Abstract
The chaperone-like activity of human lens α-crystallin in inhibiting the aggregation of denatured proteins suggests a role for α-crystallin in cataract prevention. Although a variety of techniques have generated structural information relevant to its chaperone-like activity, the size and heterogeneity of α-crystallin have prevented determination of its crystal structure. Even though synthetic cross-linkers have provided considerable information about protein structures, they have not previously been used to study the proximity and orientation of subunits within human α-crystallin. Cross-linkers provide structural insight into proteins by binding the side chains of amino acids within close proximity. To identify the cross-linked residues, the modified protein is digested and the resulting peptides are analyzed by mass spectrometry. Analysis of products from the reaction of α-crystallin with 3,3′dithiobis(sulfosuccinimidyl propionate), DTSSP, identified several modifications to both αA and αB. The most structurally informative of these modifications was a cross-link between lysine 166 of αA and lysine 175 of αB. This cross-link provides experimental evidence supporting theoretical structural models that place the C termini of αA and αB within close proximity in the native aggregate.
Keywords: structural analysis, α-crystallin, synthetic cross-linker, DTSSP, ESIMS
In humans, the native α-crystallin assembly is composed of approximately 40 subunits of two 20-kDa proteins: αA (de Jong et al. 1975; Takemoto and Emmons 1991; Miesbauer et al. 1994) and αB (Kramps et al. 1977; Dubin et al. 1990; Miesbauer et al. 1994) in a ratio of αA to αB of approximately 3:2 (Ma et al. 1998). In addition to its role as a structural protein, α-crystallin has demonstrated chaperone-like activity in suppressing the denaturation-induced aggregation of some proteins (Horwitz 1992; Raman and Rao 1994; Farahbakhsh et al. 1995; Raman et al. 1995; Wang and Spector 1995). Information regarding the three-dimensional structure of α-crystallin would help elucidate its mechanism of chaperone-like activity. Three-dimensional structures of proteins are most often determined by X-ray crystallography and NMR. Neither of these techniques has provided much information about α-crystallin because the crystals of α-crystallin needed for X-ray crystallography have not been produced, and high-resolution NMR analysis has been hampered by the large molecular mass of α-crystallin. Cross-linking residues within close proximity, another approach for obtaining three-dimensional structural information, offers limited resolution, but can be applied to sparingly soluble proteins of virtually any size. Cross-linkages may be either intramolecular or intermolecular. Intramolecular cross-linking demonstrates which regions within a protein subunit are adjacent (Young et al. 2000; Pearson et al. 2002; Kruppa et al. 2003), while intermolecular cross-linking indicates adjacent regions of subunits in a protein assembly (Callaghan et al. 1999; Luo et al. 1999; Bennett et al. 2000). There are several types of synthetic cross-linkers of various specificities and lengths.
We report data from reaction of α-crystallin with the bis-N-hydroxysuccinimidyl (NHS) ester cross-linker, DTSSP (Staros 1982; Staros and Kakkad 1983; Ehrlich et al. 1994; Jordan et al. 1997; Topham et al. 1999; Bennett et al. 2000; Chan et al. 2000). This cross-linker reacts with the primary amines of lysines, the N termini of proteins (Lomant and Fairbanks 1976), and the hydroxyl groups of tyrosines and serines (Swaim et al. 2004). To locate DTSSP modifications, the modified protein was digested, and the resulting peptides were analyzed by mass spectrometry. Products included peptides with intramolecular cross-links (the mass of the peptide plus 174 Da), peptides with intermolecular cross-links (the sum of the two peptide masses plus 174 Da), and peptides with a hydrolyzed DTSSP adduct (the mass of the peptide plus 192 Da). Reducing the central disulfide bond of DTSSP cleaved the cross-linkage and generated a mass increase of 88 Da at each modified site.
Discussion
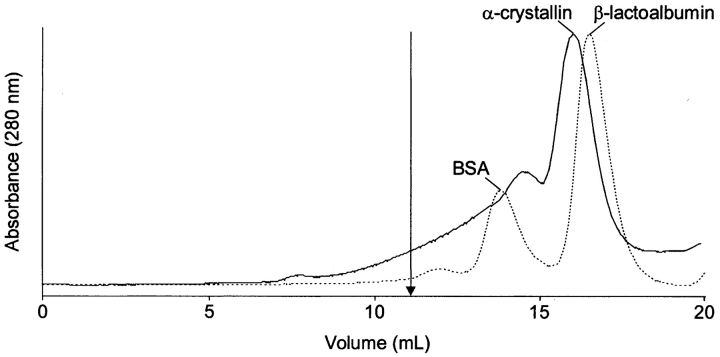
Human α-crystallin from an 11-year-old lens was modified by DTSSP using a DTSSP-to-protein ratio of 5:1 according to the procedure described previously (Swaim et al. 2004). Cross-linked α-crystallin was isolated by size-exclusion chromatography (SEC) using a denaturing buffer (6 M guanidine hydrochloride (GdnHCl), 25 mM 2-morpholinoethanesulfonic acid (MES) solution [pH 5.6]). This chromatographic separation (Fig. 1 ▶) gave a large peak at the expected elution time of α-crystallin monomers, indicating that much of the α-crystallin was not involved in intermolecular cross-links. An earlier eluting peak with a broad leading shoulder indicated the presence of higher molecular mass species, probably dimers and trimers of α-crystallin subunits. This entire peak was collected and used for our analysis.
Figure 1.
Size-exclusion separation of α-crystallin after incubation with DTSSP using GdnHCl in the running buffer (——). An arrow indicates the elution volume of native α-crystallin (11 mL) under nondenaturing conditions. The dashed line (- - -) marks the elution of the molecular weight markers BSA (66 kDa, 14.5 mL) and β-lactoalbumin (18.4 kDa, 16 mL).
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis of fractions from the SEC separation, both with and without mercaptoethanol, confirmed that the higher molecular weight species were products of DTSSP cross-linking (data not shown). After digestion of the cross-linked proteins with trypsin, the resulting peptides were analyzed by online reversed-phase HPLC electrospray ionization mass spectrometry (ESIMS) following published procedures (Swaim et al. 2004).
Several peptides with DTSSP modifications were detected in this analysis. There were masses corresponding to peptides with hydrolyzed adducts, a mass indicative of a peptide with an intramolecular cross-link and, the most interesting modification, a mass corresponding to peptides modified by an intermolecular cross-link.
The masses corresponding to peptides with hydrolyzed adducts indicated that this modification was at five of the seven lysines in αA (residues 11, 78, 99, 145, and 166) and eight of the 10 lysines in αB (residues 72, 82, 90, 92, 150, 166, 174, and 175). Peptides including αA Lys 88 and αB Lys 103 were only detected without modification, and no peptides including αA Lys 70 and αB Lys 121, either with or without modification, were detected. For each modified peptide, the modification was located from CID (collision-induced dissociation) ESIMS/MS analysis.
The detection of hydrolyzed adducts suggested that most lysines are in exposed regions of the native α-crystallin assembly, but the amino group is not within 12 Å of another reactive group. Recent analysis of α-crystallin by H-D exchange demonstrated relatively high reactivity at both the N and C termini (Hasan et al. 2003). These regions include the lysines at αA 11, 70, and 166 and at αB 72, 166, 174, and 175. In our analysis, all of these lysines except αA Lys 70 were found modified by DTSSP. Because peptides including αA Lys 70 were not detected in analyses of either unmodified α-crystallin or α-crystallin incubated with DTSSP, no conclusion could be made regarding its exposure to DTSSP. In contrast, αA Lys 88 and αB Lys 103, neither of which reacted with DTSSP, as well as αB Lys 121, which was not detected either with or without modification, all lie within regions that showed low H-D exchange. The low reactivity at αB Lys 88 toward DTSSP is also consistent with bis-ANS binding to αA 79–88 from bovine lenses, which suggested that αB Lys 88 is part of a hydrophobic region (Sharma et al. 1998). Spin labeling studies have demonstrated a region of low exposure beginning at residue 76 of αA followed by α-sheets through residue 119 (Koteiche and Mchaourab 1999). The homologous region in αB is residues 80–123. The α-sheets are thought to be the interface between molecules as they form the α-crystallin assemblies. If αA Lys 88, αB Lys 103, and αB Lys 121 were sequestered in the dimer interface, they might not be available for reaction with DTSSP, and would therefore not form cross-links even though they were in close proximity to other lysines.
There was also evidence suggesting hydrolyzed adducts at some serines in αB. However, because the CID spectra did not show conclusively which residues were modified, it was determined only that the modified residue(s) was probably among serines 135, 136, 138, and 139. The absence of reactivity at tyrosines and serines cannot be attributed to their position within the structure of α-crystallin because DTSSP has less reactivity with these residues than with lysines (Swaim et al. 2004).
An intramolecular cross-link was indicated by the presence of an ion at m/z 721.82+, corresponding to αB peptide 164–175 (MW 1267.6) with a mass increase of 174 Da. The CID mass spectrum of this ion demonstrated an intramolecular cross-link between Lys 174 and Lys 175, consistent with their close proximity in the αB subunit (data not shown).
The ion at m/z 676.82+ suggested an intermolecular cross-link between αA and αB. This mass corresponded to the molecular mass of αA peptide 164–173 (MW 1031.5) with a cross-link to a single lysine residue (MW 146.1). A single lysine plus the DTSSP cross-linker (174 Da) would generate a mass increase of 320 Da to the peptide. The point of attachment was determined from the CID mass spectrum of the modified peptide, m/z 676.82+ (Fig. 2 ▶). Unmodified fragments y2 through y4 and y7 demonstrated that residues 167 to 173 of αA peptide 164–173 were not modified. No unmodified b fragments were detected, while the fragments b3, b4, and b7 were detected with mass increases of 320 Da. These modified fragments indicated that the intermolecular cross-link to the single lysine was at Lys 166, residue 3 of αA 164–173. This assignment was supported by the ion at m/z 449.21+, corresponding to the MH+ for an internal fragment consisting of αA Lys 166 (128 Da) cross-linked (174 Da) to a single lysine (146 Da).
Figure 2.
HPLC ESIMS/MS analysis of αA peptide 164–173 (MW 1031.5) with an intermolecular cross-link to a single lysine (MW 146.1). The mass spectrum of the ion analyzed (m/z 676.82+) is inset. The sequence of αA 164–173 is shown with the b and y fragments that would result from a mass increase of 320 Da to Lys 166.
The residue attached to αA 164–173 was deduced by considering what single lysines could be generated by tryptic digestion of α-crystallin. Although no single lysine can be generated by tryptic digestion of αA, digestion of αB could generate Lys 121, Lys 150, and Lys 175. However, neither Lys 121 nor Lys 150 is likely to be the single lysine cross-linked to αA 164–173 because trypsin usually does not cleave C-terminal to a modified lysine. Because it is preceded by a lysine (Lys 174) and it is the last residue of αB, isolated Lys 175 can be produced by trypsin digestion without C-terminal cleavage. Therefore, αB Lys 175 is most likely the single residue bound to Lys 166 of αA peptide 164–173. This intermolecular crosslink supports structural models proposing that α-crystallin exists as either a protein micelle (Augusteyn and Koretz 1987; Radlick and Koretz 1992) or a GroEL-like assembly (Carver et al. 1994). Both models are based on the amphipathic nature of αA and αB (Puri et al. 1983) and involve sequestering of the hydrophobic N termini and solvent-exposure of the hydrophilic C-termini. Formation of this intermolecular cross-link between αA 164–173 and αB 175 is the first conclusive evidence that lysine amino groups in the C termini of αA and αB are within 12 Å in the native assembly.
Acknowledgments
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.04910004.
References
- Augusteyn, R.C. and Koretz, J.F. 1987. A possible structure for α-crystallin. FEBS Lett. 222 1–5. [DOI] [PubMed] [Google Scholar]
- Bennett, K.L., Kussmann, M., Bjork, P., Godzwon, M., Mikkelsen, M., Sorensen, P., and Roepstorff, P. 2000. Chemical cross-linking with thiol-cleavable reagents combined with differential mass spectrometric peptide mapping—A novel approach to assess intermolecular protein contacts. Protein Sci. 9 1503–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan, J., Simonsen, A., Gaullier, J.-M., Toh, B.-H., and Stenmark, H. 1999. The endosome fusion regulator early-endosomal autoantigen 1 (EEA1) is a dimer. Biochem. J. 338 539–543. [PMC free article] [PubMed] [Google Scholar]
- Carver, J.A., Aquilina, J.A., and Truscott, R.J. 1994. A possible chaperone-like quaternary structure for α-crystallin. Exp. Eye Res. 59 231–234. [DOI] [PubMed] [Google Scholar]
- Chan, F.K.-M., Chun, H.J., Zheng, L., Siegel, R.M., Bui, K.L., and Lenardo, M.J. 2000. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science 288 2351–2354. [DOI] [PubMed] [Google Scholar]
- de Jong, W.W., Terwindt, E.C., and Bloemendal, H. 1975. The amino acid sequence of the A chain of human α-crystallin. FEBS Lett. 58 310–313. [DOI] [PubMed] [Google Scholar]
- Dubin, R.A., Ally, A.H., Chung, S., and Piatigorsky, J. 1990. Human α B-crystallin gene and preferential promoter function in lens. Genomics 7 594–601. [DOI] [PubMed] [Google Scholar]
- Ehrlich, L.S., Agresta, B.E., Gelfand, C.A., Jentoft, J., and Carter, C.A. 1994. Spectral analysis and tryptic susceptibility as probes of HIV-1 capsid protein structure. Virology 204 515–525. [DOI] [PubMed] [Google Scholar]
- Farahbakhsh, Z.T., Huang, Q.L., Ding, L.L., Altenbach, C., Steinhoff, H.J., Horwitz, J., and Hubbell, W.L. 1995. Interaction of α-crystallin with spin-labeled peptides. Biochemistry 34 509–516. [DOI] [PubMed] [Google Scholar]
- Hasan, A., Yu, J., Smith, D.L., and Smith, J.B. 2003. Thermal stability of human α-crystallins sensed by amide hydrogen exchange. Protein Sci. 13 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz, J. 1992. α-crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. 89 10449–10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan, J., Murciano, J., Lotero, A., Herraez, A., and Diez, J. 1997. In vitro properties and organ uptake of rat band 3 cross-linked erythrocytes. Biochimie 79 53–61. [DOI] [PubMed] [Google Scholar]
- Koteiche, H.A. and Mchaourab, H.S. 1999. Folding pattern of the α-crystallin domain in αA-crystallin determined by site-directed spin labeling. J. Mol. Biol. 294 561–577. [DOI] [PubMed] [Google Scholar]
- Kramps, J.A., de Man, B.M., and de Jong, W.W. 1977. The primary structure of the B2 chain of human α-crystallin. FEBS Lett. 74 82–84. [DOI] [PubMed] [Google Scholar]
- Kruppa, G.H., Schoeniger, J., and Young, M.M. 2003. A top down approach to protein structural studies using chemical cross-linking and fourier transform mass spectrometry. Rapid Commun. Mass Spectrom. 17 155–162. [DOI] [PubMed] [Google Scholar]
- Lomant, A.J. and Fairbanks, G. 1976. Chemical probes of extended biological structures: Synthesis and properties of the cleavable protein cross-linking reagent [35S]dithiobis(succinimidyl propionate). J. Mol. Biol. 104 243–261. [DOI] [PubMed] [Google Scholar]
- Luo, Y., Leszyk, J., Qian, Y., Gergely, J., and Tao, T. 1999. Residues 48 and 82 at the n-terminal hydrophobic pocket of rabbit skeletal muscle troponin-c photo-cross-link to met121 of troponin-I. Biochemistry 38 6678–6688. [DOI] [PubMed] [Google Scholar]
- Ma, Z., Hanson, S.R., Lampi, K.J., David, L.L., Smith, D.L., and Smith, J.B. 1998. Age-related changes in human lens crystallins identified by HPLC and mass spectrometry. Exp. Eye Res. 67 21–30. [DOI] [PubMed] [Google Scholar]
- Miesbauer, L.R., Zhou, X., Yang, Z., Sun, Y., Smith, D.L., and Smith, J.B. 1994. Post-translational modifications of water-soluble human lens crystallins from young adults. J. Biol. Chem. 269 12494–12502. [PubMed] [Google Scholar]
- Pearson, K.M., Pannell, L.K., and Fales, H.M. 2002. Intramolecular cross-linking experiments on cytochrome c and ribonuclease a using an isotope multiplet method. Rapid Commun. Mass Spectrom. 16 149–159. [DOI] [PubMed] [Google Scholar]
- Puri, N., Augusteyn, R.C., Owen, E.A., and Siezen, R.J. 1983. Immunochemical properties of vertebrate α-crystallins. Eur. J. Biochem. 134 321–326. [DOI] [PubMed] [Google Scholar]
- Radlick, L.W. and Koretz, J.F. 1992. Biophysical characterization of α-crystallin aggregates: Validation of the micelle hypothesis. Biochim. Biophys. Acta 1120 193–200. [DOI] [PubMed] [Google Scholar]
- Raman, B. and Rao, C.M. 1994. Chaperone-like activity and quaternary structure of α-crystallin. J. Biol. Chem. 269 27264–27268. [PubMed] [Google Scholar]
- Raman, B., Ramakrishna, T., and Rao, C.M. 1995. Rapid refolding studies on the chaperone-like α-crystallin. Effect of α-crystallin on refolding of β- and γ-crystallins. J. Biol. Chem. 270 19888–19892. [DOI] [PubMed] [Google Scholar]
- Staros, J.V. 1982. N-Hydroxylsulfosuccinimide active esters: Bis(n-hydroxy-sulfosuccinimide) esters of two dicarboxylic acids are hydrophilic, membrane-impermeant, protein cross-linkers. Biochemistry 21 3950–3955. [DOI] [PubMed] [Google Scholar]
- Staros, J.V. and Kakkad, B.P. 1983. Cross-linking and chymotryptic digestion of the extracytoplasmic domain of the anion exchange channel in intact human erythrocytes. J. Membr. Biol. 74 247–254. [DOI] [PubMed] [Google Scholar]
- Swaim, C.L., Smith, D.L., and Smith, J.B. 2004. Unexpected products from the reaction of the synthetic cross-linker 3,3′-dithiobis(sulfosuccinimidyl propionate), DTSSP with peptides. J. Am. Soc. Mass Spectrom. 15 736–749. [DOI] [PubMed] [Google Scholar]
- Takemoto, L. and Emmons, T. 1991. Truncation of α A-crystallin from the human lens. Exp. Eye Res. 53 811–813. [DOI] [PubMed] [Google Scholar]
- Topham, P.S., Kawachi, H., Haydar, S.A., Chugh, S., Addona, T.A., Charron, K.B., Holzman, L.B., Shia, M., Shimizu, F., and Salant, D.J. 1999. Nephritogenic mAb 5–1–6 is directed at the extracellular domain of rat nephrin. J. Clin. Invest. 104 1559–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, K. and Spector, A. 1995. α-crystallin can act as a chaperone under conditions of oxidative stress. Invest. Ophthalmol. Vis. Sci. 36 311–321. [PubMed] [Google Scholar]
- Young, M.M., Tang, N., Hempel, J.C., Oshiro, C.M., Taylor, E.W., Kuntz, I.D., Gibson, B.W., and Dollinger, G. 2000. High throughput protein fold identification by using experimental constraints derived from intramolecular cross-links and mass spectrometry. Proc. Natl. Acad. Sci. 97 5802–5806. [DOI] [PMC free article] [PubMed] [Google Scholar]