Abstract
Objectives
In the mammalian brain, G protein-coupled MT1 and MT2 melatonin receptors may be involved in Alzheimer's pathology, long-term potentiation, depression, and in the behavioral effects of psychoactive drugs. These drugs; e.g. antidepressants and drugs of abuse, are typically used over long periods of time and may alter neuroplasticity and gene expression. We hypothesized that such antidepressant- and cocaine-altered expression of melatonin receptor mRNA may occur in the hippocampus and striatum.
Methodology
Male C3H/HeJ mice were treated with the antidepressants fluoxetine, desipramine, and clomipramine, with the psychostimulant cocaine, and with a vehicle either a single time or once a day for 14 days. Brain samples were collected 24 h after the last injection and the content of MT1 and MT2 mRNA was assayed.
Results
A single drug injection did not alter the MT1 and MT2 mRNA content. In the hippocampus, protracted treatment with antidepressants increased the amount of MT1 mRNA (with the exception of fluoxetine) but decreased MT2 mRNA content; cocaine did not produce any alterations. In the striatum, antidepressants produced the opposite effect on MT1 mRNA content; they decreased it. They did not significantly alter striatal MT2 mRNA (we observed a nonsignificant trend to a decrease). Cocaine also decreased striatal MT1 mRNA content without affecting MT2 mRNA.
Conclusion
These results suggest that drug- and region-specific alterations of MT1/MT2 mRNA produced by protracted antidepressants and cocaine treatment may alter MT1/MT2 expression and contribute to long-term neuroplastic effects of these drugs.
Keywords: Melatonin receptors, fluoxetine, desipramine, clomipramine, hippocampus, striatum
Introduction
It has been suggested that the pineal hormone melatonin may be neuroprotective via the antioxidant actions of the melatonin molecule 1. However, the G protein-coupled metabotropic melatonin receptors MT1 and MT2 2 are the primary mediators of the physiological actions of melatonin. The function of these receptors appears to be characterized by MT1/MT2 homo- and hetero-dimerization 3. These receptors are expressed in the mammalian central nervous system (CNS) including humans 4-8, where they may play a role in CNS pathobiology and brain functioning, and in the mechanisms influencing the molecular and behavioral effects of psychoactive drugs.
For example, significant alterations in the intensity and distribution of MT1 and MT2 immunoreactivities were observed in postmortem brain from Alzheimer's patients; MT1 immunoreactivity was significantly increased in Alzheimer's hippocampi 4, whereas MT2 immunoreactivity in Alzheimer's hippocampi was decreased 5. Interestingly, transgenic mice deficient in MT2 receptors demonstrated deficient hippocampal long-term potentiation (LTP) 9. Furthermore, when wild-type mice were tested in an elevated plus maze on two consecutive days, they showed shorter transfer latencies to enter a closed arm on the second day; this experience-dependent behavior did not occur in MT2 knockouts 9. These results suggest that MT2 receptors may participate in hippocampal synaptic plasticity and in memory processes.
A putative role for melatonin has also been proposed in mechanisms of depressive behaviors 10, 11 and in actions of antidepressant drugs 12. More recently, it was shown in a mouse model of forced swimming that the antidepressant-like behavioral effects of a melatonin receptor ligand require the expression of MT2 but not MT1 receptors 13.
MT1 but not MT2 receptors are required for nighttime inhibition of behavioral actions of cocaine; i.e., locomotor sensitization 14. Repeated injections of cocaine trigger locomotor sensitization only if delivered during the day (low melatonin) but not at night (high melatonin) 15. The nighttime inhibition of cocaine sensitization is reversed by pinealectomy, suggesting that the pineal gland, i.e., the main source of melatonin, may be responsible for this inhibition 16. In fact, it was shown that the nighttime inhibition of cocaine sensitization that occurs in wild-type mice is preserved in MT2 knockout mice but is absent in MT1 knockouts, suggesting that MT1 receptors are required for inhibitory action of melatonin on the development of cocaine sensitization 14.
Objectives
CNS-acting drugs such as antidepressants and cocaine typically are used over prolonged periods of time when they cause significant and lasting alterations in neuroplasticity and in the expression of brain genes including those encoding a number of brain receptors. In this work, we hypothesized that such drug-altered expression of melatonin receptor mRNA may occur in brain regions involved in the actions of antidepressants and cocaine; i.e., the hippocampus and striatum. We tested this hypothesis in experiments with mice.
Methods
Animals and drug treatment
C3H/HeJ mice (6−8 per group), 8 weeks old and weighing 25−30 g were purchased from Jackson Laboratory (Bar Harbor, Maine). Animals were housed in groups of three and had free access to laboratory chow and water except during behavioral experiments. They were kept in a temperature-controlled room under conditions of 14 h light: 10 h dark cycle (lights on at 5 am; ZT00). Fluoxetine (10 mg/kg), desipramine (10 mg/kg), clomipramine (10 mg/kg), and cocaine hydrochloride (20 mg/kg) (Sigma Chemical Co., St. Louis, Missouri) were dissolved in sterile saline (0.9% NaCl) with 5% dimethyl sulfoxide (DMSO; Sigma Chemical Co.) and administered intraperitoneally (i.p.) in an injection volume of 50 μl/25 g body weight. Controls were injected with the same volume of vehicle. Mice were treated at 10 a.m. either for one day (single treatment) or for 14 days once a day (protracted treatment). The drug doses and injection schedules were chosen based on previous reports 15, 17, 18. The experimental protocol was approved by the Institutional Animal Care and Ethics Committee.
Reverse transcription - polymerase chain reaction (RT-PCR) assay
Striatal (i.e., caudate-putamen) and hippocampal tissue samples were collected 24 h after the last injection. They were excised from 1-mm-thick coronal brain slices as described elsewhere 19. Briefly, the caudate-putamen of the striatum was excised from the slices between the bregma 1.54 to −0.46mm, whereas the hippocampi were excised from the slices between the bregma −1.58 to −3.52mm. The total RNA was extracted using the chemical isolation method with TRIzol® reagent (Invitrogen, Carlsbad, California) following the manufacturer's instructions. To eliminate possible DNA contamination, RNA samples were treated with a DNase reagent, DNA-freeTM (Ambion, Inc., Austin, Texas). The yield of total RNA was determined by measuring the absorbance of an aliquot of the precipitated stock at a wavelength of 260/280 nm. Furthermore, to test possible DNA contamination, each sample was run by RT-PCR without adding the reverse transcriptase enzyme (see below). Amplification primers for MT1 (Gene Bank accession #U52222, forward (F): 5’-GTC AGC GCA TTT CTA ATG GGC TTG and reverse (R): 3’-GCT GTT GAA GTA CGC CAG GTA GTA), MT2 (Gene Bank accession #.AY145850, F: 5’-TGA TAG CCT TGT ACC CTT ACC CAC and R: 3’-CCT GGA GCA CCA GTA CCC AGA TT), and for cyclophilin (Gene Bank accession #X52803, F: 5’-ATT TGG CTA TAA GGG TTC CTC and R: 3’-ACG CTC CAT GGC TTC CAC AAT) were purchased from Integrated DNA Technologies, Inc. (Coralville, IA). To allow co-amplification of either MT1 or MT2 mRNA with the more abundant cyclophilin mRNA, pilot studies were conducted to determine optimal relative primer concentrations and cycle numbers whereby the PCR would still be within the exponential (i.e., linear) range for both transcripts. The assay was performed as described elsewhere 7. Briefly, total RNA (0.5 μg of starting amount) was denaturated and then reverse transcribed. The cDNA aliquots containing reverse-transcribed material and specific primer pairs for either MT1 or MT2 along with cyclophilin (the ratio to cyclophilin is 1:10) were amplified with Hot Tub DNA polymerase (Amersham, Piscataway, NJ) in a thermal cycler (9600, Perkin Elmer, Wellesley, MA). The PCR mixture was amplified for 34 cycles with denaturation (94°C, 15 s), annealing (65°C for MT1 and 60°C for MT2, 30 s), and elongation (72°C, 30 s). The reaction was terminated with a 5-min final elongation step. Trace amounts of [32P]dCTP (0.5 μCi/sample; Amersham, Piscataway, NJ) were included during the PCR step for subsequent quantification. For each sample, the ethidium bromide-stained bands for MT1, MT2 and cyclophilin were excised from the electrophoresis gels and the radioactivity was counted 7. Results were calculated as the ratio of either MT1 or MT2 to cyclophilin (mean ± S.E.M.).
Statistical analysis
A one-way between-group analysis of variance was followed by the 2-sided Dunnett test for a multiple comparison with control (SPSS 12.0 for Windows) and the mean difference was accepted as significant at the 0.05 level.
Results
Protracted administration of antidepressants but not cocaine produces differential effects on MT1 vs. MT2 expression in the hippocampus
Twenty four hours after the fourteenth injection of each of the three antidepressants we used, the amount of hippocampal MT2 mRNA was reduced in comparison with vehicle-treated controls (Fig. 1). In contrast, the amount of MT1 mRNA was increased by antidepressants (except for fluoxetine). The same schedule of cocaine administration failed to alter hippocampal MT1 and MT2 mRNA levels (Fig. 1).
Figure 1.
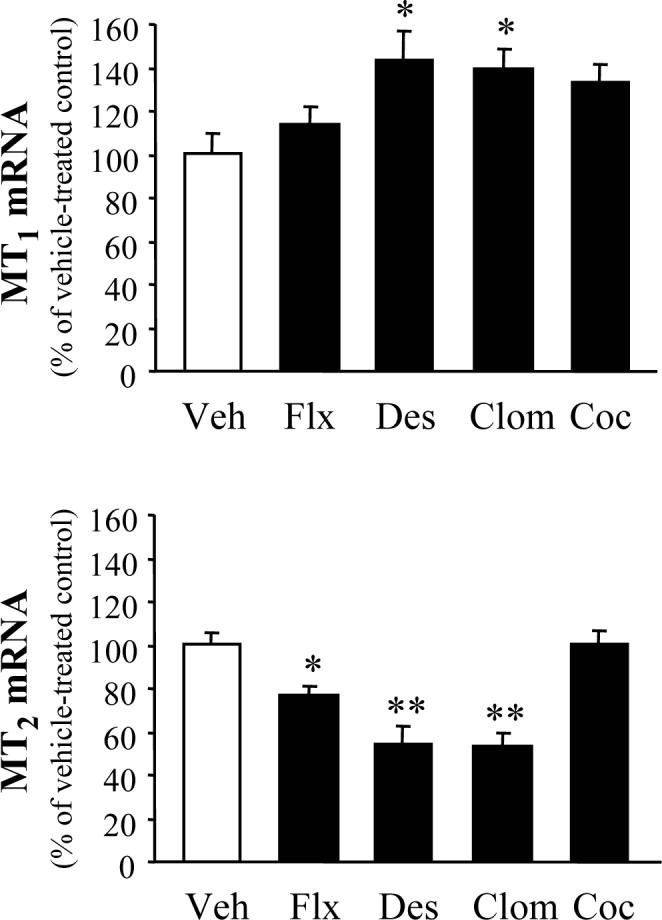
Effects of protracted antidepressant and cocaine treatment on hippocampal melatonin receptor mRNA levels. Samples were collected 24 h after the 14th injection of vehicle (Veh), fluoxetine (Flx), desipramine (Des), clomipramine (Clom), and cocaine (Coc). Results were calculated as the ratio of either MT1 or MT2 to cyclophilin. The ratios in vehicle-treated controls were: MT1/cyclophilin = 0.22±0.02; MT2/cyclophilin = 0.30±0.02. Results are expressed as a percentage of the corresponding vehicle-treated controls (mean ± S.E.M.). *P<0.05, **P<0.001 vs. Veh (Dunnett test).
Protracted administration of antidepressants and cocaine decreases MT1 expression in the striatum
The protracted (i.e., 14-day) treatment with antidepressants did not significantly alter the striatal MT2 mRNA content (Fig. 2) although, similar to hippocampal findings, it did produce a trend to a decrease. Unlike in the hippocampus, the antidepressants decreased and not increased the striatal content of MT1 mRNA. Protracted treatment with cocaine also decreased striatal MT1 but not MT2 mRNA levels (Fig. 2).
Figure 2.
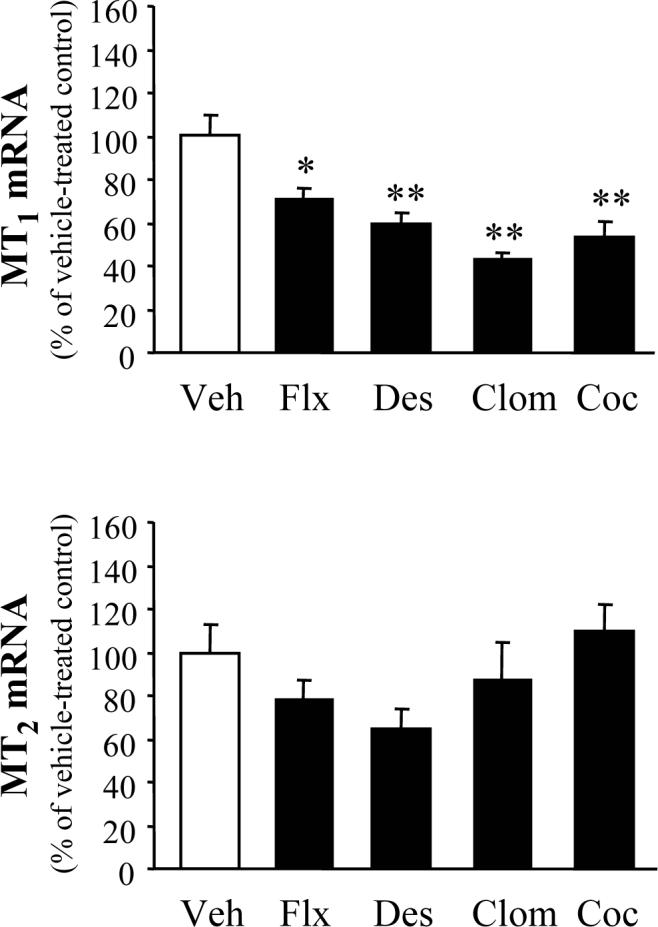
Effects of protracted antidepressant and cocaine treatment on striatal melatonin receptor mRNA levels. Samples were collected 24 h after the 14th injection of vehicle (Veh), fluoxetine (Flx), desipramine (Des), clomipramine (Clom), and cocaine (Coc). Results were calculated as the ratio of either MT1 or MT2 to cyclophilin. The ratios in vehicle-treated controls were: MT1/cyclophilin = 0.37±0.03; MT2/cyclophilin = 0.30±0.04. Results are expressed as a percentage of the corresponding vehicle-treated controls (mean ± S.E.M.). *P<0.05, **P<0.01 vs. Veh (Dunnett test).
A single injection of antidepressants and cocaine fails to alter MT1 and MT2 expression
In contrast to the altered MT1/MT2 expression observed after protracted drug treatment, a single injection of antidepressants and cocaine did not significantly alter MT1 and MT2 mRNA levels either in the hippocampus (Fig. 3) or the striatum (Fig. 4).
Figure 3.
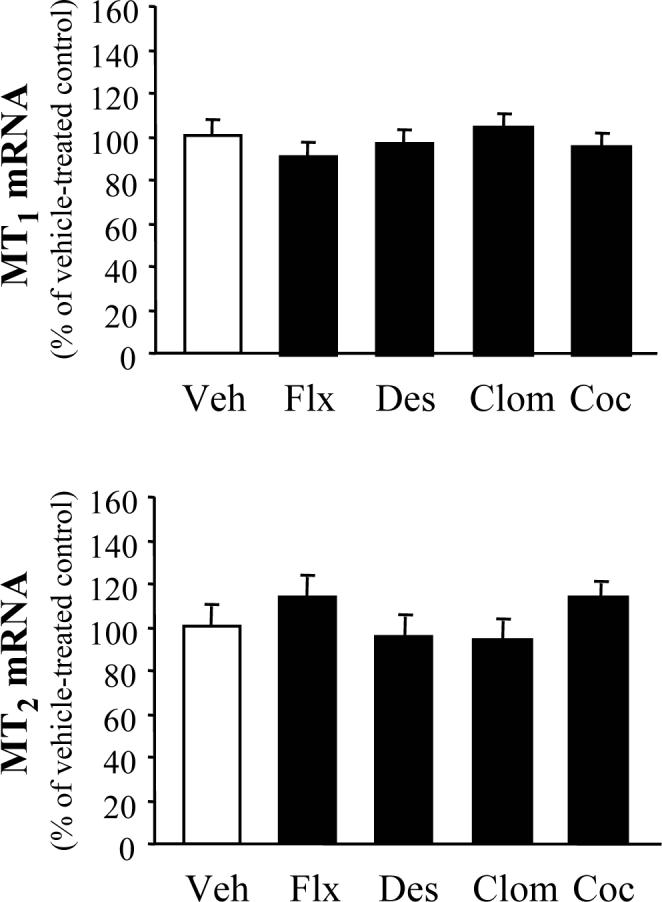
Hippocampal melatonin receptor mRNA levels after single injections of antidepressant and cocaine. Mice (6−8 per group) were injected with vehicle (Veh), fluoxetine (Flx), desipramine (Des), clomipramine (Clom), and cocaine (Coc) 24 h prior to sample collection. Results (mean ± S.E.M.) were calculated as MT1/cyclophiline and MT2/cyclophiline mRNA ratios, and are expressed as a percentage of the corresponding vehicle-treated group. No significant differences were observed between drug- vs. vehicle-treated groups; MT1= [F (4, 30) = 0.345, P = 0.845], MT2 = [F (4, 34) = 0.502, P = 0.734].
Figure 4.
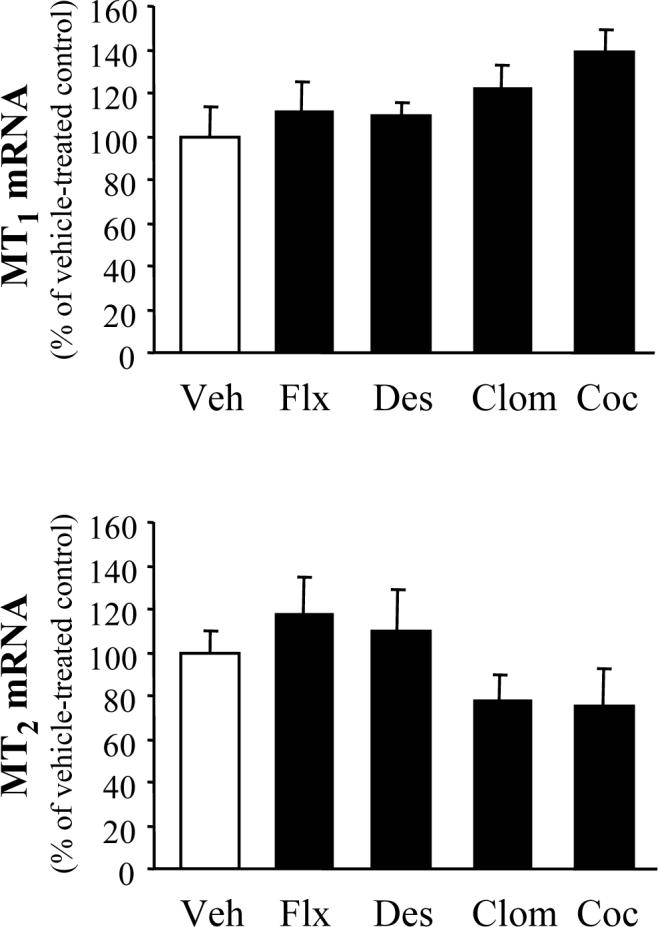
Striatal melatonin receptor mRNA levels after single injections of antidepressant and cocaine. Mice (6−8 per group) were injected with vehicle (Veh), fluoxetine (Flx), desipramine (Des), clomipramine (Clom), and cocaine (Coc) 24 h prior to sample collection. Results (mean ± S.E.M.) were calculated as MT1/cyclophiline and MT2/cyclophiline mRNA ratios, and are expressed as a percentage of the corresponding vehicle-treated group. No significant differences were observed between drug- vs. vehicle-treated groups; MT1= [F (4, 29) = 1.113, P = 0.370], MT2 = [F (4, 30) = 1.782, P = 0.159].
Discussion
In this work, we found evidence that the CNS content of melatonin receptor mRNA is significantly modified by prolonged treatment with antidepressants and cocaine. Interestingly, the effects of these drugs on MT1/MT2 mRNAs were brain region-specific. For example, the effect of antidepressants on MT1 expression was opposite in the hippocampus vs. the striatum. The expression of the melatonin receptor mRNA is reduced/downregulated upon exposure to melatonin 20. Thus, it is unlikely that the differential region-specific effect of antidepressants on MT1 mRNA levels; i.e., a concomitant up-regulation (hippocampus) and down-regulation (striatum), is mediated by antidepressant-altered levels of circulating melatonin. In fact, the antidepressants we used in this study apparently do not affect melatonin levels in-vivo 21. Hence, the regional differences we observed may relate to region-specific presence of the primary (e.g., serotonin, noradrenaline and dopamine transporters) or secondary (e.g., clock genes)18 targets for the CNS actions of drugs used in our study.
Also obvious was the difference between the effects of antidepressants and cocaine on MT2 expression; cocaine did not alter this expression whereas all three antidepressants significantly reduced MT2 expression in the hippocampus and caused a non-significant trend to MT2 mRNA decreases in the striatum. The similar effect of all three antidepressants on melatonin receptor expression is rather surprising because the drugs we used operate via different primary mechanisms of action. Fluoxetine is a predominant serotonin reuptake inhibitor, desipramine is a tricyclic predominantly noradrenaline reuptake inhibitor, and clomipramine is a tricyclic serotonin/noradrenaline reuptake inhibitor. It is possible that a common second or third messenger intracellular mechanism, which is triggered by protracted but not single drug application that may be responsible for the common therapeutic, i.e., antidepressant, activity of these drugs, also regulates the expression of melatonin receptors.
Interestingly, the effect of antidepressants on hippocampal MT1/MT2 mRNA content is similar to the alterations in hippocampal MT1/MT2 protein content observed in Alzheimer's disease 4,5. In both cases, MT1 receptors appear to be up-regulated and MT2 receptors are down-regulated. It was shown that melatonin can inhibit/impair hippocampal LTP (a model for learning/memory) and that this impairment is reduced by a MT2 deficiency 22. Hence, it is possible that a relative shift toward reduced hippocampal MT2 receptors could be beneficial. If so, the findings in postmortem Alzheimer's brain could reflect a compensatory mechanism. Further studies are needed to investigate whether mRNA changes observed in our study are accompanied by corresponding alterations in the content of melatonin receptor proteins.
If drug-induced changes in hippocampal and striatal expression of melatonin receptor mRNA are accompanied by similar changes in receptor protein content and also occur in humans, our study may point to several clinically important implications. For example, depression is often associated with sleep disturbances and a new MT1/MT2 agonist, ramelteon, has recently been introduced as a therapy for insomnia 23. Since it is likely that ramelteon will be used in combination with antidepressants, clinical studies may be warranted to clarify whether antidepressant treatment would interfere with the therapeutic effects of ramelteon. Similarly, it might be worth exploring whether ramelteon should be used in combination with antidepressants. If antidepressant effects include changes in the CNS expression of melatonin receptors, ramelteon might cause unforeseen interactions with antidepressant drugs, both beneficial and detrimental to antidepressant therapy. Finally, considering the participation of melatonin receptors in the long-term effects of cocaine 14, a possible role for melatonin receptor agonists in drug abuse should also be considered.
Acknowledgments
We acknowledge the support of the NIH grants R01 MH61572 (H.M.) and R01 DA15072 (T.U.).
References
- 1.Reiter RJ, Tan XD, Pappolla MA. Melatonin relieves the neural oxidative burden that contributes to dementias. Ann N Y Acad Sci. 2004;1035:179–96. doi: 10.1196/annals.1332.012. [DOI] [PubMed] [Google Scholar]
- 2.Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels: 7 TM receptors. Br J Pharmacol. 2005;144:S4–S62. doi: 10.1038/sj.bjp.0706158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayoub MA, Levoye A, Delagrange P, Jockers R. Preferential formation of MT1/MT2 melatonin receptor heterodimers with distinct ligand interaction properties compared with MT2 homodimers. Mol Pharmacol. 2004;66:312–21. doi: 10.1124/mol.104.000398. Erratum in: Mol Pharmacol 2004;66:771. [DOI] [PubMed] [Google Scholar]
- 4.Savaskan E, Olivieri G, Meier F, Brydon L, Jockers R, Ravid R, Wirz-Justice A, Muller-Spahn F. Increased melatonin 1a-receptor immunoreactivity in the hippocampus of Alzheimer's disease patients. J Pineal Res. 2002;32:59–62. doi: 10.1034/j.1600-079x.2002.00841.x. [DOI] [PubMed] [Google Scholar]
- 5.Savaskan E, Ayoub MA, Ravid R, Angeloni D, Fraschini F, Meier F, Eckert A, Muller-Spahn F, Jockers R. Reduced hippocampal MT2 melatonin receptor expression in Alzheimer's disease. J Pineal Res. 2005;38:10–6. doi: 10.1111/j.1600-079X.2004.00169.x. [DOI] [PubMed] [Google Scholar]
- 6.Torres-Farfan C, Rocco V, Monso C, Valenzuela FJ, Campino C, Germain A, Torrealba F, Valenzuela GJ, Serono-Ferre M. Maternal melatonin effects on clock gene expression in a nonhuman fetus. Endocrinology. 2006;147:4618–26. doi: 10.1210/en.2006-0628. [DOI] [PubMed] [Google Scholar]
- 7.Jilg A, Moek J, Weaver DR, Korf HW, Stehle JH, von Gall C. Rhythms in clock proteins in the mouse pars tuberalis depend on MT1 melatonin receptor signaling. Eur J Neurosci. 2005;22:2845–54. doi: 10.1111/j.1460-9568.2005.04485.x. [DOI] [PubMed] [Google Scholar]
- 8.Uz T, Arslan AD, Kurtuncu M, Imbesi M, Akhisaroglu M, Dwivedi Y, Pandey GN, Manev H. The regional and cellular expression profile of the melatonin receptor MT1 in the central dopaminergic system. Brain Res Mol Brain Res. 2005;136:45–53. doi: 10.1016/j.molbrainres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Zahn PK, Lansmann T, Berger E, Speckmann EJ, Musshoff U. Gene expression and functional characterization of melatonin receptors in the spinal cord of the rat: implications for pain modulation. J Pineal Res. 2003;35:24–31. doi: 10.1034/j.1600-079x.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 10.Larson J, Jessen RE, Uz T, Arslan AD, Kurtuncu M, Imbesi M, Manev H. Impaired hippocampal long-term potentiation in melatonin MT2 receptor-deficient mice. Neurosci Lett. 2006;393:23–6. doi: 10.1016/j.neulet.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 11.Uz T, Manev H. Prolonged swim-test immobility of serotonin N-acetyltransferase (AANAT)-mutant mice. J Pineal Res. 2001;30:166–70. doi: 10.1034/j.1600-079x.2001.300305.x. [DOI] [PubMed] [Google Scholar]
- 12.Kurtuncu M, Luka LJ, Dimitrijevic N, Uz T, Manev H. Reliability assessment of an automated forced swim test device using two mouse strains. J Neurosci Methods. 2005;149:26–30. doi: 10.1016/j.jneumeth.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Uz T, Manev H. Chronic fluoxetine administration increases the serotonin N-acetyltransferase messenger RNA content in rat hippocampus. Biol Psychiatry. 1999;45:175–9. doi: 10.1016/s0006-3223(98)00032-8. [DOI] [PubMed] [Google Scholar]
- 14.Sumaya IC, Masana MI, Dubocovich ML. The antidepressant-like effect of the melatonin receptor ligand luzindole in mice during forced swimming requires expression of MT2 but not MT1 melatonin receptors. J Pineal Res. 2005;39:170–7. doi: 10.1111/j.1600-079X.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- 15.Uz T, Kurtuncu M, Manev H. Program No. 799.11. 2005 Abstract Viewer/Itinerary Planner. Society for Neuroscience; Washington, DC: Differential role of melatonin receptors MT1 and MT2 in the development of diurnal locomotor sensitization induced by cocaine. Online. [Google Scholar]
- 16.Uz T, Javaid JI, Manev H. Circadian differences in behavioral sensitization to cocaine: putative role of arylalkylamine N-acetyltransferase. Life Sci. 2002;70:3069–75. doi: 10.1016/s0024-3205(02)01559-x. [DOI] [PubMed] [Google Scholar]
- 17.Uz T, Akhisaroglu M, Ahmed R, Manev H. The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice. Neuropsychopharmacology. 2003;28:2117–23. doi: 10.1038/sj.npp.1300254. [DOI] [PubMed] [Google Scholar]
- 18.Thome J, Sakai N, Shin K, Steffen C, Zhang YJ, Impey S, Storm D, Duman RS. cAMP response element-mediated gene transcription is upregulated by chronic antidepressant treatment. J Neurosci. 2000;20:4030–6. doi: 10.1523/JNEUROSCI.20-11-04030.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uz T, Ahmed R, Akhisaroglu M, Kurtuncu M, Imbesi M, Arslan AD, Manev H. Effect of fluoxetine and cocaine on the expression of clock genes in the mouse hippocampus and striatum. Neuroscience. 2005;134:1309–16. doi: 10.1016/j.neuroscience.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ. A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res. 1991;548:100–10. doi: 10.1016/0006-8993(91)91111-d. [DOI] [PubMed] [Google Scholar]
- 21.Guerrero HY, Gauer F, Schuster C, Pevet P, Masson-Pevet M. Melatonin regulates the mRNA expression of the mt(1) melatonin receptor in the rat Pars tuberalis. Neuroendocrinology. 2000;71:163–69. doi: 10.1159/000054533. [DOI] [PubMed] [Google Scholar]
- 22.Hartter S, Wang X, Weigmann H, Friedberg T, Arand M, Oesch F, Hiemke C. Differential effects of fluvoxamine and other antidepressants on the biotransformation of melatonin. J Clin Psychopharmacol. 2001;21:167–74. doi: 10.1097/00004714-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Wang LM, Suthana NA, Chaudhury D, Weaver DR, Colwell CS. Melatonin inhibits hippocampal long-term potentiation. Eur J Neurosci. 2005;22:2231–7. doi: 10.1111/j.1460-9568.2005.04408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGechan A, Wellington K. Ramelteon. CNS Drugs. 2005;19:1057–65. doi: 10.2165/00023210-200519120-00007. [DOI] [PubMed] [Google Scholar]


