Abstract
During the first few minutes of fibrillation of a 14-residue peptide homologous to the hydrophobic C-terminal part of the Aβ-peptide, EM micrographs reveal small crystalline areas (100 to 150 nm, repeating unit 47 Å) scattered in more amorphous material. On a longer time scale, these crystalline areas disappear and are replaced by tangled clusters resembling protofilaments (hours), and eventually by more regular amyloid fibrils of 60 Å to 120 Å diameter (days). The transient population of the crystalline areas indicates the presence of ordered substructures in the early fibrillation process, the diameter of which matches the length of the 14-mer peptide in an extended β-strand conformation.
Keywords: peptide aggregation, protein aggregation, amyloid fibrils, peptide crystal, electron microscopy
The deposition of insoluble protein fibrils or amyloids is associated with a number of fatal diseases, such as Alzheimer’s, prion diseases, and systemic amyloidoses (Rochet and Lansbury 2000). X-ray fiber diffraction and electron microscopy studies of insoluble deposits from proteins of very diverse origins reveal a remarkable structural homogeneity (Sunde et al. 1997), possibly linked to the near-universal ability of protein to fibrillate (Dobson 2001). The fibrillation process is typically characterized by a lag phase followed by a relatively rapid build-up of fibrils. This implies that fibrillation proper is preceded by other events such as conformational changes at the monomer level and the formation of a fibrillation nucleus (Harper and Lansbury 1997). A variety of such transiently populated structures have been revealed. For example, Ig light-chain fibrillation yielded single 2.4-nm-diameter filaments in the first few hours of incubation, which over several days combined to form protofibrils of 40 Å diameter and finally mature fibrils of intertwined protofibrils (Ionescu-Zanetti et al. 1999). Similar hierarchical patterns have been found also for other proteins (Chamberlain et al. 2000; Kad et al. 2001). Intermediate fibrillar species arouse interest at two levels. First, they are expected to provide a molecular understanding of the fibrillation process, and second, they may shed light on the mechanism of cytotoxicity. Toxicity studies suggest that structures observed at earlier stages of the aggregation process, viz. oligomeric intermediates (Lambert et al. 1998), early aggregates (Bucciantini et al. 2002), or protofibrils (Rochet and Lansbury 2000; Volles et al. 2001), may be intrinsically pathogenic to living cells (Harper et al. 1999; Lansbury 1999; Walsh et al. 1999), whereas the mature fibrils yield no significant effect (Chamberlain et al. 2000).
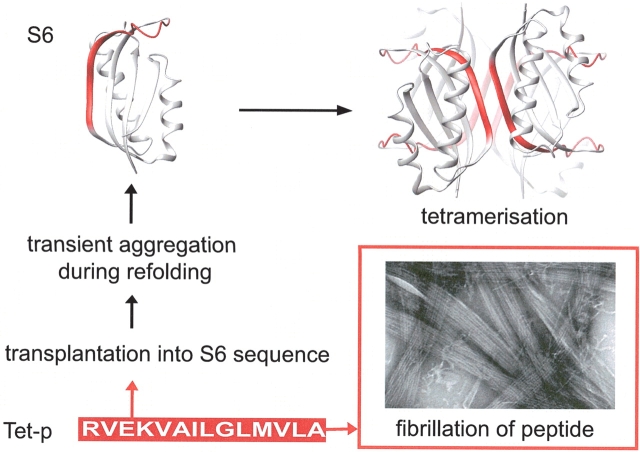
In an attempt to shed more light on the sequence of events occurring during fibrillation, we have studied the evolution of different morphological species during the aggregation of the 14-residue peptide AcNH-RVEKVAILGLMVLA-CONH2 (Tet-p). This peptide has previously been analyzed in the context of a soluble scaffold, the globular protein S6, to provide detailed crystallographic and mechanistic data on its oligomerization behavior (Otzen et al. 2000). The peptide was grafted into the position of an exposed β-strand of the S6 structure (residues 40–53) and contains four point mutations relative to wild-type S6. The mutations increase the homology with the hydrophobic C-terminal part of the 42-residue Aβ-peptide and trigger complex aggregation behavior of S6 without significantly affecting folding or stability of the monomeric protein. Under physiological conditions, the altered S6 construct assembled into tetramers linked by the modified β-strand, and during refolding, the denatured state formed transient aggregates (Fig. 1 ▶; Otzen et al. 2000). The simple recipe for this induced aggregation is the replacement of key charged residues in the original sequence, the so-called aggregation gatekeepers (Otzen et al. 2000), with hydrophobic ones. Analysis of the aggregation behavior of acyl phosphatase mutants (Bucciantini et al. 2002) has subsequently confirmed this observation.
Figure 1.
The Tet-p peptide is homologous to the C-terminal part of the Aβ-peptide (aa 25–38) and displays a complex aggregation behavior, both on its own and upon insertion into other soluble proteins. Transplantation of the Tet-p peptide into the sequence of the ribosomal protein S6 triggers transient aggregation of the coil state during refolding. The transient aggregates are short-lived (20 msec) and ripped apart upon refolding of the S6 structure. On a longer time scale, however, the Tet-p sequence (red) promotes ordered tetramerization of the S6 monomers by the formation of intermolecular β-strands that are antiparallel. The different species accumulating in the fibrillation process of the free Tet-p peptide are show in Fig. 2 ▶.
We have incubated samples of Tet-p at a concentration of 1 mg/mL in aqueous buffer and monitored their aggregation process over a period of several days by electron microscopy negative staining. During this period, the peptide goes through several aggregation states with distinct morphological properties (Fig. 2 ▶).
Figure 2.
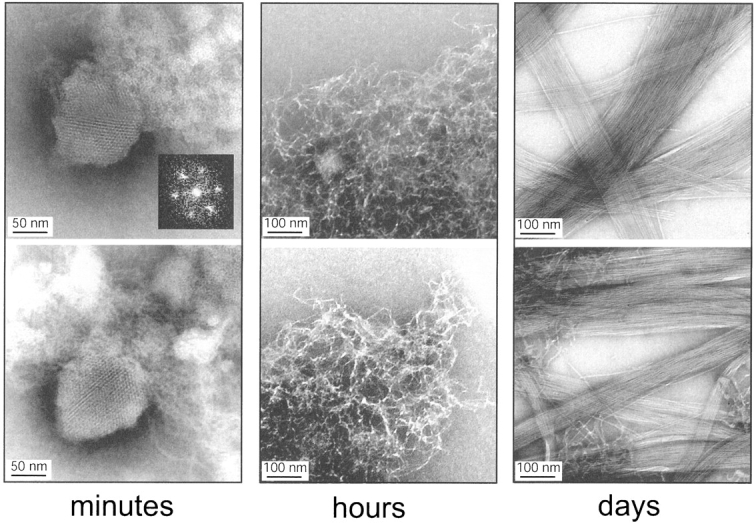
Representative transmission electron microscopy images of species formed during fibrillation of the peptide Tet-p. (Left to right) First forms crystalline areas embedded in seemingly amorphous material (minutes). The inlay represents a Fourier Transform of the crystalline species. The crystalline areas are then replaced by tangled species (hours), leading eventually to amyloid fibrils (days). As controls we have used the peptides AcNH-RVEKVEELGLMVLA-CONH2 (S6-p) and AcNH-RVEKVAILGLRRLA-CONH2 (41/42-p), which are similar to Tet-p but contain additional charges (“aggregation gatekeepers” from the S6 wild-type sequence; Otzen et al. 2000) that decrease their susceptibility to form fibrils. S6-p corresponds to the S6 wild-type sequence (residues 36–49), whereas 41/42-p has Glu41 and Glu42 mutated to Ala and Ile, respectively. S6-p does not fibrillate, whereas 41/42-forms more irregular fibrils than does Tet-p and no nanocrystals (data not shown). This is consistent with the idea that the nanocrystals arise transiently because the bulk transport of peptides is too slow to allow direct formation of the fibril: The energetically more frustrated control peptide 41/42-p is not as quickly trapped.
The first aggregation state, which is only seen during the first few minutes of incubation, involves structures with a crystalline appearance, which we, for ease of reference, term nanocrystals. These nanocrystals represent ~2% to 10% of the deposited material in terms of grid area (based on several hundred scans). Quantification is intrinsically difficult, because the nanocrystals show a rather clustered distribution on the grid, often in connection to large patches of seemingly amorphous material. The micrographs of these structures are likely to arise from direct density effects and not from the negative staining of the sample. Fourier transforms of the crystal-like image imply that the structural units are organized in a hexagonal pattern with a repeating unit of ~50 Å (Fig. 2 ▶). The dimension of the repeating unit is inconsistent with salt crystals or uniformly stacked peptides. Rather, it suggests the existence of ordered peptide substructures with a diameter that corresponds to the length of the 14-mer Tet-p peptide in an extended conformation with a translation of ~3.4 Å per residue. In this respect, these substructures seem more uniform than do the transient aggregates that form during refolding of the Tetp-S6 construct: Kinetic evidence suggest that the transient aggregates involve contacts in multiple registers (Otzen et al. 2000). In general, the crystalline areas are embedded in more amorphous aggregates. The regular absorbance pattern and the even density of the crystalline areas indicate that they have flake-like dimensions rather than being spherical. The formation of these nanocrystals is reproducible in independent experiments and has been detected both with peptides produced commercially (by Synt:em) and by ourselves.
Over the next few hours and days, the crystalline species is replaced by tangled clusters, leading in the course of several weeks to more regular fibrils of 60 Å to 120 Å in diameter (Fig. 2 ▶). The tangles and fibrils probably corre spond to the protofibrils and fibrils observed for other proteins (Rochet and Lansbury 2000; Volles et al. 2001).
It is not inconceivable that the rapid formation and decay of the preceding crystalline areas have prevented their earlier detection by EM in other systems. However, nanocrystals of the scrapie prion protein, similar in appearance to those of Tet-p, have been reported by Wille and Prusiner (1999). These crystals were crystallized from reverse micelles, and in addition, to detergent their formation required uranyl salts. The detergent conditions under which the protein was stored prior to EM did not allow fibrils to form, and it is not clear whether the nanocrystals would be able to form under conditions in which the prion protein fibrillates.
Judging by its size and morphology, the crystalline intermediate of Tet-p does not appear to be on the most direct pathway to the mature fibrils. More likely it constitutes a dead-end trap. Dead-end traps are not unprecedented in fibrillation. Phase partitioning between soluble precursor elements and a sparingly soluble off-pathway species has previously been proposed to regulate the fibrillation of islet amyloid polypeptide (Padrick and Miranker 2002) by forming a reservoir of precipitated protein that slowly releases monomers for fibrillation. Likewise, oligomeric species that do not lead to fibrillation have been observed during the aggregation of the Aβ-peptide (Pallitto and Murphy 2001) and immunoglobulin light-chain (Souillac et al. 2002). The transient Aβ oligomers reached a maximum occupancy after 40 min of aggregation and were detected by fluorescence correlation spectroscopy (Tjernberg et al. 1999).
Upon closer examination of Figure 2 ▶, it is apparent that the tangled clusters in several cases spread out from the crystalline area as lateral or radial outgrowth. This behavior hints at the possibility that the crystal surface acts as template for seeding more linear growth of fibrillar structures. In support of this scenario, Serrano and coworkers (L. Serrano, pers. comm.) have recently observed a similar hairlike growth from spherical particles early in the aggregation process of a de novo designed peptide.
Zhu and coworkers (2002) have recently reported that surface-catalyzed fibril formation of SMA (on mica grids) proceeds via preformed “amorphous cores” from which the fibrillar growth originates. These SMA cores appear by AFM after 4 h of incubation and could well be related to the nanocrystalline species formed by the Tet-p peptide. Moreover, their size (100 to 200 nm) matches well the nanocrystalline species (100 to 150 nm).
What is then the nature of the 50 Å substructures forming the crystalline areas? In an earlier time-resolved study of the full-length Aβ-peptide, Harper and coworkers (1999) observed that a spherical species of similar dimensions (diameter ~40 Å) accumulated early in the aggregation process and then seemed to coalesce into protofibrils. More recently, the same group reported that the Aβ-peptide may also produce ring-like assemblies with a diameter of 70 Å to 100 Å resembling βbarrels (Lashuel et al. 2002). An attractive feature of barrel-like structures as early intermediates in protein aggregation is that they, at least conceptually, provide a simple rationale for toxicity. In analogy with cytolytic toxins, βbarrels may form membrane-spanning pores, leading to erroneous permeabilization and cell dysfunction (Gouaux 1997). For example, the membrane-spanning parts of the barrel-forming toxins α-hemolysin (Gouaux 1998) and aerolysin (Rossjohn et al. 1998) both have diameters ~100 Å, and individual membrane-bound β-barrels, such as the 22-stranded FepA transporter (Buchanan et al. 1999), have diameters ~40 Å. Consistently, protofilaments of Aβ and α-synuclein, but not their mature fibrils (Volles et al. 2001; Volles and Lansbury 2002), have been observed to permeabilize lipid vesicles and display pore-like activity in vitro. The crystalline areas in Figure 2 ▶ are currently too small to be subjected to X-ray analysis, and we do not observe isolated spherical substructures, so the connection of the nanocrystalline array to possible βbarrel structures must remain speculative at present. However, we note that the size of the Tet-p substructure (50 Å in both dimensions) is fully consistent with a βbarrel with a length corresponding to the extended peptide and a width similar to a membrane-bound β-barrel’s such as the FepA transporter (~40 Å; Buchanan et al. 1999). Further, it is easy to envisage how such regular barrel structures may organize into flake-like crystals. Even so, it remains to be established whether the Tet-p sub structures are present also in the mature fibrils. If not, both the crystalline areas and the substructures that they are composed of will constitute kinetic traps in the fibrillation process. Such kinetic traps could arise either because of an inherent consequence of the aggregation energy landscape (cf. intermediates in protein folding; Dill and Chan 1997), or because the bulk transport of peptides is too slow to directly allow the formation of the thermodynamically most advantageous species (cf. spinodal decomposition; Schmeltzer et al. 1999; Vaiana et al. 2003). Thus, the convergent evidence that toxicity arises during the early aggregation events need not automatically imply the involvement of fibril precursors or lack of cooperativity in the fibrillation process. It is equally possible that the adverse gain of function arises from off-pathway conformations that are kinetically trapped.
Materials and methods
The peptide Tet-p (AcNH-RVEKVAILGLMVLA-CONH2) was synthesized by Synt:em (France) to 90% purity. The peptide was dissolved in 50% acetonitrile and 50% water to a concentration of 10 mg/mL. Fibrillation was induced by 10-fold dilution into 25 mM sodium phosphate (pH 7.0) and 50 mM NaCl. Electron microscopy was carried out as follows: 2 to 3 μL peptide solution was deposited on a copper grid covered by a carbon-stabilized formvar film, excess liquid was removed by blotting after 30 sec, and the sample was left to dry for a few minutes. Following this, 2 to 3 μL 1% phosphotungstic acid in water was added, excess liquid was again removed after 30 sec, and the solution left to dry before transferring the grid to the microscope (a Philips CM120 BioTWINCryo Biocryo transmission electron microscope). Imaging was performed with a multi-scan CCD camera.
Acknowledgments
We thank Håkan Wennerström for stimulating discussions and Gunnel Karlsson for expert technical assistance at the Biomicroscopy Unit, Lund University. The work was supported by an EMBO long-term fellowship (D.E.O.), the Danish Technical Science Research Council (D.E.O.), and the Swedish Research Council (M.O.).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.03538904.
References
- Bucciantini, M., Giannoni, E., Chiti, F., Baroni, F., Formigli, L., Zurdo, J., Taddei, N., Ramponi, G., Dobson, C.M., and Stefani, M. 2002. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 416 507–511. [DOI] [PubMed] [Google Scholar]
- Buchanan, S.K., Smith, B.S., Venkatramani, L., Xia, D., Esser, L., Palnitkar, M., Chakraborty, R., van der Helm, D., and Deisenhofer, J. 1999. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat. Struct. Biol. 6 56–63. [DOI] [PubMed] [Google Scholar]
- Chamberlain, A.K., MacPhee, C.E., Zurdo, J., Morzova-Roche, L.A., Hill, H.A.O., Dobson, C.M., and Davis, J.J. 2000. Ultrastructural organization of amyloid fibrils by atomic force microscopy. Biophys. J. 79 3282–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dill, K.A. and Chan, H.S. 1997. From Levinthal to pathways to funnels. Nat. Struct. Biol. 4 10–19. [DOI] [PubMed] [Google Scholar]
- Dobson, C.M. 2001. The structural basis of protein folding and its links with human disease. Phil. Trans. R. Soc. Lond. B 356 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouaux, E. 1997. Channel-forming toxins: Tales of transformation. Curr. Opin. Struct. Biol. 7 566–573. [DOI] [PubMed] [Google Scholar]
- ———. 1998. α-Hemolysin from Staphylococcus aureus: An archetype of β-barrel, channel-forming toxins. J. Struct. Biol. 121 110–122. [DOI] [PubMed] [Google Scholar]
- Harper, J.D. and Lansbury, P.T.J. 1997. Models of amyloid seeding in Alzheimer’s disease and scrapie: Mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Ann. Rev. Biochem. 66 385–407. [DOI] [PubMed] [Google Scholar]
- Harper, J.D., Wong, S.S., Lieber, C.M., and Lansbury, P.T.J. 1999. Assembly of Ab amyloid protofibrils: An in vitro model for a possible early event in Alzheimer’s disease. Biochemistry 38 8972–8980. [DOI] [PubMed] [Google Scholar]
- Ionescu-Zanetti, C., Khurana, R., Gillespie, J.R., Petrick, J.S., Trabachino, L.C., Minert, L.J., Carter, S.A., and Fink, A.L. 1999. Monitoring the assembly of Ig light-chain amyloid fibrils by atomic force microscopy. Proc. Natl. Acad. Sci. 96 13175–13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kad, N.M., Thomson, N.H., Smith, D.P., Smith, D.A., and Radford, S.E. 2001. β2-Microglobulin and its deamidated variant, N17D form amyloid fibrils with a range of morphologies in vitro. J. Mol. Biol. 313 559–571. [DOI] [PubMed] [Google Scholar]
- Lambert, M.P., Barlow, A.K., Chromy, B.A., Edwards, C., Freed, R., Liosatos, M., Morgan, T.E., Rozovsky, I., Trommer, B., Viola, K.L., et al. 1998. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. 95 6448–6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansbury, P.T. 1999. Evolution of amyloid: What normal protein folding may tell us about fibrillogenesis and disease. Proc. Natl. Acad. Sci. 96 3342–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashuel, H.A., Hartley, D., Petre, B.M., Weals, T., and Lansbury, P.T. 2002. Amyloid pores from pathogenic mutations. Nature 418 291. [DOI] [PubMed] [Google Scholar]
- Otzen, D.E., Kristensen, P., and Oliveberg, M. 2000. Designed protein tetramer zipped together with an Alzheimer sequence: A structural clue to amyloid assembly. Proc. Natl. Acad. Sci. 97 9907–9912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padrick, S.B. and Miranker, A.D. 2002. Islet amyloid: Phase partitioning and secondary nucleation are central to the mechanism of fibrillogenesis. Biochemistry 41 4694–4703. [DOI] [PubMed] [Google Scholar]
- Pallitto, M.M. and Murphy, R.M. 2001. A mathematical model of the kinetics of β-amyloid fibril growth from the denatured state. Biophys. J. 81 1805–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochet, J.-C. and Lansbury, P.T. 2000. Amyloid fibrillogenesis: Themes and variations. Curr. Opin. Struct. Biol. 10 60–68. [DOI] [PubMed] [Google Scholar]
- Rossjohn, J., Feil, S.C., McKinstry, W.J., Tsernoglous, D., van der Goot, G., Buckley, J.T., and Parker, M.W. 1998. Aerolysin: A paradigm for membrane insertion of β-sheet protein toxins? J. Struct. Biol. 121 92–100. [DOI] [PubMed] [Google Scholar]
- Schmeltzer, J., Röpke, G., and Mahnke, R. 1999. Aggregation phenomena in complex systems. Wiley-VCH Verlag GmbH, Weinheim, Germany.
- Souillac, P.O., Uversky, V.N., Millett, I.S., Khurana, R., Doniach, S., and Fink, A.L. 2002. Elucidation of the molecular mechanism during the early events in immunoglobulin light chain amyloid fibrillation. J. Biol. Chem. 277 12666–12679. [DOI] [PubMed] [Google Scholar]
- Sunde, M., Serpell, L.C., Bartlam, M., Fraser, P.E., Pepys, M.B., and Blake, C.C.F. 1997. Common core structure of amyloid fibrils by synchrotron X-ray diffraction. J. Mol. Biol. 273 729–739. [DOI] [PubMed] [Google Scholar]
- Tjernberg, L.O., Pramanik, A., Bjorling, S., Thyberg, P., Thyberg, J., Nordstedt, C., Berndt, K.D., Terenius, L., and Rigler, R. 1999. Amyloid β-peptide polymerization studied using fluorescence correlation spectroscopy. Chem. Biol. 6 53–62. [DOI] [PubMed] [Google Scholar]
- Vaiana, S.M., Palma-Vittorelli, M.B., and Palma, M.U. 2003. Time scale of protein aggregation dictated by liquid–liquid demixing. Proteins 51 147–153. [DOI] [PubMed] [Google Scholar]
- Volles, M.J. and Lansbury, P.T. 2002. Vesicle permeabilization by protofibrillar α-synuclein is sensitive to Parkinson’s disease–linked mutations and occurs by a pore-like mechanism. Biochemistry 41 4595–4602. [DOI] [PubMed] [Google Scholar]
- Volles, M.J., Lee, S.J., Rochet, J.C., Shtilerman, M.D., Ding, T.T., Kessler, J.C., and Lansbury, P.T. 2001. Vesicle permeabilization by protofibrillar a-synuclein: Implications for the pathogenesis and treatment of Parkinson’s disease. Biochemistry 40 7812–7819. [DOI] [PubMed] [Google Scholar]
- Walsh, D.M., Hartley, D.M., Kusumoto, Y., Fezoui, Y., Condron, M.M., Lomakin, A., Benedek, G.B., Selkoe, D.J., and Teplow, D.B. 1999. Amyloid β-protein fibrillogenesis: Structure and biological activity of protofibrillar intermediates. J. Biol. Chem. 274 25945–25952. [DOI] [PubMed] [Google Scholar]
- Wille, H. and Prusiner, S.B. 1999. Ultrastructural studies on scrapie prion protein crystals obtained from reverse micellar solutions. Biophys. J. 76 1048–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, M., Souillac, P.O., Ionescu-Zanetti, C., Carter, S.A., and Fink, A.L. 2002. Surface-catalyzed amyloid fibril formation. J. Biol. Chem. 277 50914–50922. [DOI] [PubMed] [Google Scholar]