Abstract
Peptidyl arms extending from one protein domain to another protein domain mediate many important interactions in biology. A well-studied example of this type of protein–protein interaction occurs between the yeast homeodomain proteins, MAT α2 and MAT a1, which form a high-affinity heterodimer on DNA. The carboxyl-terminal arm extending from MAT α2 to MAT a1 has been proposed to produce an allosteric conformational change in the a1 protein that generates a very large increase in the DNA binding affinity of a1. Although early studies lent some support to this model, a more recent crystal structure determination of the free a1 protein argues against any allosteric change. This note presents a thermodynamic argument that accounts for the proteins’ binding behavior, so that allosteric conformational changes are not required to explain the large affinity increase. The analysis presented here should be useful in analyzing binding behavior in other systems involving arm interactions.
Keywords: binding cooperativity, allostery, arm–domain, protein interactions, local concentration, mating type, peptidyl arm, peptidyl tail
Peptidyl arms or tails mediate interactions between proteins in a wide variety of processes, including DNA replication (Berdis et al. 1996; Sanders et al. 1997), signaling (Sano et al. 1997), vesicle transport (ter Haar et al. 2000), apoptosis (Shi 2002), and regulation by proteins like AraC (Saviola et al. 1997; Soisson et al. 1997; Seabold and Schleif 1998), and homeodomain proteins (Mak and Johnson 1993; Piper et al. 1999; LaRonde-LeBlanc and Wolberger 2003). An important paradigm for peptidyl arm–domain interactions is found in the interaction between the yeast homeodomain proteins, MAT α2 and MAT a1. The proteins bind to adjacent sites on the DNA and contacts between the proteins are mediated by a flexible carboxyl-terminal tail of α2 that becomes ordered upon binding to a1 (Wolberger et al. 1991; Mak and Johnson 1993). The binding of the carboxyl-terminal tail of MAT α2 to the globular protein domain of MAT a1 dramatically increases the DNA binding affinity of both proteins, and the large increase in binding affinity has been attributed to allosteric effects of the bound peptidyl tail that reshape the MAT a1 protein so as to increase its DNA binding affinity (Stark et al. 1999). Although structures of the two proteins have been interpreted to suggest the existence of such changes (Anderson et al. 2000; Ke et al. 2002), a more recent report of the structure of MAT a1 protein has raised the possibility that allosteric structural changes may not be required (Ke and Wolberger 2003). We show here that a very large cooperativity in DNA binding by two proteins interacting via an arm can result from the binding energetics of the arm, independent of any allosteric effects. Such a cooperative effect, whether mediated by an arm or not, can arise when the two interacting components are held near one another. This general mechanism for generating cooperativity has been well described (Creighton 1993) and analyzed, and is termed the chelate effect (Page and Jencks 1971). We present here an application of this analysis that readily accounts for the contribution to cooperative binding by a flexible peptidyl arm.
Consider two proteins, P and Q, whose dissociation constants from their respective DNA binding sites are Kp and Kq, and whose binding sites on DNA are positioned such that, when P and Q are both bound, an arm extending from P can bind to Q and that the apparent solution binding affinity of the arm to Q is Karm. Let P, Q, and D represent the concentrations of the free proteins and of DNA, PD and QD the concentrations of the individual complexes, (PQ)D the complex of P and Q on DNA without the arm binding to Q, and (P ~ Q)D the concentration of PQ complex on DNA with the arm from P bound to Q (Fig. 1 ▶). Directly from the definition of the dissociation constants,
Figure 1.
Pathways of complex formation. Each of these reactions are assumed to be in equilibrium. Only the (PQ)D complex is considered in the formation of the arm-bound complex (P~4Q) because the concentrations of P and Q are assumed to be much lower than Ceff, the effective concentration of the arm in the vicinity of its binding site on Q when both P and Q are bound to DNA.
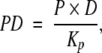 |
and
 |
Similarly, (PQ)D is obtained from the reaction of PD with Q, that is,
 |
and hence
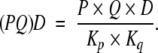 |
Binding of the arm to Q is governed by the effective concentration of the arm in the immediate vicinity of its binding site on Q, Ceff, and Karm. In solution, the fraction of the protein molecules that bind a ligand is
 |
if the concentration of ligand is Ceff and it binds to the protein with a dissociation constant of Karm. Equivalently, the fraction of time that an individual protein molecule in the solution binds the ligand is described by the same equation. Hence, (P ~ Q)D is proportional to
 |
and (PQ)D is proportional to
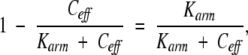 |
making the ratio
 |
Substituting from above,
 |
Hence, the apparent binding affinity is increased by the ratio of
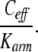 |
In the case of a1–α2, NMR data (Phillips et al. 1994) and calculation of the interaction energies from the crystal structure coordinates (Li et al. 1995), both show that the length of the linker from the end of the structured region of α2 to the position of the first significant contact with a1 is two residues, about 10 Å. If we approximate the linker as being flexible, then the nearest binding site on the tail is confined to the volume within a hemisphere of 10 Å radius (Fig. 2 ▶). Hence, the binding target on a1 is exposed to the binding site at a concentration of 1 molecule in a 10 Å radius hemisphere, which is an effective solution concentration of about 1 M. Because the binding affinity of the arm is about 2 × 10−4 M (Phillips et al. 1994), the cooperativity from energetic effects can be as large as
Figure 2.
Representation of the hemisphere within which the first binding region of the carboxyl tail of α2 is confined.
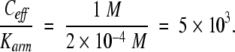 |
It has been found experimentally that Ka1 ≥ 10−5 M, and Kα2 ≈10−6 M (Phillips et al. 1994). Using the relationship derived above, the bimolecular dissociation constant can be estimated to be greater than
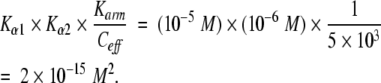 |
This is consistent with experimental data showing that the constant is 10−14 M2 to 10−15 M2 (Phillips et al. 1994). Thus, binding energetics are capable of providing all of the cooperativity that is observed experimentally.
The above calculation, together with the recent crystallographic study of the a1 homeodomain in the absence of α2 or DNA (Ke and Wolberger 2003) supports that idea that binding energetics are sufficient to account for the observed ability of the α2 protein to raise the affinity of a1 for DNA. Because there are many other examples of macromolecular interactions that are mediated by flexible peptide arms, the estimation we present here based on the concept of local concentration (Crothers and Metzger 1972; Margossian and Lowey 1978; Klemm and Pabo 1996) should be instructive in evaluating interactions in other systems and determining whether it is necessary to invoke factors other than simple cooperativity to account for binding behavior.
Acknowledgments
This work was supported by NIH Grant GM18277 to R.S. and NSF Grant MCB-0220191 to C.W.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.04908404.
References
- Anderson, J., Forman, M., Modleski, W., Dahlquist, F., and Baxter, S. 2000. Cooperative ordering in homeodomain–DNA recognition: Solution structure and dynamics of the MATa1 homeodomain. Biochemistry 39 10045–10054. [DOI] [PubMed] [Google Scholar]
- Berdis, A., Soumillion, P., and Benkovic, S. 1996. The carboxyl terminus of the bacteriophage T4 DNA polymerase is required for holoenzyme complex formation. Proc. Natl. Acad. Sci. 93 12822–12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton, T.E. 1993. Proteins—Structures and molecular properties. W. H. Freeman and Co., New York.
- Crothers, D.M. and Metzger, H. 1972. The influence of polyvalency on the binding properties of antibodies. Immunochemistry 9 341–357. [DOI] [PubMed] [Google Scholar]
- Ke, A. and Wolberger, C. 2003. Insights into binding cooperativity of MATa1/MATα2 from the crystal structure of a MATa1 homeodomain-maltose binding protein chimera. Protein Sci. 12 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke, A., Mathias, J., Vershon, A., and Wolberger, C. 2002. Structural and thermodynamic characterization of the DNA binding properties of a triple alanine mutant of MATα2. Structure 10 961–971. [DOI] [PubMed] [Google Scholar]
- Klemm, J.D. and Pabo, C.O. 1996. Oct-1 POU domain–DNA interactions: Cooperative binding of isolated subdomains and effects of covalent linkage. Genes & Dev. 10 27–36. [DOI] [PubMed] [Google Scholar]
- LaRonde-LeBlanc, N. and Wolberger, C. 2003. Structure of HoxA9 and Pbx1 bound to DNA: Hox hexapeptide and DNA recognition anterior to posterior. Genes & Dev. 17 2060–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, T., Stark, M., Johnson, A., and Wolberger, C. 1995. Crystal structure of the MATa1/MAT α 2 homeodomain heterodimer bound to DNA. Science 270 262–269. [DOI] [PubMed] [Google Scholar]
- Mak, A. and Johnson, A. 1993. The carboxy-terminal tail of the homeo domain protein α2 is required for function with a second homeo domain protein. Genes & Dev. 7 1862–1870. [DOI] [PubMed] [Google Scholar]
- Margossian, S. and Lowey, S. 1978. Interactions of myosin subfragments with F-actin. Biochemistry 17 5431–5439. [DOI] [PubMed] [Google Scholar]
- Page, M.I. and Jencks, W.P. 1971. Entropic contributions to rate accelerations in enzymic and intramolecular reactions and the chelate effect. Proc. Natl. Acad. Sci. 68 1678–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, C., Stark, M., Johnson, A., and Dahlquist, F. 1994. Heterodimerization of the yeast homeodomain transcriptional regulators α2 and a1 induces an interfacial helix in α2. Biochemistry 33 9294–9302. [DOI] [PubMed] [Google Scholar]
- Piper, D., Batchelor, A., Chang, C., Clearly, C., and Wolberger, C. 1999. Structure of a HoxB1-Pbx1 heterodimer bound to DNA: Role of the hexapeptide and a fourth homeodomain helix in complex formation. Cell 96 587–597. [DOI] [PubMed] [Google Scholar]
- Sanders, G., Kassavetis, G., and Geiduschek, E. 1997. Dual targets of a transcriptional activator that tracks on DNA. EMBO J. 16 1862–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano, T., Ohyama, K., Yamano, Y., Nakogomi, Y., Nakazawa, S., Kikyo, M., Shirai, H., Blank, J., Exton, J., and Inagami, T. 1997. A domain for G protein coupling in carboxyl-terminal tail of rat angiotensin II receptor type 1A. J. Biol. Chem. 272 23631–23636. [DOI] [PubMed] [Google Scholar]
- Saviola, B. and Schleif, R. 1997. Arm–domain interactions in AraC. J. Mol. Biol. 278 539–548. [DOI] [PubMed] [Google Scholar]
- Seabold, R. and Schleif, R. 1998. Apo-AraC actively seeks to loop. J. Mol. Biol. 278 529–538. [DOI] [PubMed] [Google Scholar]
- Shi, Y. 2002. A conserved tetrapeptide motif: Potentiating apoptosis through IAP-binding. Cell Death Differ. 9 93–95. [DOI] [PubMed] [Google Scholar]
- Soisson, S., MacDougall-Shackleton, B., Schleif, R., and Wolberger, C. 1997. Structural basis for ligand-regulated oligomerization of AraC. Science 276 421–425. [DOI] [PubMed] [Google Scholar]
- Stark, M., Escher, D., and Johnson, A. 1999. A trans-acting peptide activates the yeast a1 repressor by raising its DNA-binding affinity. EMBO J. 18 1621–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Haar, E., Harrison, S., and Kirchausen, T. 2000. Peptide-in-groove interactions link target proteins to the β-propeller of clathrin. Proc. Natl. Acad. Sci. 97 1096–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolberger, C., Vershon, A., Liu, B., Johnson, A., and Pabo, C. 1991. Crystal structure of a MAT α2 homeodomain–operator complex suggests a general model for homeodomain–DNA interactions. Cell 67 517–528. [DOI] [PubMed] [Google Scholar]




