
| C2B-A | C2B-B | C2B-C | C2B-D | |
| Space group: P3221 | ||||
| Cell dimensions | ||||
| a = b (Å) | 54.5 | 54.7 | 54.6 | 54.6 |
| c (Å) | 104.2 | 103.8 | 103.6 | 103.3 |
| Data collection | ||||
| Resolution (Å) | 1.50 (1.53–1.50) | 1.04 (1.06–1.04) | 1.65 (1.69–1.65) | 1.18 (1.20–1.18) |
| Observation | 203,470 | 477,779 | 162,737 | 618,517 |
| Unique reflections | 28,428 (1,201) | 85,544 (4,126) | 22,306 (1,430) | 56,844 (1639) |
| Completeness | 96.3% (61.6%) | 99.0% (96.6%) | 99.9% (98.7%) | 95.5% (56.2%) |
| I/σ(I) | 33.6 (3.0) | 19.3 (1.6) | 24.2 (4.1) | 31.1 (3.4) |
| Rmergea | 0.052 (0.302) | 0.103 (0.416) | 0.095 (0.386) | 0.048 (0.190) |
| Redundancy | 7.2 | 5.6 | 7.3 | 10.9 |
| Refinement | ||||
| Resolution (Å) | 20.0–1.50 | 20.0–1.04 | 20.0–1.65 | 20.0–1.18 |
| Completeness | 96.22% | 99.04% | 99.83% | 95.47% |
| Reflections | 26,931 | 81,211 | 21,129 | 53,935 |
| R-factorb | 0.188 | 0.165 | 0.163 | 0.155 |
| Rfree | 0.214 | 0.183 | 0.197 | 0.173 |
| Mean B-factor | 20.1 | 12.3 | 15.4 | 13.4 |
| RMS bonds (Å) | 0.014 | 0.018 | 0.015 | 0.017 |
| RMS angles (°) | 1.5 | 1.7 | 1.4 | 1.7 |
| PDB code | — | 1TJX | 1UOV | 1TJM |
aRmerge = ∑|I - 〈| 〉|/∑|I|.
bR-factor = ∑hkl|Fo − Fc|/∑hkl|Fo|.
Overall structure
The four C2B crystal structures have the same overall topology as the NMR-based solution structure (Fernandez et al. 2001). For example, the 1.04 Å C2B-B structure shows that protein is composed of two layers of anti-parallel β sheets, along with two short α helices (Fig. 1A ▶). The β-stranded core of the C2B domain adopts the same topology as its related C2A domain (Sutton et al. 1995) and presents a convex surface for further recognition. Two additional α-helices, one between strand β7–β8 and the other at the C terminus, make one face of the C2B domain significantly different from that observed for the corresponding C2A-domain. Three loops, designated 1, 2, and 3, encompass the calcium binding sites. The terminal α-helix, which is rich in acidic residues (E410, E411, E412, and D414), introduces a negatively charged patch, which is close to a region that is rich in basic residues (K313, R322, K325, K327, and K375; Fig. 1B ▶).
Figure 1.

Structure of C2B-B. (A) Front and side view of ribbon diagrams of the structure of C2B-B with 2 Ca2+ ions. β-Strands are labeled from 1 to 8, while helices are labeled H1 and H2. Ca2+ ions are labeled Ca1 and Ca2. (B) Front and back view of electrostatic potential surface of C2B-B. The acidic and basic residues are colored green and orange, respectively.
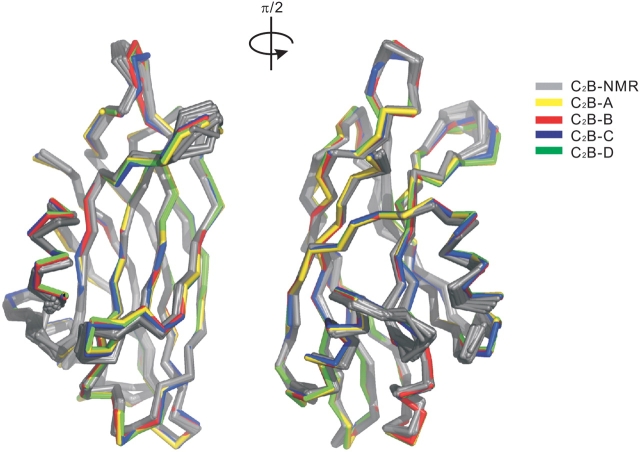
Not surprisingly, the backbone folds of all four crystal structures of C2B are very similar (Fig. 2 ▶), except for the disordered peptide segment observed in C2B-A. The root-mean-square deviation (RMSD) of the Cα atoms for the four C2B crystal structures is less than 0.34 Å. This result is also consistent with NMR studies, where it was shown that cation-binding does not impact on the global structure of C2B, but rather stabilized the conformation of loop 1 (Fernandez et al. 2001). The greatest variability among the three divalent cation-coordinated crystal structures is found in the loop connections that encircle the Ca2+ binding sites. The X-ray (C2B-B) and NMR (C2B-NMR; Fernandez et al. 2001) structures of C2B coordinated by two bound Ca2+ ions exhibit an RMSD for all Cα atoms of 0.91 Å.
Figure 2.
Superimposition of crystal structures C2B-A, C2B-B, C2B-C and C2B-D and 20 C2B-NMR structures. The Cα traces of C2B-A, C2B-B, C2B-C and C2B-D are colored yellow, red, blue, and green, respectively. The C2B-NMR structures are colored gray. The orientations are the same as in Figure 1A ▶.
Cation-binding sites
Loop 1 was disordered in the C2B-A crystal structure in the absence of Ca2+ ions, but could be traced in the C2B-B and C2B-C crystal structures in the presence of Ca2+ ions, consistent with the conformational stabilization of loop 1 by bound Ca2+ (Shao et al. 1998). Two Ca2+ binding sites, designated 1 and 2, are common to the C2B-B (Fig. 3B ▶) and C2B-C (Fig. 3C ▶) crystal structures and the C2B-NMR (Fig. 3A ▶) structure. In the NMR structure, where Ca2+ coordination is deduced from NOE patterns and divalent cation-induced chemical shift perturbations, supplemented by mu-tagenesis studies, site 1 is coordinated by the side-chain oxygens of D303, D309, D363, and D365, and main chain oxygen of Y364, while site 2 is coordinated with side-chain oxygens of D303, D363, D365, and D371, and the main chain oxygen of M302 (Fig. 3A ▶). The coordination geometries of sites 1 and 2 in the C2B-B (Fig. 3B ▶) and C2B-C (Fig. 3C ▶) crystal structures exhibit pentagonal-bipyramidyl coordination (Fig. 4B ▶), with site 1 coordinated to five protein residues (side-chain oxygens of D303, D309, D363, and D365 and main-chain oxygen of Y364) and one water molecule, while site 2 is coordinated to four protein residues (side-chain oxygens of D303, D363, and D365 and main chain oxygen of Y302) and three water molecules (Table 2). A third pentagonal-bipyramidyl Ca2+ coordination site, designated site 3, was identified in the C2B-C crystal structure (Fig. 3C ▶) soaked with high CaCl2 concentration, where the side chain of N333 appears to rotate and cooperate with D309 to bind the third Ca2+ ion. Site 3 is coordinated to three protein residues (side-chain oxygens of D309 and N333) and four water molecules (Figs. 3C ▶, 4B ▶). The most noteworthy difference between the C2B-B and C2B-C crystal structures and the C2B-NMR structure is that D371, which is involved in coordination to Ca2 in the NMR structure (Fig. 3A ▶), is not involved in Ca2+-binding in the crystal structures (Fig. 3B, C ▶).
Figure 3.

Cation-binding sites in (A) superpositioned C2B-NMR structures and (B) C2B-B, (C) C2B-C, and (D) C2B-D crystal structures. The Ca2+ and Sr2+ ions are colored orange. The oxygen atoms that coordinate the cations are colored red. Water molecules are represented as small red spheres. Dashed lines indicate pentagonal bipyramidyl coordination to Ca2+ sites (B,C) and tetragonal bipyramidyl coordination to Sr2+ site (D) in the crystal structures.
Figure 4.
(A) Partial amino acid sequences alignment of Syt homology group. This includes Syt I: synaptotagmin I (GenBank accession no. P21707); Syt II: synaptotagmin II (P29101); Syt III: synaptotagmin III (P40748); Syt IV: synaptotagmin IV (P50232); Syt V: synaptotagmin V (P47861); Syt VI: synaptotagmin VI (AAA87724); Syt VII: synaptotagmin VII (AAA87725), from Rattus norvegicus. The secondary structural elements of Syt I are also indicated. Schematic of Ca2+-binding sites 1, 2 and 3 (B) and Sr2+ binding site 1 (C). The cation binding sites are indicated by larger pink balls, while their coordination to amino acids and waters are indicated by smaller pink and yellow balls, respectively. The three Ca2+ coordination sites in B are of the pentagonal bipyramidyl type, while the single Sr2+ coordination site in C is of the tetragonal bipyramidyl type. These coordinations (distances of ≈2.5 Å) are indicated by blue lines. One additional, slightly longer coordination (distances of ≈2.7 Å) was observed for Ca2+ sites 1 and 2 (dashed green lines), D363:OD2 to Ca1 and D365:OD1 to Ca2, respectively (Table 2). These two interactions are from the bidentate aspartate, which is in coordination with the Ca2+-binding site (D363:OD1 to Ca1 and D365:OD2 to Ca2), and therefore excluded from the coordination system. Two hydrogen bonds are indicated by dashed blue lines.
Table 2.
Summary of the oxygen-ion distances (Å)
| C2B-NMRa | C2B-B | C2B-C | ||||||
| Ca1 | Ca2 | Ca1 | Ca2 | Ca1 | Ca2 | Ca3 | C2B-D Sr | |
| D303:OD1 | 2.80~2.83 | 2.57 | 2.54 | |||||
| D303:OD2 | 2.80~2.84 | 2.49~2.80 | 2.51 | 2.46 | 2.43 | 2.37 | 2.50 | |
| D309:OD1 | 2.18~2.64 | 2.50 | 2.35 | 2.60 | 2.44 | |||
| D309:OD2 | 2.37 | |||||||
| D363:OD1 | 2.52 | 2.50 | 2.41 | |||||
| D363:OD2 | 2.80~2.81 | 2.80~2.83 | 2.67b | 2.44 | 2.70b | 2.35 | 2.68 | |
| D365:OD1 | 2.60~2.81 | 2.46~2.80 | 2.48 | 2.75b | 2.42 | 2.74b | ||
| D365:OD2 | 2.25~2.81 | 2.62 | 2.52 | |||||
| N333:OD1 | 2.30 | |||||||
| M302:O | 2.81~2.83 | 2.48 | 2.37 | |||||
| Y364:O | 2.80~2.83 | 2.41 | 2.35 | 2.49 | ||||
| D371:OD1 | 2.81~2.83 | |||||||
| H2O | 2.41 | 2.35, 2.43, 2.62 | 2.45 | 2.33, 2.35, 2.52 | 2.25, 2.32, 2.33, 2.35 | 2.28 | ||
a Distances were measured for each of the NMR models individually. These distances are given to two decimal places, with the caveat that they represent estimates only, given the indirect nature of the measurements for these NMR structures.
b Distances between noncoordination pairs are indicated as italic.
In the presence of Sr2+ ions, C2B shows a different pattern of divalent cation binding. The Sr2+ ion is hexa-coordinated, with Sr2+ occupying site 1, where it is coordinated to four protein residues (side-chain oxygens of D303, D309, and D363 and main-chain oxygen of Y364), and one water molecule, thereby adopting an approximate tetragonal bi-pyramidal geometry (Fig. 4C ▶).
Discussion
We have solved four crystal structures of the C2B domain, three of which are in the presence of divalent cations. Two of these structures were solved at high resolution, namely 1.04 Å for C2B-B containing two bound Ca2+ ions (Fig. 3B ▶) and 1.18 Å for C2B-D containing one bound Sr2+ ion (Fig. 3D ▶). These structures therefore provide details of coordination within three separate divalent cation-binding sites (Table 2), the order of occupancy by bound divalent cations, and insights into the minimum number of divalent cations necessary for triggering exocytosis.
Our crystallographic studies establish that loop 1 is disordered in the divalent cation-free state (C2B-A structure) or in the presence of Mg2+, but becomes ordered in the presence of divalent cations Ca2+ (C2B-B and C2B-C structures) and Sr2+ (C2B-D structure). Divalent cation binding site 1 is occupied in both Ca2+-bound structures (Fig. 3B, C ▶) and the Sr2+-bound structure (Fig. 3D ▶). Divalent-cation site 2 is occupied in both Ca2+-bound structures (Fig. 3B, C ▶), but not in the Sr2+-bound structure (Fig. 3D ▶). It is therefore likely that site 1 is the highest-affinity cation-binding site, followed closely by site 2. NMR titration studies have estimated dissociation constants of 300–400 μM and 500–600 μM, for Ca2+-binding sites 1 and 2 on C2B, respectively (Fernandez et al. 2001). It should be noted that despite both Ca2+ sites exhibiting pentahedral-bipyramidyl coordination, site 1 is coordinated to six oxygens from protein residues, while site 2 is coordinated to four oxygens from protein residues. Four aspartic acid residues (D303, D309, D363, and D365) participate in divalent cation coordination of conserved sites 1 and 2. These four acidic residues are invariant among Syt I–VII (Fig. 4A ▶). It remains unclear at this time whether site 3, which is the weakest of the three Ca2+-binding sites, plays a functional role in Ca2+-mediated synaptic transmission.
The identification of a single bound Sr2+ in divalent cation-binding site 1 in the C2B-D crystal structure (Fig. 3D ▶) following soaking of the C2B-A crystal in 200 mM SrCl2, contrasts with the identification of Ca2+ ions bound in cation-binding site 1 and 2 in the C2B-B crystal structure (Fig. 3B ▶) following soaking of the C2B-A crystal in 200 mM CaCl2. The side chain of D365 is not involved in coordinating the Sr2+ in site 1 in the C2B-D crystal structure (Fig. 3D ▶), as it is in coordinating the Ca2+ in site 1 in the C2B-B crystal structure (Fig. 3B ▶). Nevertheless, Sr2+ can trigger fast exocytosis (Goda and Stevens 1994; Shin et al. 2003), which implies that a single bound divalent cation in site 1 is sufficient for maintaining the C2B fold, and therefore is competent for triggering membrane fusion. However, since transmitter release in hippocampal neurons has been shown to have an Sr2+ cooperativity of 4, other factors such as the presence of lipids, the tandem C2A domain, or synaptotagmin oligomerization may provide additional Sr2+-binding sites (Goda and Stevens 1994).
There is in general good overall agreement for coordination of Ca2+ at sites 1 and 2 on C2B from NMR (Fernandez et al. 2001) and crystallographic (this study) approaches. The only difference relates to the D371, which coordinates to Ca2 in the NMR structure (Fig. 3A ▶), but not in any of the crystal structures (Fig. 3B, D ▶). High resolution crystal structures unambiguously identify the divalent cation binding sites and the amino acid and water molecules involved in coordination. Nevertheless, there is always a concern that a key contact in crystal structures could be perturbed by packing interactions. In this regard, there exists a potential weak hydrogen bond (N•••O distance ≈3.12 Å) between one of the carboxylate oxygens of D371 and the side chain NH3+ of K324 from an adjacent molecule in the crystal lattice (Fig. 4B ▶). In our view, C2B would have to undergo a main-chain conformational change that D371 could directly coordinate to Ca2, since this would not be achievable solely through side-chain rotation of D371. The NMR approach does not directly monitor Ca2+ ions or the amino acid ligands coordinated to them. Rather, it uses a combination of NOE and chemical shift titration patterns to deduce this information, supplemented where available by relevant mutational data. In this regard, the D371N mutation appears to have a less severe effect on Ca2+ binding to the C2B domain than does the D309N mutation (Fernandez et al. 2001). Thus, there appears to be no obvious explanation for the difference between the crystallographic and NMR approaches regarding the role of D371 in Ca2 coordination at this time. Position 371 is occupied by either aspartic acid or glutamine among C2B homologs (Fig. 4A ▶). It is therefore conceivable that the longer glutamine side chain at position 371 could reach and coordinate Ca2+ in site 2, in different Syt iso-forms.
In summary, our high-resolution crystal structures have identified the protein groups and water molecules that coordinate the Ca2+ and Sr2+ cations in each of the three sites, as well as the local conformational changes within the C2B domain on proceeding from the free to the divalent cation-bound forms. The distinct cation-binding patterns observed for the C2B domain could provide insights into the mechanism of Ca2+-dependent synaptotagmin/SNARE complex interaction and Sr2+-triggered membrane fusion.
Materials and methods
Gene cloning, protein expression, and purification
The cDNA encoding the rat Syt I C2B domain (amino acids 271–421) was amplified by PCR and inserted into pGEX-KG plasmid between EcoRI and XhoI cleavage sites. A single colony of BL21 (λDE3) bacteria harboring the expression vector was cultured in 80 mL Luria-Bertani broth overnight, and was then used to inoculate 4 L media containing 50 μg/mL ampicillin. Cells were grown at 37°C until an OD600 of 0.8 was reached, and protein expression was induced overnight with 0.3 mM IPTG at room temperature. The bacteria were collected and resuspended in 250 mL buffer A (25 mM HEPES at pH 7.5, 300 mM NaCl). After disrupting the cells with a cell disruptor, bacteria were centrifuged at 235,000g for 1 h. The recovered clean lysate was loaded onto a 25 mL GSTrap column (Amersham) that was pre-equilibrated by buffer A. The column was washed with buffer A and the fusion protein was eluted with 25 mM Tris buffer (pH 8.0) in the presence of 10 mM glutathione. Digestion of the fusion protein was performed with 500 U of thrombin at 4°C, for 3 h. Syt I C2B domain was further purified by a heparin column and the main fraction was eluted with 720 mM NaCl and then applied to a Superdex-75 column. Pooled fractions containing the C2B domain were finally concentrated to ≈22 mg/mL.
Crystallization
Crystals were grown using the hanging-drop vapor diffusion method at 4°C. The crystallization drop was made by mixing 1 μL protein sample and 1 μL well solution (1.8 M ammonium sulfate, 100 mM sodium acetate at pH 5.0, and 5 mM DTT). The crystal was cryoprotected by soaking with 40% glycerol and 60% well solution. This crystal form, named C2B-A, belongs to P3221 space group with one molecule in the asymmetric unit (18.3 kDa; 2.439 Å3/Da; 48% solvent volume). The remaining crystals were obtained by soaking in divalent cation solutions. C2B-A crystals were washed four times with cryoprotectant solution (40% [v/v] glycerol, 100 mM sodium acetate at pH 5.0), and then transferred to cryoprotectant solution containing defined concentrations of calcium or strontium cations for 1 min, prior to freezing them in liquid nitrogen. The C2B-A crystals soaked in 200 and 600 mM CaCl2 were designated C2B-B and C2B-C, respectively, whereas the one soaked in 200 mM SrCl2 was designated C2B-D.
X-ray data collection
All X-ray data sets were collected at 100 K. The data set for C2B-A and C2B-C were collected on our in-house Rigaku R-AXIS HTC with 10 min exposure per 0.5° frame. The data set for C2B-B was collected at Advanced Photon Source, 14BM-C, Argonne National Laboratory, using 10 sec exposure per 0.2° frame for high-resolution data, followed by 0.65 sec exposure per 0.5° frame for low-resolution data. The data set for C2B-D was collected at APS 19BM, Argonne National Laboratory using 4 sec exposure per 0.25° frame. The statistics for each data set are given in Table 1.
Structure determination
The crystal structures were determined by a molecular replacement procedure using the program Molrep from the CCP4 suite (Collaborative Computational Project 1994). The coordinates of the NMR-based C2B solution structure (1K5W; Fernandez et al. 2001) was used as the search model. Crystallographic refinement was done with CNS (Brunger et al. 1998) and Refmac5 (Murshudov et al. 1997). We used 5% of the data for the Rfree calculation. Iterative cycles of interactive manual refitting of the model were carried out using the program Turbo, which made use of maps created with CNS or Refmac5 to complete and correct the model. Restrained refinement was carried out using a maximum likelihood target function and anisotropic temperature factors for individual atoms. Additional alternate conformations and water molecules were added. During the later stages of refinement, difference (Fo–Fc) maps were used to place the divalent atoms.
Coordinates deposition
The crystal structures of C2B-B, C2B-C and C2B-D have been deposited in PDB as 1TJX, 1UOV, and 1TJM, respectively.
Acknowledgments
We acknowledge access to the Advanced Photon Source beam-lines 14BM-C and 19BM at the Argonne National Laboratory, supported by the US Department of Energy, Office of Energy Research, under contract no. W-31-109-ENG-38.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.04832604.
References
- Brunger, A.T., Adams, P.D., Clore, G.M., DeLano, W.L., Gros, P., Grosse-Kunstleve, R.W., Jiang, J.S., Kuszewski, J., Nilges, M., Pannu, N.S., et al. 1998. Crystallography & NMR system: A new software suite for macro-molecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54 (Pt 5): 905–921. [DOI] [PubMed] [Google Scholar]
- Collaborative Computational Project, No. 4. 1994. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50 760–763. [DOI] [PubMed] [Google Scholar]
- Fernandez, I., Arac, D., Ubach, J., Gerber, S.H., Shin, O., Gao, Y., Anderson, R.G., Sudhof, T.C., and Rizo, J. 2001. Three-dimensional structure of the synaptotagmin 1 C2B-domain: Synaptotagmin 1 as a phospholipid binding machine. Neuron 32 1057–1069. [DOI] [PubMed] [Google Scholar]
- Fernandez-Chacon, R., Konigstorfer, A., Gerber, S.H., Garcia, J., Matos, M.F., Stevens, C.F., Brose, N., Rizo, J., Rosenmund, C., and Sudhof, T.C. 2001. Synaptotagmin I functions as a calcium regulator of release probability. Nature 410 41–49. [DOI] [PubMed] [Google Scholar]
- Geppert, M., Goda, Y., Hammer, R.E., Li, C., Rosahl, T.W., Stevens, C.F., and Sudhof, T.C. 1994. Synaptotagmin I: A major Ca2+ sensor for transmitter release at a central synapse. Cell 79 717–727. [DOI] [PubMed] [Google Scholar]
- Goda, Y. and Stevens, C.F. 1994. Two components of transmitter release at a central synapse. Proc. Natl. Acad. Sci. 91 12942–12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh, T.W. and Bellen, H.J. 2003. Synaptotagmin I, a Ca2+ sensor for neuro-transmitter release. Trends Neurosci. 26 413–422. [DOI] [PubMed] [Google Scholar]
- Mackler, J.M., Drummond, J.A., Loewen, C.A., Robinson, I.M., and Reist, N.E. 2002. The C2B Ca2+-binding motif of synaptotagmin is required for synaptic transmission in vivo. Nature 418 340–344. [DOI] [PubMed] [Google Scholar]
- Murshudov, G.N., Vagin, A.A., and Dodson, E.J. 1997. Refinement of macro-molecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53 240–255. [DOI] [PubMed] [Google Scholar]
- Shao, X., Fernandez, I., Sudhof, T.C., and Rizo, J. 1998. Solution structures of the Ca2+-free and Ca2+ -bound C2A domain of synaptotagmin I: Does Ca2+ induce a conformational change? Biochemistry 37 16106–16115. [DOI] [PubMed] [Google Scholar]
- Shin, O.H., Rhee, J.S., Tang, J., Sugita, S., Rosenmund, C., and Sudhof, T.C. 2003. Sr2+ binding to the Ca2+ binding site of the synaptotagmin 1 C2B domain triggers fast exocytosis without stimulating SNARE interactions. Neuron 37 99–108. [DOI] [PubMed] [Google Scholar]
- Sudhof, T.C. 2002. Synaptotagmins: Why so many? J Biol Chem 277 7629–7632. [DOI] [PubMed] [Google Scholar]
- Sutton, R.B., Davletov, B.A., Berghuis, A.M., Sudhof, T.C., and Sprang, S.R. 1995. Structure of the first C2 domain of synaptotagmin I: A novel Ca2+/ phospholipid-binding fold. Cell 80 929–938. [DOI] [PubMed] [Google Scholar]
- Sutton, R.B., Ernst, J.A., and Brunger, A.T. 1999. Crystal structure of the cytosolic C2A–C2B domains of synaptotagmin III. Implications for Ca+2-independent snare complex interaction. J. Cell. Biol. 147 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker, W.C. and Chapman, E.R. 2002. Role of synaptotagmin in Ca2+-triggered exocytosis. Biochem. J. 366 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C.T., Grishanin, R., Earles, C.A., Chang, P.Y., Martin, T.F., Chapman, E.R., and Jackson, M.B. 2001. Synaptotagmin modulation of fusion pore kinetics in regulated exocytosis of dense-core vesicles. Science 294 1111–1115. [DOI] [PubMed] [Google Scholar]
- Wang, C.T., Lu, J.C., Bai, J., Chang, P.Y., Martin, T.F., Chapman, E.R., and Jackson, M.B. 2003. Different domains of synaptotagmin control the choice between kiss-and-run and full fusion. Nature 424 943–947. [DOI] [PubMed] [Google Scholar]
- Yoshihara, M., Adolfsen, B., and Littleton, J.T. 2003. Is synaptotagmin the calcium sensor? Curr. Opin. Neurobiol. 13 315–323. [DOI] [PubMed] [Google Scholar]