Abstract
Hydrogen-bonded β-turns in proteins occur in four categories: type I (the most common), type II, type II’, and type I’. Asx-turns resemble β-turns, in that both have an NH. . .OC hydrogen bond forming a ring of 10 atoms. Serine and threonine side chains also commonly form hydrogen-bonded turns, here called ST-turns. Asx-turns and ST-turns can be categorized into four classes, based on side chain rotamers and the conformation of the central turn residue, which are geometrically equivalent to the four types of β-turns. We propose asx- and ST-turns be named using the type I, II, I’, and II’ β-turn nomenclature. Using this, the frequency of occurrence of both asx- and ST-turns is: type II’ > type I > type II > type I’, whereas for β-turns it is type I > type II > type I’ > type II’. Almost all type II asx-turns occur as a recently described three residue feature named an asx-nest.
Keywords: asx-turn, β-turn, hydrogen-bond, nest, ST-turn
Hydrogen-bonded β-turns are considered to have four residues, i, i + 1, i + 2, and i + 3, with the defining hydrogen bond between the main chain CO group of residue i and the main chain NH group of residue i + 3. The division into four categories (Venkatachalam 1968) for peptides with trans peptide bonds has been confirmed (Richardson 1981; Baker and Hubbard 1984; Wilmot and Thornton 1988, 1990; Hutchinson and Thornton 1994, 1996; Gunasekharan et al. 1998).
Richardson (1981) pointed to the frequent occurrence of asx-turns and their resemblance to β-turns. The term asx means either aspartate or asparagine, which behave similarly in that both have side chain γ-carbonyl groups. Both asx-turns and β-turns form a 10-atom, hydrogen-bonded ring. In asx-turns, the main chain atoms in residue i and the NH group of residue i + 1 in the β-turn are replaced by the side chain of an asx residue. Hence the asx side-chain atoms mimic the main-chain ones of the β-turn. Asx-turns have three residues, i(asx), i + 1, and i + 2, so the residue numbering differs from that of homologous β-turns, such that i + 1 of asx-turns corresponds to i + 2 of β-turns. Several authors (Tainer et al. 1982; Rees et al. 1983; Richardson and Richardson 1989; Eswar and Ramakrishnan 1999; Wan and Milner-White 1999a; Chakrabarti and Pal 2001) discuss the asx-turn and others (Presta and Rose 1988; Richardson and Richardson 1988; Bordo and Argos 1994; Doig et al. 1997; Aurora and Rose 1998; Pal et al. 2003) show it is common at the N terminus of α-helices.
The side chain oxygen atoms of serine and threonine residues (i) often form a hydrogen bond with the main chain NH groups of the residue two ahead (i + 2). Such features are like asx-turns except that they are 9-atom, instead of 10-atom, hydrogen-bonded rings. Data have been assembled (Baker and Hubbard 1984; Eswar and Ramakrishnan 1999, 2000) showing their common occurrence and pointing to their similarity with asx-turns. We refer to these collectively as ST-turns (Wan and Milner-White 1999b). Sequence comparisons of homologous proteins (Vijayakumar et al. 1999; Wan and Milner-White 1999b) show that, within and between asx- and ST-turns, the four residues in question often substitute each other.
Previous work on ST-turns has noted their prevalence and grouped them into three geometrically distinct categories, but their similarity to β-turns has not been explicitly discussed. Asx-turns have been more extensively studied, and of particular relevance is the work of Eswar and Ramakrishnan (1999) who categorized asx-turns into four geometrically distinct classes noting some similarities with the β-turn classes. However, their insights regarding the four classes were not presented in summary form and careful reading of the entire paper is required to absorb them. There is a need for making these important findings more accessible so that the general reader has a realistic opportunity to appreciate them. We also expand on them by noting that there are four categories of ST-turns, which also, despite having one less carbon atom, clearly resemble the four types of β-turns. It therefore seems appropriate that both asx- and ST-turns should in future be named using the type I, I’, II, and II’ β-turn nomenclature.
A commonly occurring anion-binding motif has been described (Watson and Milner-White 2002a, b) as a nest because it consists of a concavity made from the main chain NH groups of three successive amino-acid residues. The anionic group bound is often a single oxygen atom with either a whole or partial negative charge. A recurring variant of this was called an asx-nest because the first nest residue is either aspartate or asparagine and its side chain oxygen is the anion in the nest. A similar distribution is found for ST-nests, which are like asx-nests except that either serine or threonine is the first residue and its side chain oxygen occupies the nest. They are relevant because it emerges that asx-and ST-turns mimicking type II β-turns are the same as asx- and ST-nests.
Results
In a database of 500 proteins, Table 1 shows the numbers of each N, D, S or T residue and of those involved in asx- or ST-turns. Both asx-turns and β-turns consist of a 10-atom, hydrogen-bonded ring closed by a CO. . .H-N hydrogen bond. ST-turns are similar but the ring is 9-membered. Figure 1 ▶ provides a comparison of the relevant torsion angles of the two types of turn. To facilitate this, a new set of angles called ωe, φe, and ψe are defined in Figure 1 ▶, A–C. These are angles in the asx- or ST-turn equivalent to corresponding angles in the β-turn. If there is mimicry, the angles φe and ψe in asx- and ST-turns should resemble φ and ψ of residue i + 1 in β-turns, whereas φ and ψ of residue i + 1 of asx- and ST-turns should resemble φ and ψ of residue i + 2 of β-turns.
Table 1.
Proportions of residues involved in β-turn mimics
| Residue type | Asn | Asp | Ser | Thr | N/D/S/T |
| Number in database | 5096 | 6617 | 6728 | 6345 | 24,786 |
| Number forming asx- or ST-turns | 569 | 1071 | 491 | 367 | 2498 |
| Percentage forming asx- or ST-turns | 11.2 | 16.2 | 7.3 | 5.8 | 10.1 |
The total numbers of aspartate, asparagine, serine, and threonine residues in the dataset of 500 proteins are given, along with the numbers and percentages of each of that form of asx- and ST-turns (i.e., occupy position i of an asx- or ST-turn).
Figure 1.
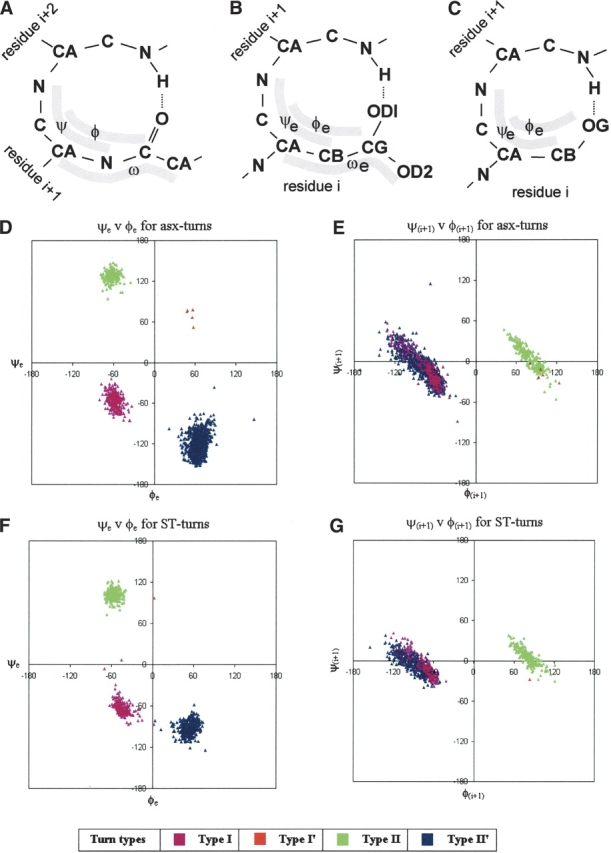
Distributions of torsion angles of asx- and ST-turns. (A–C) Diagrams of the β-turn, asx-turn (with aspartate side chain), and ST-turn (with serine side chain), respectively.φe, ψe, and ωe are the angles equivalent to φ, ψ, and ω of β-turns; they are also defined in B and C. Residue positions are indicated, and it should be noted that position i + 1 of an asx- or ST-turn is equivalent to i + 2 of a β-turn. ψe, φe, and ωe have a fixed approximate relationship to ψ, χ1, and χ2: ψe ≈ψ+ 120°; φe ≈χ1 - 120°; ωe ≈χ2 + 180°. (D–E) Turns of different types are represented by data points of different colors, as indicated by the legend. D and E show asx-turn angles whereas F and G show ST-turn angles. D and F plot the asx- or ST-turn angles φe vs. ψe, equivalent to φ and ψ of residue i + 1 of a β-turn; E and G plot φ against ψ for asx- or ST-turn residue i + 1, equivalent to residue i + 2 of a β-turn.
Figure 1 ▶, D and F, reveals that the φe,ψe values for asx- and ST-turns cluster into four groups placed symmetrically on the Ramachandran plot, making allowance for one of the groups being sparsely populated. In Figure 1 ▶, E and G, the φ,ψ values for residue i + 1 are plotted; here two clusters are observed, which is consistent with the four groupings. Finding four groups leads to a consideration of the extent to which they are related to the four known groups of β-turns. Torsion angle values were used to group the asx- and ST-turns into types equivalent to the four types of hydrogen-bonded β-turn. The data points in Figure 1 ▶, D–G, have been colored to allow comparison of distributions of different types across the asx- and ST-turns.
Table 2 provides a comparison of the average values of theφ,ψ angles of residues i + 1 and i + 2 of types I, I’, II, and II’ β-turns with those of the corresponding φe,ψe angles, and residue i + 1 φ,ψ angles, of asx- and ST-turns. It is evident that the four groups of both asx- and ST-turns have average torsion angle values that resemble those of β-turns. For asx-turns the similarity is most striking whereas slight differences are observed between the ST-turns and β-turns. The average ψe angles of the type II and II’ ST-turns differ from ψ of the type II and II’ β-turns by 18° and 27°, respectively. However, the similarity is still apparent and for this reason it is appropriate that the nomenclature for the four β-turn types be used for asx- and ST-turns.
Table 2.
Comparing torsion angles between β-turns and their minics
| Torsion angle values | |||||||||||
| Turn type | No. | ω | ωe | φ | φe | ψ | ψe | φ (i+2) | φ (i+1) | ψ (i+2) | ψ (i+1) |
| Type I β-turn | trans | −60 | −30 | −90 | 0 | ||||||
| Type I asx-turn | 302 | −143 | −58 | −55 | −72 | −13 | |||||
| Type I ST-turn | 205 | N/A | −44 | −62 | −75 | −9 | |||||
| Type I’ β-turn | trans | 60 | 30 | 90 | 0 | ||||||
| Type I’ asx-turn | 5 | 102 | 53 | 70 | 98 | −18 | |||||
| Type I’ ST-turn | 0 | N/A | — | — | — | — | |||||
| Type II β-turn | trans | −60 | 120 | 80 | 0 | ||||||
| Type II asx-turn | 203 | −176 | −62 | 128 | 80 | 5 | |||||
| Type II ST-turn | 207 | N/A | −57 | 102 | 80 | 7 | |||||
| Type II’ β-turn | trans | 60 | −120 | −80 | 0 | ||||||
| Type II’ asx-turn | 1130 | 155 | 65 | −123 | −73 | −14 | |||||
| Type II’ ST-turn | 445 | N/A | 53 | −93 | −89 | −5 | |||||
Numbers and average torsion angles of four different types of asx- and ST-turns are given, along with ideal β-turn torsion angle values (Lewis et al. 1973; Wilmot and Thornton, 1990). The widely quoted so-called ideal values, from the original β-turn analysis of Venkatachalam (1968), are included. More recent β-turn definitions have often involved distance criteria rather than hydrogen bonding so comparison with such values might be misleading. Ideal torsion angle values of β-turns and values of equivalent torsion angles of asx- and ST-turns share the same columns. The shorter side chains of ST-turns do not have ωe angles.
The numbers of different types of asx- and ST-turns in the database are shown in Table 2. In β-turns the most common occurrence is of type I; this might be regarded as a 3/10-helix with only one hydrogen bond; such a conformation is energetically favorable. In asx and ST-turns the most common is type II’, whereas the next most common is type I. The structures of type II asx- and ST-turns are the same as those previously identified as asx-nest or ST-nest motifs (Watson and Milner-White 2002a). The tight clustering of φe and ψe angles in type II asx- and ST-turns, seen in Figure 1 ▶, D and F, may result from the constrained nest conformation. One factor allowing asx-turns (but not ST-turns) to exhibit minor conformational differences from β-turns is the fact that ωe, corresponding to ω of residue i + 1 of β-turns, is a side chain angle (χ2), so is not restrained to the trans conformation of ω. The flexibility of ωe provides extra conformational possibilities for asx-turns compared to β-turns.
Discussion
Four types of asx- and ST-turns are observed that are geometrically equivalent to the four main types of β-turns: I, I’, II, and II’. We propose they be named in the same way. The most common of the asx- and ST-turn conformations is type II’, characterized by a side chain χ1 value around 180° (angle interconversion is discussed in the legend to Fig. 1 ▶). Types I and II are less common. Type I has χ1 of about 60° and mimics the main chain part of a 3/10-helix. Type II also has χ1 in the region of 60° and has a central residue (i + 1) with the αL or γL conformation. The rarest is type I’.
Considering that ST-turns consist of a hydrogen-bonded ring of 9, rather than 10, atoms, it is at first sight surprising they occur in the same classes as asx-turns with similar structures. However, the chemical properties of the four residues (D, N, S, and T) are not dissimilar and they exhibit a tendency in such situations to substitute each other over evolutionary time (Vijayakumar et al. 1999; Wan and Milner-White 1999b).
The greater flexibility of asx-turns compared with β-turns, conferred by the side chain χ2 angle, suggests the asx-turn can adopt a conformation with a more favorable hydrogen bond. On the other hand, φe of the asx-turn (χ1 of the side chain) is more constrained than φ of the β-turn, as it must adopt a rotamer conformation. Considering this, it is interesting to reverse our perspective on how β-turns are mimicked by asx- and ST-turns and instead ponder mimicry of the asx side chain rotamer angle by φ of the β-turn. It could be said that this φ angle mimics the rotamer conformation of the asx-turn.
A common motif incorporating asx- or ST-turns is the asx- or ST-nest. This is a recently identified, yet by no means uncommon, protein motif (Watson and Milner-White 2002a). The observation that almost all type II asx- and ST-turns occur as asx- or ST-nest motifs is an unexpected finding of the present work. Conversely, all asx- or ST-nests are also type II asx- or ST-turns so they are nearly synonymous.
Materials and methods
A data set of 500 PDB files was obtained from the kinemage Web site (http://kinemage.biochem.duke.edu). The proteins are at a resolution <1.8 Å and homology is <50%. Selection emphasizes data quality by removing structures not meeting criteria such as B-factor, clash score, and main chain torsion angles (Lovell et al. 2003). Hydrogen atoms were added as needed using the Reduce program (Word et al. 1999a) and the program was also used to correct side-chain orientations (Word et al. 1999b). Hydrogen bond definition is based on the method recommended by Baker and Hubbard (1984). Asx- and ST-turns are identified by a single hydrogen-bond, that between the side chain oxygen of an aspartate, asparagine, serine, or threonine residue (i) and the main chain NH group of a residue two ahead (i + 2). β-Turns are identified by a hydrogen bond between the main chain CO of residue i and the main chain NH of residue i + 3. Dihedral angles used are defined in Figure 1 ▶. In asx- and ST-nests the first residue is D, N, S, or T and its side chain oxygen atom is within 4.0 Å of at least two of the main chain nitrogen atoms of residues i, i + 1, or i + 2.
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.04920904.
References
- Aurora, R. and Rose, G.D. 1998. Helix capping. Protein Sci. 7 21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, E.N. and Hubbard, R.E. 1984. Hydrogen bonding in globular proteins. Prog. Biophys. Mol. Biol. 44 97–179. [DOI] [PubMed]
- Bordo, D. and Argos, P. 1994. The role of side-chain hydrogen bonds in the formation and stabilization of secondary structures in soluble proteins. J. Mol. Biol. 243 504–519. [DOI] [PubMed] [Google Scholar]
- Chakrabarti, P. and Pal, D. 2001. The inter-relationships of side-chain and main-chain conformations in proteins. Prog. Biophys. Mol. Biol. 76 1–102. [DOI] [PubMed] [Google Scholar]
- Doig, A.J., Macarthur, M.W., Stapley, B.J., and Thornton, J.M. 1997. Structures of N-termini of helices in proteins. Protein Sci. 6 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswar, N. and Ramakrishnan, C. 1999. Secondary structures without backbone: An analysis of backbone mimicry by polar side chains in proteins. Protein Eng. 12 447–455. [DOI] [PubMed] [Google Scholar]
- ———. 2000. Deterministic features of side-chain hydrogen bonds in globular protein structures. Protein Eng. 13 27–238. [DOI] [PubMed] [Google Scholar]
- Gunasekharan, K., Gomalki, L., Ramakrishnan, C., Chandrasekhar, J., and Balaram, P. 1998. Conformational interconversions in peptide β-turns. Analysis of turns in proteins and computational estimates of barriers. J. Mol. Biol. 284 1505–1516. [DOI] [PubMed] [Google Scholar]
- Hutchinson, E.G. and Thornton, J.M. 1994. A revised set of potentials for β-turn formation in proteins. Protein Sci. 3 2207–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ———. 1996. PROMOTIF—A program to identify and analyze structural motifs in proteins. Protein Sci. 5 212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, P.N., Momany, F.A., and Scheraga, H.A. 1973. Chain reversals in proteins. Biochem. Biophys. Acta 303 211–229. [DOI] [PubMed] [Google Scholar]
- Lovell, S.C., Word, J.M., Richardson, J.S., and Richardson, D.C. 2000. The penultimate rotamer library. Proteins 40 389–408. [PubMed] [Google Scholar]
- Lovell, S., Davis, I.W., Arendall III, W.B., de Bakker, P.I.W., Word, J.M., Prisant, M.G., Richardson, J.S., and Richardson, D.C. 2003. Structure validation by Cα geometry: φ?ψ and Cβ deviation. Proteins 50 437–450. [DOI] [PubMed] [Google Scholar]
- Pal, L., Chakrabarti, P., and Batu, G. 2003. Sequence and structure patterns in proteins from an analysis of the shortest helices: Implications for helix nucleation. J. Mol. Biol. 326 273–291. [DOI] [PubMed] [Google Scholar]
- Presta, L.G. and Rose, G.D. 1988. Helix signals in proteins. Science 240 1632–1641. [DOI] [PubMed] [Google Scholar]
- Rees, D.C., Lewis, M., and Lipscomb, W.N. 1983. Crystal structure of carboxy-peptidase A. J. Mol. Biol. 168 367–387. [DOI] [PubMed] [Google Scholar]
- Richardson, J.S. 1981. Protein anatomy. Adv. Prot. Chem. 34 167–339. [DOI] [PubMed] [Google Scholar]
- Richardson Richardson, J.S. and Richardson, D.C. 1988. Amino acid preferences for specific locations at the ends of α-helices. Science 240 1648–1652. [DOI] [PubMed] [Google Scholar]
- ———. 1989. Prediction of protein structure and the principles of protein conformation (ed. G.D. Fasman), pp. 1–98. Plenum Press, New York.
- Tainer, J.A., Getzoff, E.D., Beem, K.M., Richardson, J.S., and Richardson, D.C. 1982. Structure of copper zinc superoxide dismutase. J. Mol. Biol. 160 181–217. [DOI] [PubMed] [Google Scholar]
- Venkatachalam, C.M. 1968. Stereochemical criteria for polypeptides and proteins. V. Conformation of a system of 3 linked peptide units. Biopolymers 6 1425–1436. [DOI] [PubMed] [Google Scholar]
- Vijayakumar, M., Quian, H., and Zhou, H.X. 1999. Hydrogen bonds between short polar side chains and peptide backbone: Prevalence in proteins and effects on helix-forming propensities. Proteins 34 497–507. [PubMed] [Google Scholar]
- Wan, W.Y. and Milner-White, E.J. 1999a. A natural grouping of motifs with an aspartate or asparagine forming two hydrogen bonds to residues ahead in sequence: Their occurrence at α-helical N termini and in other situations. J. Mol. Biol. 286 1633–1649. [DOI] [PubMed] [Google Scholar]
- ———. 1999b. A recurring two-hydrogen-bond motif incorporating a serine or threonine residue is found both at α-helical N-termini and in other situations. J. Mol. Biol. 286 1650–1666. [DOI] [PubMed] [Google Scholar]
- Watson, J.D. and Milner-White, E.J. 2002a. A novel main-chain anion binding site in proteins: A particular combination of φ?ψ angles in successive residues gives rise to anion binding sites that occur commonly and are often found at functionally important regions. J. Mol. Biol. 315 171–182. [DOI] [PubMed] [Google Scholar]
- ———. 2002b. The conformations of polypeptide chains where the φ?ψ values of alternating residues are enantiomeric. J. Mol. Biol. 315 183–191. [DOI] [PubMed] [Google Scholar]
- Wilmot, C.M. and Thornton, J.M. 1988. Analysis and prediction of the different types of β-turns in proteins. J. Mol. Biol. 203 221–232. [DOI] [PubMed] [Google Scholar]
- ———. 1990. β-Turns and their distortions: A proposed new nomenclature. Protein Eng. 3 479–493. [DOI] [PubMed] [Google Scholar]
- Word, J.M., Lovell, S.C., LaBean, T.H., Taylor, H.C., Zalis, M.E., Presley, B.K., Richardson, J.S., and Richardson, D.C. 1999a. Asparagine and glutamine: Using hydrogen bond contacts in the choice of sidechain amide orientation. J. Mol. Biol. 285 1711–1733. [DOI] [PubMed] [Google Scholar]
- Word, J.M., Lovell, S.C., Richardson, J.S., and Richardson, D.C. 1999b. Visualizing and quantifying molecular goodness-of-fit: Small probe contact dots with explicit hydrogens. J. Mol. Biol. 285 1735–1747. [DOI] [PubMed] [Google Scholar]


