Abstract
α-Synuclein is the main component of the intracellular protein aggregates in neurons of patients with Parkinson’s disease. The occurrence of the disease is associated with oxidative damage. Although it is known that peroxidative chemistry leads to the aggregation of α-synuclein in vitro, the specific amino acid types of α-synuclein involved in this type of aggregation have not been identified. We show, using human cytochrome c plus H2O2 as the source oxidative stress, that the tyrosines of α-synuclein are required for aggregation. The studies reveal the chemical basis for a crucial step in the aggregation process.
Keywords: aggregation, Lewy bodies, oxidative stress, Parkinson’s disease, α-synuclein, tyrosine
Parkinson’s disease is one of the most common age-related neurodegenerative disorders, affecting almost 3% of the population more than 65 years of age. This devastating disease, clinically characterized by tremor, rigidity, and bradykinesia, affects more than one million persons in the United States alone (Lang and Lozano 1998). The symptoms are caused by the loss of dopaminergic neurons in the substantia nigra region of the brain. The few surviving neurons in this region contain Lewy bodies (Holdorff 2002), intracytoplasmic aggregates composed largely of the protein α-synuclein (Spillantini et al. 1997).
α-Synuclein became the focus of Parkinson’s disease research after the discovery of two rare, familial forms of the disease caused by point mutations in the α-synuclein gene (Polymerpoulos et al. 1997). Although the exact functions of α-synuclein are unknown, two main biochemical activities have been proposed: regulation of dopamine neuro-transmission and regulation of dopaminergic synaptic vesicles. α-Synuclein is natively disordered (Weinreb et al. 1996) and has a random-coil circular dichroism spectrum (Kim 1997), but its α-helical content increases from 3% to 63%-70% on binding lipid membranes (Davidson et al. 1998).
The cause of α-synuclein aggregation to form Lewy bodies is unknown. However, the production of reactive oxygen species (ROS) has been suggested as a central event. Oxidative stress is an intracellular imbalance in the pro-oxidant/antioxidant equilibrium, favoring pro-oxidants (Sies 1991). The main source of intracellular ROS is the mitochondria (Boveris et al. 1972; Boveris and Chance 1973), where ROS are produced during the reduction of oxygen to water. Under conditions of oxidative stress, both ROS and the respiratory chain protein cytochrome c can leak from the mitochondria into the cytoplasm (Shigenaga et al. 1994). It has been suggested that this leakage is facilitated by pores in the mitochondrial membranes formed by α-synuclein (Lashuel et al. 2002; Volles and Lansbury Jr. 2002). Whatever the mechanism, cytochrome c co-localizes with α-synuclein in Lewy bodies (Hashimoto et al. 1999).
All cellular components are vulnerable to oxidative stress. The damage to proteins includes side chain modification and main chain fragmentation. Two biological markers for oxidative damage in proteins are accumulation of carbonyl groups and dityrosine (Berlett and Stadtman 1997). Dityrosine is a useful marker for protein oxidation (Giulivi and Davies 1993; Heinecke et al. 1993; Huggins et al. 1993) and is found in amino acid hydrosylates of brain tissue affected by Parkinson’s disease (Pennathur et al. 1999).
There are two proposed mechanisms for dityrosine formation: Fenton chemistry and peroxidase-mediated tyrosyl radical formation. Fenton chemistry, which generates hydroxyl radicals (HO•) from simple transition metal plus H2O2, was believed (Stadtman 1993) to play a role in dityrosine formation. However, recent studies (Kato et al. 2001) show that HO• is not involved in dityrosine formation, and may even decrease the yield of dityrosine (Atwood et al. 2004). Oxidative cross-linking of proteins by tyrosine residues probably occurs through a peroxidase-like mechanism. The proposed mechanism for the reaction of H2O2 with ferricytochrome c owes a great deal to what is known about the reaction of H2O2 with the heme-containing peroxidase enzymes, especially cytochrome c peroxidase (Erman and Vitello 2002). This enzyme donates two electrons and two protons to H2O2, producing two molecules of water and leaving two free radicals on the enzyme, one heme-based and the other on a buried tryptophan. The final location of the radicals on cytochrome c with reaction with H2O2 is less clear, but spin trap experiments, which indirectly detect free radicals, suggest that the unpaired electrons end up on tyrosines (Barr et al. 1996; Deterding et al. 1998; Chen et al. 2002; Qian et al. 2002). If two adjacent tyrosine radical-containing molecules interact, a dityrosine-linked dimer forms. Highlighting the importance of understanding peroxidative chemistry is the recent observation that cyclooxygenase (a peroxidase family member) plus H2O2 induces aggregation of Aβ, the protein associated with Alzheimer’s disease (Nagano et al. 2004).
Hashimoto et al. (1999) were the first to show the aggregation of α-synuclein by cytochrome c and H2O2. They also showed that cytochrome c co-localizes with α-synuclein in intracellular aggregates in neurons from subjects with Parkinson’s disease. These results suggest that cytochrome c plays a role in the oxidative stress-induced aggregation of α-synuclein in Parkinson’s disease. However, the mechanism by which cytochrome c leads to α-synuclein aggregation, including the types of amino acids involved, is unknown. Barr et al. (1996) and Qian et al. (2002) showed that radicals formed by the reaction of horse cytochrome c and H2O2 are centered on tyrosine residues using electron spin trapping. Deterding et al. (1998) first showed with mass spectrometry and spin trapping studies that a free radical formed on horse cytochrome c under oxidative conditions can be transferred to tyrosine-containing peptides and that tyrosines are required for transfer. Nevertheless, the residue types involved in peroxidative aggregation of α-synuclein remained unknown.
From these data about cytochrome c oxidation and tyrosine we hypothesized that tyrosine residues on α-synuclein are important for the peroxidative aggregation of α-synuclein. The hypothesis was tested by making an α-synuclein variant in which all the tyrosines were changed to phenylalanines and testing the variant under the same conditions used by Hashimoto et al. (1999).
Results
Wild-type α-synuclein aggregates in the presence of cytochrome c and H2O2
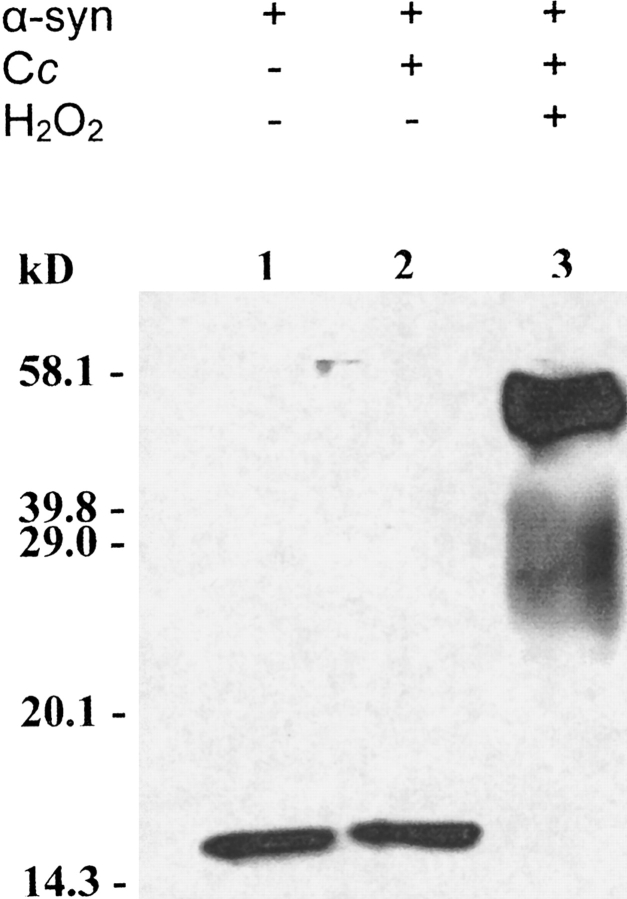
Recombinant wild-type α-synuclein was incubated with cytochrome c in the presence or absence of H2O2. Coomassie Brilliant Blue staining (Fig. 1 ▶) and immunoblot analysis (Fig. 2 ▶) showed that α-synuclein aggregates to an SDS-stable species in the presence of H2O2 plus recombinant human cytochrome c. The data in lane 2 of both figures show that H2O2 is required for the aggregation of α-synuclein. Comparing the data in Figures 1 ▶ and 2 ▶ indicates that the band at just above 20.1 kD in lane 3 of Figure 1 ▶ is the cytochrome c dimer from self-induced peroxidative aggregation, as was observed by Hashimoto et al. (1999). Also, as observed by these investigators and confirmed by us (data not shown) α-synuclein fails to aggregate in the presence of 1 mM H2O2 alone. To prove that the interaction between cytochrome c and H2O2 is required for α-synuclein aggregation we performed the complete reaction in the presence of cyanide, which binds the heme iron of cytochrome c, blocking the reaction of cytochrome c with H2O2. Aggregation is inhibited by cyanide (data not shown). The results reported here with human cytochrome c and wild-type α-synuclein confirm and extend the results of Hashimoto et al. (1999), who used horse heart cytochrome c.
Figure 1.

α-Synuclein aggregates in the presence of H2O2 and recombinant human cytochrome c. Purified recombinant wild-type human α-synuclein (α-syn) (10 μM) was incubated at 37°C for 1 h either alone (lane 1) or with 100 μM recombinant human cytochrome c (Cc; lanes 2, 3) in the presence (lane 3) or absence (lanes 1, 2) of 1 mM H2O2. The gel was stained with Coomassie Brilliant Blue.
Figure 2.
Anti-α-synuclein immunoblot analysis for the aggregation of wild-type α-synuclein in the presence of cytochrome c and 1 mM H2O2. Recombinant wild-type human α-synuclein (10 μM) was incubated at 37°C for 1 h either alone (lane 1) or with 100 μM recombinant human cytochrome c (lanes 2, 3) in the presence (lane 3) or absence (lanes 1, 2) of 1 mM H2O2.
The no-tyrosine variant of α-synuclein fails to aggregate in the presence of cytochrome c and H2O2
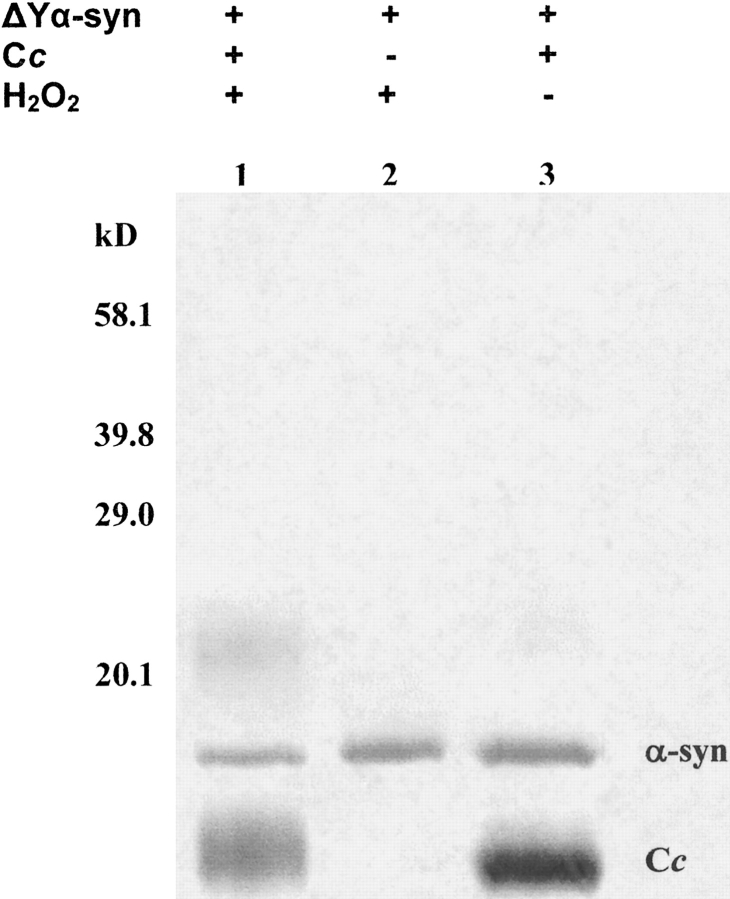
Recombinant no-tyrosine α-synuclein was incubated with recombinant human cytochrome c under the same conditions as the experiments shown in Figure 1 ▶. Coomassie Brilliant Blue staining showed that no-tyrosine α-synuclein does not aggregate in the presence of recombinant human cytochrome c and H2O2 (Fig. 3 ▶). As described above, the presence of the cytochrome c dimer at just above 20.1 kD in lane 1 of Figure 3 ▶ proves the reaction was aggregation-competent, and, like the wild-type protein, the variant failed to aggregate in the presence of H2O2 alone. Our results establish for the first time that tyrosine residues are required for peroxidative aggregation of α-synuclein.
Figure 3.
No-tyrosine α-synuclein does not aggregate in the presence of cytochrome c and H2O2. No-tyrosine (ΔY, 10 μM) α-synuclein plus recombinant cytochrome c (Cc, 10 μM) were incubated at 37°C for 1 h, with 1 mM H2O2 (lane 1). No-tyrosine α-synuclein (10 μM) was incubated at 37°C for 1 h with 1 mM H2O2 alone (lane 2) or with 50 μM recombinant human cytochrome c alone (lane 3). The gel was stained with Coomassie Brilliant Blue.
Discussion
α-Synuclein aggregation is associated with several neuro-degenerative disorders, but despite intense investigation, the mechanism of aggregation and the residue types involved are unknown. It is known, however, that α-synuclein forms SDS stable oligomers when exposed to oxidizing agents (Souza et al. 2000). Hashimoto et al. (1999) showed that α-synuclein aggregates in the presence of cytochrome c and H2O2. Cytochrome c forms tyrosyl radicals when exposed to H2O2 (Barr et al. 1996), and these radicals can be transferred to tyrosine residues on other proteins (Deterding et al. 1998). On the basis of these observations we hypothesized that tyrosines are key residues in the aggregation of α-synuclein in the presence of cytochrome c and H2O2. The data in Figures 1 ▶ and 2 ▶, which were acquired under the same conditions used by Hashimoto et al., shows that both cytochrome c and H2O2 are required for α-synuclein aggregation, and the inhibition of the reaction by cyanide shows direct involvement of the heme in peroxidative aggregation of α-synuclein by cytochrome c.
α-Synuclein has four tyrosines (at positions 39, 125, 133, and 136). To investigate the role of these tyrosines in the reaction, we converted the four tyrosines in α-synuclein to phenylalanines. To rule out structural changes in the no-tyrosine variant, we analyzed its secondary structure by using circular dichroism spectropolarimetry (data not shown). The no-tyrosine variant has a random coil spectrum, identical to that of wild-type α-synuclein.
As shown in Figure 3 ▶, no-tyrosine α-synuclein remains monomeric in the presence of cytochrome c and H2O2. These results prove that tyrosines are required for the peroxidative aggregation of α-synuclein in the presence of H2O2 and cytochrome c and are consistent with results from studies of cyclooxygenase-induced peroxidative aggregation of Aβ, a protein associated with Alzheimer’s disease (Nagano et al. 2004). On the other hand, when Cu(II) and H2O2 are used to produce ROS through the Fenton reaction, tyrosines are not required for the oxidative aggregation of α-synuclein (Norris et al. 2003). This discrepancy points to the importance of the ROS source in the mechanism of aggregation. We have assumed that the aggregates observed in SDS gels are covalently cross-linked. We are currently testing this assumption and determining which of the four tyrosines are responsible for aggregation.
In summary, we have shown that tyrosines in α-synuclein are required for α-synuclein’s peroxidative aggregation, and that the source of ROS plays a key role in the mechanism of aggregation.
Materials and methods
Protein purification
Recombinant human cytochrome c and human α-synuclein were expressed in Escherichia coli and purified as described (Morar et al. 2001; Olteanu et al. 2003).
Site-directed mutagenesis
The human α-synuclein variant with all the tyrosines mutated to phenylalanines, no-tyrosine α-synuclein, was made by using the QuikChange Site-Directed Mutagenesis Kit (Stratagene). The conversion was performed in three steps: (Y39F), (Y125F), and (Y133, 136F). The mutagenic oligonucleotides were synthesized at the Nucleic Acid Core Facility in the UNC Lineberger Cancer Center. The nucleotide sequence was confirmed by the UNC Automated Sequencing Facility. The concentration of the variant was determined by using the same absorbance coefficient as for the wild-type protein (5800 M−1 cm−1 at 276 nm calculated by using ExPASy (http://us.expasy.org). Because the no-tyrosine variant contains neither tyrosine nor tryptophan, the protein concentration was confirmed by SDS-PAGE analysis.
Aggregation assays of cytochrome c and α-synuclein in the presence of H2O2
Aggregation assays were performed as described by Hashimoto et al. (1999). Briefly, 100-μM cytochrome c and 10-μM α-synuclein (the wild-type protein or the no-tyrosine variant) were incubated in a total volume of 100 μL of PBS (100 mM NaCl, 20 mM KH2PO4, 80 mM Na2HPO4 • 2 H2O at pH 7.4), with or without 1 mM H2O2, at 37°C, for 1 h. The reaction mixture was analyzed by SDS-PAGE, at 200 V, for 45 min, in a 12% gel. Immunoblot analysis was performed as described by Hashimoto et al. (1999). Samples were electrophoresed at 200 V. The gel was blotted onto a nitro-cellulose membrane that had been soaked for 10 min in methanol. The gel was blotted for 1 h in transfer buffer (3.02 g of Tris base, 14.4 g of glycine, 1 L of H2O). The membrane was blocked in 5% (w/v) dried milk in TBST (10 mL 1 M Tris at pH 7.5, 30 mL 5 M NaCl, 500 μL 0.05% Tween 20), at room temperature, for 1 h, followed by incubation with mouse anti-α-synuclein antibody (LB509, dilution 1:1000) (Zymed Laboratories Inc.), for 1 h, at 37°C, in TBST plus 5% (w/v) dried milk. The membrane was then incubated with horseradish peroxidase rabbit antimouse secondary antibody (dilution 1:3000) (Zymed Laboratories Inc.) for 1 h, at room temperature. Protein bands were visualized by using the ECL Western blot detection kit (Amersham).
Acknowledgments
We thank the members of the Pielak laboratory and Mark O. Lively, Matthew R. Redinbo, and Elizabeth C. Pielak for helpful discussions. This work was funded by the NIH (R21ES10774) and the NSF (MCB 0109366 and 0212939).
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.04947204.
References
- Atwood, C.S., Perry, G., Zeng, H., Kato, Y., Jones, W.D., Ling, K.Q., Huang, X., Moir, R.D., Wang, D., Sayre, L.M., et al. 2004. Copper mediates dityrosine cross-linking of Alzheimer’s amyloid-α. Biochemistry 43 560–568. [DOI] [PubMed] [Google Scholar]
- Barr, D.P., Gunther, M.R., Deterding, L.J., Tomer, K.B., and Mason, R.P. 1996. ESR spin-trapping of a protein-derived tyrosyl radical from the reaction of cytochrome c with hydrogen peroxide. J. Biol. Chem. 271 15498–15503. [DOI] [PubMed] [Google Scholar]
- Berlett, B.S. and Stadtman, E.R. 1997. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 272 20313–20316. [DOI] [PubMed] [Google Scholar]
- Boveris, A. and Chance, B. 1973. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem. J. 134 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris, A., Oshino, N., and Chance, B. 1972. The cellular production of hydrogen peroxide. Biochem. J. 128 617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.R., Deterding, L.J., Sturgeon, B.E., Tomer, K.B., and Mason, R.P. 2002. Protein oxidation of cytochrome c by reactive halogen species enhances its peroxidase activity. J. Biol. Chem. 277 29781–29791. [DOI] [PubMed] [Google Scholar]
- Davidson, W.S., Jonas, A., Clayton, D.F., and George, J.M. 1998. Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. J. Biol. Chem. 273 9443–9449. [DOI] [PubMed] [Google Scholar]
- Deterding, L.J., Barr, D.P., Mason, R.P., and Tomer, K.B. 1998. Characterization of cytochrome c free radical reactions with peptides by mass spectrometry. J. Biol. Chem. 273 12863–12869. [DOI] [PubMed] [Google Scholar]
- Erman, J.E. and Vitello, L.B. 2002. Yeast cytochrome c peroxidase: Mechanistic studies via protein engineering. Biochim. Biophys. Acta. 1597 193–220. [DOI] [PubMed] [Google Scholar]
- Giulivi, C. and Davies, K.J. 1993. Dityrosine and tyrosine oxidation products are endogenous markers for the selective proteolysis of oxidatively modified red blood cell hemoglobin by (the 19 S) proteasome. J. Biol. Chem. 268 8752–8759. [PubMed] [Google Scholar]
- Hashimoto, M., Takeda, A., Hsu, L.J., Takenouchi, T., and Masliah, E. 1999. Role of cytochrome c as a stimulator of α-synuclein aggregation in Lewy body disease. J. Biol. Chem. 274 28849–28852. [DOI] [PubMed] [Google Scholar]
- Heinecke, J.W., Li, W., Daehnke 3rd, H.L., and Goldstein, J.A. 1993. Dityrosine, a specific marker of oxidation, is synthesized by the myeloperoxidase-hydrogen peroxide system of human neutrophils and macrophages. J. Biol. Chem. 268 4069–4077. [PubMed] [Google Scholar]
- Holdorff, B. 2002. Friedrich Heinrich Lewy (1885–1950) and his work. J. Hist. Neurosci. 11 19–28. [DOI] [PubMed] [Google Scholar]
- Huggins, T.G., Wells-Knecht, M.C., Detorie, N.A., Baynes, J.W., and Thorpe, S.R. 1993. Formation of o-tyrosine and dityrosine in proteins during radiolytic and metal-catalyzed oxidation. J. Biol. Chem. 268 12341–12347. [PubMed] [Google Scholar]
- Kato, Y., Kitamoto, N., Kawai, Y., and Osawa, T. 2001. The hydrogen peroxide/copper ion system, but not other metal-catalyzed oxidation systems, produces protein-bound dityrosine. Free Radic. Biol. Med. 31 624–632. [DOI] [PubMed] [Google Scholar]
- Kim, J. 1997. Evidence that the precursor protein of non-Aβ component of Alzheimer’s disease amyloid (NACP) has an extended structure primarily composed of random-coil. Mol. Cell. 7 78–83. [PubMed] [Google Scholar]
- Lang, A.E. and Lozano, A.M. 1998. Parkinson’s disease. Second of two parts. N. Engl. J. Med. 339 1130–1143. [DOI] [PubMed] [Google Scholar]
- Lashuel, H.A., Hartley, D., Petre, B.M., Walz, T., and Lansbury Jr., P.T. 2002. Amyloid pores from pathogenic mutations. Nature 418 291. [DOI] [PubMed] [Google Scholar]
- Morar, A.S., Olteanu, A., Young, G.B., and Pielak, G.J. 2001. Solvent-induced collapse of α-synuclein and acid-denatured cytochrome c. Protein. Sci. 10 2195–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano, S., Huang, X., Moir, R.D., Payton, S.M., Tanzi, R.E., and Bush, A.I. 2004. Peroxidase activity of cyclooxygenase-2 (COX-2) cross-links α-amyloid (Aβ) and generates Aβ-COX-2 hetero-oligomers that are increased in Alzheimer’s disease. J. Biol. Chem. 279 14673–14678. [DOI] [PubMed] [Google Scholar]
- Norris, E.H., Giasson, B.I., Ischiropoulos, H., and Lee, V.M. 2003. Effects of oxidative and nitrative challenges on α-synuclein fibrillogenesis involve distinct mechanisms of protein modifications. J. Biol. Chem. 278 27230–27240. [DOI] [PubMed] [Google Scholar]
- Olteanu, A., Patel, C.N., Dedmon, M.M., Kennedy, S., Linhoff, M.W., Minder, C.M., Potts, P.R., Deshmukh, M., and Pielak, G.J. 2003. Stability and apoptotic activity of recombinant human cytochrome c. Biochem. Biophys. Res. Commun. 312 733–740. [DOI] [PubMed] [Google Scholar]
- Pennathur, S., Jackson-Lewis, V., Przedborski, S., Heinecke, J.W., Polacino, P.S., Stallard, V., Klaniecki, J.E., Montefiori, D.C., Langlois, A.J., Richardson, B.A., et al. 1999. Mass spectrometric quantification of 3-nitrotyrosine, orthotyrosine, and o,o′-dityrosine in brain tissue of 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine-treated mice, a model of oxidative stress in Parkinson’s disease. J. Biol. Chem. 274 34621–34628. [DOI] [PubMed] [Google Scholar]
- Polymerpoulos, M.H., Lavedan, C., Leroy, E., Ide, S.E., Dehejia, A., Dutra, A., Pike, B., Root, H., Rubenstein, J., Boyer, R., et al. 1997. Mutations in the α-synuclein gene identified in families with Parkinson’s disease. Science 276 2045–2047. [DOI] [PubMed] [Google Scholar]
- Qian, S.Y., Chen, Y.R., Deterding, L.J., Fann, Y.C., Chignell, C.F., Tomer, K.B., Mason, R.P., Barr, D.P., and Gunther, M.R. 2002. Identification of protein-derived tyrosyl radical in the reaction of cytochrome c and hydrogen peroxide: Characterization by ESR spin-trapping, HPLC and MS ESR spin-trapping of a protein-derived tyrosyl radical from the reaction of cytochrome c with hydrogen peroxide. Biochem. J. 363 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigenaga, M.K., Hagen, T.M., and Ames, B.N. 1994. Oxidative damage and mitochondrial decay in aging. Proc. Natl. Acad. Sci. 91 10771–10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies, H. 1991. Oxidative stress: From basic research to clinical application. Am. J. Med. 91 31S–38S. [DOI] [PubMed] [Google Scholar]
- Souza, J.M., Giasson, B.I., Chen, Q., Lee, V.M., and Ischiropoulos, H. 2000. Dityrosine cross-linking promotes formation of stable α-synuclein polymers. Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J. Biol. Chem. 275 18344–18349. [DOI] [PubMed] [Google Scholar]
- Spillantini, M.G., Schmidt, M.L., Lee, V.M.-Y., Trojanowski, J.Q., Jakes, R., and Goedert, M. 1997. α Synuclein in Lewy bodies. Nature 388 839–840. [DOI] [PubMed] [Google Scholar]
- Stadtman, E.R. 1993. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu. Rev. Biochem. 62 797–821. [DOI] [PubMed] [Google Scholar]
- Volles, M.J., and Lansbury Jr., P.T. 2002. Vesicle permeabilization by proto-fibrillar α-synuclein is sensitive to Parkinson’s disease-linked mutations and occurs by a pore-like mechanism. Biochemistry 41 4595–4602. [DOI] [PubMed] [Google Scholar]
- Weinreb, P.H., Zhen, W., Poon, A.W., Conway, K.A., and Lansbury Jr., P.T. 1996. NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry 35 13709–13715. [DOI] [PubMed] [Google Scholar]