Abstract
Ventral frontal cortex is commonly involved in traumatic brain injury (TBI). The Smell Identification Test (SIT), Object Alternation (OA), and the Iowa Gambling Task (IGT) are associated with this brain region in experimental and neuropsychological research. We examined the relationship of performance on these tests to residual structural brain integrity quantified from MRI in 58 TBI patients, including 18 patients with focal cortical contusions and 40 patients with diffuse injury only. Image analysis yielded regional volumetric measures of gray matter, white matter and cerebrospinal fluid. Multivariate analyses identified distributed patterns of regional volume loss associated with test performance across all three behavioral measures. The tasks were sensitive to effects of TBI. In multivariate analyses, performance in all three tasks was related to gray matter loss including ventral frontal cortex, but the SIT was most sensitive to ventral frontal cortex damage, even in patients without focal lesions. The SIT was further related to temporal lobe and posterior cingulate/retrosplenial volumes. OA and the IGT were associated with superior medial frontal volumes. Complex tasks, such as OA and the IGT, do not consistently localize to a single cortical region. The SIT is associated with the integrity of ventral frontal regions, but it is also affected by distributed damage, although the contribution of undetected olfactory tract or bulb damage could not be ruled out. This study illustrates the scope and limitations of functional localization in human ventral frontal cortex.
Keywords: Head injury, diffuse axonal injury, focal lesions, neuropsychology, orbitofrontal cortex, partial least squares analysis
INTRODUCTION
Traumatic brain injury (TBI) poses a particular challenge for the investigation of anatomical-behavioral correlations because of its unique combination of diffuse and focal damage. Due to excessive tissue strain in areas where the brain is confined by ridges of the inner skull, focal contusional damage in TBI predominates in ventral and polar frontal and lateral anterior temporal lobe regions (Gentry, Godersky, & Thompson, 1988; Holbourn, 1943). Diffuse axonal injury (DAI) resulting from acceleratory/deceleratory forces, is more ubiquitous and leads to white matter atrophy and deafferentiation of widespread axonal projections, including those from and to frontal and temporal brain regions (Gentry et al., 1988). Both focal and diffuse brain damage seen in traumatic brain injury can cause abnormal signal intensity on MR images and may result in atrophy, even in mild-moderate TBI (MacKenzie et al., 2002). Brain volumetric studies of TBI patients are therefore ideally suited to the quantification of these atrophic changes and their potential impact on behavior. Although focal lesions following TBI are associated with cognitive impairment, diffuse injury may have a similar or higher impact (Wallesch, Curio, Galazky, Jost, & Synowitz, 2001). Even without focal structural lesions of the brain in TBI, cerebral hypometabolism in frontal and cingulate cortex caused by DAI is associated with cognitive impairment (Fontaine, Azouvi, Remy, Bussel, & Samson, 1999). The goal of this study is to assess the effects of TBI neuropathology as quantified by high resolution structural MRI on specific cognitive problems associated with the ventral frontal cortex.
Among long-term cognitive impairment resulting from TBI, the most common are problems in information processing speed and attention (Zahn & Mirsky, 1999), memory (Levin & Goldstein, 1986), and executive functions (Levin, 1998; Stuss & Gow, 1992). Moreover, TBI patients may experience psychosocial problems, emotional and personality changes, and difficulties in interpersonal relationships (Cicerone & Tanenbaum, 1997; Prigatano, 1992). These behavioral problems in patients with TBI parallel the effects of non-traumatic focal frontal lobe lesions, where damage affects ventral prefrontal cortex disrupting signal transfer to the limbic system involved in emotional processing (Pandya & Barnes, 1987).
Only a few standardized neuropsychological test instruments are purported to measure ventral rather than dorsal frontal functions. Among these are tests of olfactory functions such as Smell Identification Test™ (Doty, 1995), Object Alternation (OA) tests that measure acquisition of a task rule in which the stimulus-reward association is contingent upon the outcome of the last trial, and decision making, most commonly assessed with the Iowa Gambling Task (Bechara, Damasio, Damasio, & Anderson, 1994; Bechara, Tranel, & Damasio, 2000). Previous studies showed sensitivity of these behavioral tasks to focal lesions in ventral frontal cortex (Bechara et al., 1994; Freedman, Black, Ebert, & Binns, 1998; Hornak et al., 2004; Hulshoff Pol et al., 2002; Potter & Butters, 1980), although specificity of these lesion-behavior associations has been called into question (Dunn, Dalgleish, & Lawrence, 2006; Turner & Levine, 2006). However, the differential or combined sensitivity of the tasks in TBI remains unclear, since few studies have investigated performance on these tests in TBI (Gansler, Covall, McGrath, & Oscar-Berman, 1996; Levine et al., 2005; Yousem, Geckle, Bilker, Kroger, & Doty, 1999), with no studies investigating all three tests simultaneously. Moreover, beyond relating large ventral frontal lesions to cognitive/behavioral problems, assessment of DAI effects may yield more precise predictions about anatomical-behavioral relationships in TBI. Therefore, we investigated how residual structural brain integrity of TBI patients with and without focal lesions corresponded to cognitive problems in three neuropsychological tests commonly associated with ventral frontal cortex functions.
METHODS
Participants
Fifty-eight patients spanning the entire range of trauma severity were recruited approximately one year post-injury from consecutive admission lists to Sunnybrook Health Sciences Centre, Toronto, Canada. Injury severity was determined by the Glasgow Coma Scale (GCS: Teasdale & Jennett, 1974) score as documented by the trauma team leader at the point of transfer from the Trauma Unit, approximately six hours post-injury in most patients. The GCS was used to code TBI severity as mild (scores: 13−15), moderate (scores: 9−12), or severe (scores: 3−8). Severity classification (mild/moderate/severe) was upgraded in eight patients where extended loss of consciousness (> 2 hr), post-traumatic amnesia (> 48 hr) or focal lesions suggested more severe injury than indicated by the initial GCS score. Approximately 78% of the patients sustained their TBI's as a driver or a passenger in a motor vehicle accident (N= 45). The remainder were injured as pedestrians in motor vehicle accidents (N= 7), falls (N= 4) or other causes (N= 2).
For comparison of behavioral performance levels only, twenty-five comparison participants were recruited from friends and family members of the patients. Exclusion criteria for all participants included self-reported anosmia, psychiatric history, previous or current substance abuse, learning disabilities, previous TBI's with loss of consciousness greater than five minutes, history of neurological disease, major medical problems or medication impacting cognition, and lack of English proficiency.
As can be seen in Table 1, participants were well-matched for age, education, and vocabulary knowledge.
Table 1.
Demographic variables of comparison subjects and TBI patients separated by severity group
| Comparison subjects N=25 | Mild TBI N=12 | Moderate TBI N=27 | Severe TBI N=19 | Significance | ||
|---|---|---|---|---|---|---|
| Sex | Women | 16 | 5 | 10 | 6 | Chi2 [3] = 4.12 |
| Men | 9 | 7 | 17 | 13 | p> 0.1 | |
| Sex distribution in patients with/without lesions | Women | n/a | 0/5 | 4/6 | 4/2 | Chi2 [2] = 5.12 |
| p> 0.05 | ||||||
| Men | n/a | 0/7 | 5/12 | 5/8 | Chi2 [2] = 3.50 | |
| p> 0.1 | ||||||
| Age in years | Mean | 27.72 | 33.92 | 32.19 | 28.21 | F [3,79]= 1.69 |
| (SD1) | (7.93) | (13.64) | (11.21) | (7.35) | p> 0.1 | |
| Years of education | Mean | 15.08 | 13.42 | 14.81 | 14.74 | F [3,79]= 1.62 |
| (SD1) | (1.78) | (2.02) | (2.22) | (2.75) | p> 0.1 | |
| Vocabulary Knowledge | Mean | 30.40 | 28.77 | 29.47 | 26.54 | F [3,72]= 1.85 |
| (Zachary, 1986)* | (SD1) | (4.15) | (3.57) | (6.32) | (6.22) | p> 0.1 |
| Years since injury | Mean | n/a | 1.10 | 1.12 | 1.02 | F[2, 55]= 0.54 |
| (SD1) | (0.31) | (0.39) | (0.23) | p>0.1 | ||
| Glasgow Coma Scale | Mean | n/a | 14.54 | 11.0 | 5.68 | F[2,55]= 84.55 |
| (SD1) | (0.72) | (2.11) | (2.16) | p< 0.001 | ||
| SIT(™) | Mean | 35.92 | 35.75 | 31.93 | 28.16 | F[3,79]= 8.08, p<0.001 |
| (SD1) | (2.78) | (1.76) | (6.78) | (7.78) | ||
| OA | Mean | 4.84 | 14.08 | 14.48 | 21.42 | F[3,79]= 4.3 |
| errors | (SD1) | (6.21) | (14.34) | (16.08) | (18.85) | p<0.01 |
| IGT | Median | 12 | 0 | 1.6 | 0 | F[3,79]= 1.73 |
| Composite score across all IGT blocks: advantageous — disadvantageous decisions | (Intraquartile range) | (−2.2 − 35.7) | (−8 − 12) | (−6.3 − 14.3) | (−11.3 − 30.3) | p> 0.1 |
Standard deviation; n/a: not applicable;
available for 51 patients; SIT: Smell Identification Test; OA: Object Alternation; IGT: Iowa Gambling Task
The comparison group was typically drawn from the patients' spouses or partners. This approach resulted in more women in the comparison group. However, as can be seen in Table 1, the gender distributions between comparison subjects and TBI patients as well as within patient groups with and without focal lesions were not statistically different. The comparison subjects described above did not receive MRI scanning.
All participants were informed of the experimental aim of the study and gave their written consent to participate. The study rationale was approved by a local institutional ethics review board adhering to the Declaration of Helsinki. For a fuller description of the imaging and behavioral characteristics of these patients, see Levine et al. (submitted a, b).
Neuropsychological assessment
Testing and brain imaging were conducted concurrently in the post-acute stage at approximately one year post-injury as part of the Toronto TBI study, a large-scale study of brain-behavior relationships in TBI (Levine et al., 2006). The Smell Identification Test (SIT™: Doty, 1995), Object Alternation (OA: Freedman et al., 1998), and the Iowa Gambling Task (IGT: Bechara et al., 2000) were part of a larger neuropsychological test battery including standard and experimental measures. Performance on the Word Memory Test (Green, Allen, & Astner, 1996), a symptom validity test, indicated adequate effort in all patients.
The SIT™ (Doty, 1995) is a standardized micro-encapsulated “scratch-and-sniff” test containing 40 odorants. Odorants were presented to both nostrils simultaneously. For each odor, the participants had to choose one of four possible answers. The dependent variable was the number of correctly identified odors. Although we did not assess olfactory thresholds, it is noted that the vast majority of our patients (53/58; 91%) scored above the anosmic range on the SIT, and all performed above chance, indicating that our sample had sufficient residual thresholds to perform the test. This is contrasted to the findings of Yousem and others (1999), where 16/36 (44%) of patients recruited from a smell and taste clinic obtained scores in the anosmic range, and 8/36 (22%) were at chance levels (i.e., a score of 10 or less).
The object alternation test was administered in a modified version for use with human subjects (see Freedman et al. [1998] for details). In the test session, investigator and participant sat facing each other across a table, separated by a wood frame carrying a curtain. In front of the participant was a stimulus board containing two reinforcement wells covered by two differently shaped and colored objects. As reinforcement, a penny was placed under one of the two objects and the participant was asked to choose one object hiding a penny. After the initial response the curtain was lowered and the reinforced object was alternated, i.e., the experimenter placed the penny under the object not selected by the participant in the preceding trial. Throughout the task the two objects were placed in the left and right positions according to a modified random schedule. Disregarding the spatial positions of the objects, the participant's task was to learn the reinforced object was alternated after each correct response. Learning criterion in this task was 12 consecutive correct responses; failure criterion was 50 trials after which the task was aborted. The dependent variable was the total number of errors.
Participants were administered a computerized version of the IGT (Bechara et al., 2000). In the test session, four card decks appeared facedown on the computer screen, labeled A, B, C, and D. Participants were asked to select cards from one of the decks by naming the letter of the respective deck. The examiner operated the computer mouse to make the selection. Starting capital was fixed at $200 of fictive money “borrowed” from the examiner. The decks were stacked such that A and B produced high winnings and higher losses, whereas C and D produced more modest winnings but smaller losses. After each selection, the amount won appeared on the screen, followed by the amount lost, if any. Over time, C and D yield the highest overall winnings. The test continued until 100 cards had been drawn. Participants were neither instructed about the rules of the task, nor did they know the number of trials until completion of the task. The dependent variable in this test was the number of advantageous choices calculated as the number of cards drawn from decks C and D minus the number of cards drawn from decks A and B. For the behavioral analyses, IGT difference scores are reported per IGT block (block 1: trials 1−20, block 2: trials 21−40, block 3: trials 41−60, block 4: trials 61−80, block 5: trials 81−100). For our main multivariate brain-behavior analyses (see below), one score per test (OA, SIT, IGT) was used so that each measure contributed equally to the covariance patterns. Thus, this analysis used the composite IGT difference score (across all 100 cards). As this method may obscure potentially important block effects, we conducted ancillary brain-behavior analyses on the IGT blocks. As discussed below, these analyses did not alter our conclusions.
Image acquisition
Brain MR images were acquired at the time of behavioral testing with a 1.5 T-Signa scanner (GE Medical Systems). For each patient we obtained a T1-weighted image (3D SPGR, TE= 5 ms, TR= 35 ms, 1 NEX, flip angle= 35 degrees, FOV= 22 × 16.5 cm, 0.859 × 0.859 in-plane resolution, 1.2 − 1.4 mm slice thickness), a proton-density (PD) and a T2 weighted image (interleaved axial spin echo with TE= 30 and 80 ms, TR= 3 s, 0.5 NEX, FOV= 22 × 22 cm, 0.859 × 0.859 in-plane resolution, 3 mm slice thickness). Gradient echo T2 sequences with a slice thickness of 6 mm were obtained to emphasize hemosiderin deposits in TBI patients (TR/TE= 750/35 ms, flip angle= 20 degrees, 2.0 NEX, FOV= 22 cm). For technical reasons, gradient echo images were unavailable for 18 patients.
Our imaging parameters were optimized to the detection of parenchymal damage in TBI (Blatter et al., 1997), but not to the detection of olfactory bulb and tract damage, which in previous studies has been accomplished with a surface coil centered on the nasion and a separate coronal scan through the olfactory bulbs and tracts (Yousem et al., 1999).
Image processing
Brain MRI data were analyzed via an updated version of our previously reported image processing pipeline (Dade et al., 2004; Kovacevic et al., 2002). The main modification to this protocol involves template matching, allowing for comparison of individual images to a standard image and facilitating automation of previously semi-automated steps. The first step in the pipeline was to create an unbiased non-linear average of T1-weighted images from a set of 10 healthy age-matched comparison subjects (mean age = 26.6 years; SD = 2.8) using a modification of an algorithm previously developed for mouse brain MRI (Kovacevic et al., 2005). Each subject's T1-weighted image was then registered to the template brain (Woods, Grafton, Watson, Sicotte, & Mazziotta, 1998), preserving the original size of the brain while standardizing the position and orientation. Images were resampled into the standard space using windowed sinc interpolation. Template matching was accomplished via non-linear registration of T1-weighted images to the template image (Collins & Evans, 1997).
Removal of non-brain tissue from the image incorporated thresholding information derived from the PD and T2-weighted images, facilitating the distinction between dura mater and gray matter (Kovacevic et al., 2002). This is contrasted to methods of brain extraction based on the T1-weighted image that emphasize the cortical surface, inconsistently preserving subdural CSF.
Lesions due to focal cortical contusions were manually defined slice-by-slice in the axial plane (with occasional corroboration from the coronal plane) using Analyze® software (Biomedical Imaging Resource, Mayo Foundation, Rochester, MN, USA). Traceable lesions appeared on at least two slices, with a minimal diameter of at least 3 mm. When necessary, T2- and PD-weighted images were used to guide the tracing, but only lesions visible on T1-weighted images were traced.
The voxels on the T1-image were then classified as representing gray matter, white matter, or CSF using an automated tissue classification method correcting for radio-frequency inhomogeneity inherent to MR scanning (Kovacevic et al., 2002). Voxels within lesions were classified as lesioned tissue.
A modified Semi-Automated Brain Region Extraction (SABRE: Dade et al., 2004) method was then used to create ROIs on the template brain. Based on identification of the edges of the brain and the anterior and posterior commisures, a Talairach-like (Talairach & Tournoux, 1988) grid is automatically created. The algorithm uses this grid along with the 15 manually-identified landmark coordinates and tracing of the cingulate gyrus to divide the brain into 38 regions (19 per hemisphere; see Figure 1). Non-linear deformation field matching of the template to individual images was used to customize these regions to fit each participant's brain anatomy (as opposed to transforming images to fit the template, which can distort inter-individual topographical variability). Regional gray matter, white matter, and CSF volumes were adjusted for total intracranial capacity using a regression-based method (Arndt, Cohen, Alliger, Swayze, & Andreasen, 1991)
Figure 1.

SABRE regional cortical divisions in axial and sagittal views projected on a template brain derived from 12 healthy control subjects' T1-weighted images. Abbreviations: LSF: lateral superior frontal, MSF: medial superior frontal, LMF: lateral middle frontal, MMF: medial middle frontal, LVF: lateral ventral frontal, MVF: medial ventral frontal, GCG: genual cingulate gyrus, ACG: anterior cingulate gyrus, MCG: middle cingulate gyrus, PCG: posterior cingulate gyrus/retrosplenial cortex, AT: anterior temporal, MT: medial temporal, PT: posterior temporal, O: occipital, ABGT: anterior basal ganglia/thalamus, PBGT: posterior basal ganglia/thalamus, EC: external capsule/corona radiata, IP: inferior parietal, SP: superior parietal. Non-brain tissue, the cerebellum, and the brainstem are excluded from analyzed images.
Our tissue compartment segmentation and SABRE software are particularly well suited to analysis of brains with atrophy or lesions, as they do not require spatial transformation possibly distorting inter-individual topographical variability. These algorithms have been successfully applied to normal aging (Dade et al., 2004), dementia (Gilboa et al., 2005; Bocti et al., 2006), and multiple sclerosis populations (Carone et al., 2006; Feinstein et al., 2004).
Lesion distribution
Focal cortical contusions ranging in size from 0.07 cm3 to 77.38 cm3 (median= 2.44 cm3) were identified by a neuroradiologist (F.G.) in 18 of the 58 patients (see Figure 2). All 18 patients had lesions in the frontal lobes, predominantly in ventral frontal regions of the right hemisphere or bilaterally. In four patients these lesions were confined to frontal cortex, whereas the remaining fourteen patients had additional involvement of other brain regions, mostly showing the fronto-temporal lesion pattern characteristic for TBI. These patients will be referred to as the focal lesion group, acknowledging that they also had diffuse injury, whereas the remaining 40 patients without focal cortical contusions will be referred to as the diffuse injury group. Note the label of “diffuse injury” emphasizes the absence of focal cortical contusions, but it does not exclude microvascular lesions common in TBI.
Figure 2.
Location and degree of overlap of brain lesions. Lesions are projected on five selected axial slices of a template brain derived from 12 healthy control subjects' T1-weighted images. Areas damaged in only one subject are shown in dark red, brighter colors indicate the degree to which lesions involve the same structures in up to five individuals (see legend). Lesions were concentrated in ventral prefrontal cortex and frontal insular cortex of the right hemisphere. (R= right hemisphere).
Olfactory bulb and tract damage can co-occur with ventral frontal damage in TBI, confounding interpretation of smell deficits. The olfactory tracts and bulbs for 10 patients in our sample were inspected by a neuroradiologist (F.G.), blind to olfactory diagnosis. This was accomplished by rotating the T1-weighted images to AC-PC alignment to correct all tilts, then parallel to the long axis of the olfactory bulb in the sagittal plane. The olfactory tracts and bulbs were then examined in the axial, coronal, and sagittal planes. A qualitative grading of the olfactory bulb and olfactory tract in both left and right hemispheres was assigned using a 0−1−2 scale, with 0 representing no damage, 1 representing visible (marked-moderate) damage, and 2 representing severe damage (absence of olfactory structures) (Yousem, Geckle, Bilker, McKeown, & Doty 1996).
The 10 patients included the five lowest scoring patients on the SIT (range 12−18 of 40; the cutoff score for anosmia in the SIT is 17; Doty, 1995) and five normosmic patients according to their SIT scores (range 38−40). Two of the anosmic patients had focal cortical contusions and were thus in the focal lesion group; the remaining three were in the diffuse injury group. Olfactory bulb and tract damage was found in four of the five lowest scoring patients, although three of these had unilateral olfactory tract damage (two left – both patients with focal cortical lesions, one right). We also found olfactory tract damage in two of the five normosmic patients (one right, one bilateral). Damage was rated as “1” in all instances. We did not undertake inspection of the remaining 48 scans for several reasons. First, this pilot analysis of 10 patients suggested a lack of sensitivity and specificity of extraparenchymal damage to SIT performance in our sample. Even poorly performing patients (i.e., 12/40 on the SIT) had only unilateral left damage and bilateral damage was equally represented across anosmic and normosmic patients. Our imaging parameters were not optimized to the detection of this damage; even optimal imaging parameters would be insufficient to detect microscopic damage that could, in theory, contribute to SIT performance. Finally, olfactory tract and bulb damage is inextricably confounded with ventral prefrontal damage in TBI. It is therefore impossible to separate the effects of these two contributions to SIT performance in TBI, even assuming optimal quantification of the olfactory tracts and bulbs. These issues are addressed in the results and discussion in relation to our findings and to previous research on this issue.
Statistical analyses
Partial Least Squares (PLS) is a flexible multivariate technique extensively applied to brain imaging data (McIntosh & Lobaugh, 2004). In general terms, PLS is a multivariate analysis technique for relating two sets of variables to each other. In the present application, it was used to identify patterns of volume loss related to performance on the three identified tests purportedly sensitive to ventral frontal function. Because PLS considers the brain as a whole, it well suited to the detection of distributed patterns of volume loss covarying with test performance.
In the first step of the PLS analyses, correlations were computed between the brain imaging data (i.e., regional gray matter, white matter and CSF volumes) and the behavioral measures. Singular value decomposition was then applied to this correlation matrix to identify latent variables [LVs] which indicate optimal relations between profiles of task performance and patterns of volume loss (for details, see McIntosh & Lobaugh, [2004]).
The statistical significance of each LV was assessed by 1500 permutation tests with a threshold of p<0.05 (Edgington, 1980; McIntosh, Bookstein, Haxby, & Grady, 1996), in which behavioral observations were shuffled within subjects to calculate the probability of each LV having occurred by chance. The reliability of each brain volume's contribution to the LV was determined through bootstrap resampling (subjects were resampled 500 times) (Wasserman & Bockenholt, 1989). Brain regions in the singular images were considered reliable if they had a ratio of salience to standard error (SE) greater than 3, similar to a Z-score, which corresponds to 99% confidence limits (Efron & Tibshirani, 1986). Thus, the ratio of salience to SE derived from the bootstrap analyses reflects the consistency with which the salience of a particular brain region is non-zero across subjects. Because image-wide statistical assessment is done in a single analytic step, no correction for multiple comparisons across brain regions is required.
Owing to the heterogeneous nature of TBI neuropathology, we conducted separate brain-behavior PLS analyses for the focal lesion group and the diffuse injury group. For the diffuse injury group, separate analyses were conducted for gray matter, white matter, and CSF. As noted above, lesioned regions were excluded from segmentation analysis as tissue compartments could not be reliably estimated from voxels within these regions. For analyses involving the focal lesion group, we assumed the lesioned tissue contained no functioning gray or white matter. We were unable, however to estimate CSF volumes within these regions. As the exclusion of lesioned regions could artificially reduce CSF volumes (particularly in ventral frontal regions of interest in this study), analyses for the lesion group were restricted to gray and white matter.
The remaining statistical analyses were conducted with parametric methods (t-test, ANOVA, Pearson correlations) using Statistical Package for the Social Sciences (SPSS) for Windows (Release 12.0.0, September 4th 2003; Chicago: SPSS Inc.). In case of unequal group variances, as indicated by Levene's test, the unequal variance T-test correcting degrees of freedom, was applied (incorporated in SPSS). Post-hoc simple comparisons were Bonferroni-corrected. Where necessary, we applied square-root transformations to correct for skewness before applying parametric tests.
Because statistical assessment of PLS results is non-parametric, untransformed original data were used in the PLS analyses. For ease of interpretation, behavioral data in the PLS analyses were scaled in the same direction, inverting the error rate in the OA task into positive scores of performance.
RESULTS
Behavior
All three tests were sensitive to the effects of TBI. Relative to comparison subjects, TBI patients identified fewer odors in the SIT (T[74.7]= 4.05, p<0.001), produced more errors in OA (T[76.6]= −4.01, p<0.001) and selected more cards from the higher risk decks of the IGT as assessed by the composite score (T[81]= 2.19, p<0.05).
IGT performance was differentially implicated across blocks, mirroring previously-published results from these same patients (as part of a larger sample; Levine et al., 2005). A repeated-measures analysis of variance on IGT performance with block (1−5) and group (TBI patients, comparison subjects) as factors revealed significant main effects of block (F[4,324]= 13.2, p<0.001), group (F[1,81]= 4.8, p<0.05), and an interaction of block and group (F[4,324]= 4.95, p<0.01). The main effect of group, as noted above, indicated better performance of comparison subjects than TBI patients. The group by block interaction showed differential acquisition slopes across the five IGT blocks in patients and comparison subjects: Whereas comparison subjects' IGT performance improved from block 1 to subsequent blocks (block 2: T[24]= −4.77, block 3: T[24]= −5.11, block 4: T[24]= −4.82, block 5: T[24]= −6; all p<0.001), TBI patients performed identically in blocks 1 and 2 (T[57]= −0.19, p>0.1), and only started to improve in block 3 (block 1: T[57]= −3.18, p<0.01; block 2: T[57]= −3.07, p<0.05). Direct group comparisons at each block indicated significantly higher selection from the good decks for the comparison group than for TBI patients at block 2 (T[81]= 3.82, p<0.05) and marginally so, at block 3 (T[81]= 1.98, p=0.051). In contrast, comparison subjects selected fewer cards from the good decks compared to the TBI patients in the beginning of the IGT (block 1: T[81]= −2.34, p<0.05). Thus, healthy subjects started out with a bias towards selection of risky decks, but then quickly improved their strategy and shifted towards the advantageous decks in block 2 and subsequent blocks. TBI patients began more cautiously and started improving their selection strategy only in block 3.
As demonstrated previously (Levine et al., 2005), the IGT did not differentiate patients with varying TBI severity (see Table 1). Performance in the SIT paralleled TBI severity. Mild TBI patients had a higher performance than moderate TBI patients who scored higher than severe TBI patients (see Table 1). In post hoc-tests, severe TBI patients had significantly lower SIT performance than comparison subjects (T[26.6]= −4.71, p<0.001) and mild TBI patients (T[24.7]= −4.45, p<0.01; see also panel A of Figure 3).
Figure 3 a-b.
3 a: Smell identification performance across TBI severity groups; 3 b: Number of errors in Object Alternation performance across TBI severity groups. Error bars are 95%-confidence intervals around the mean.
OA was sensitive to effects of injury severity, with significantly elevated error rates only in severe TBI patients compared to healthy subjects (T[25.31]= −3.6, p<0.01). Although mild and moderate TBI patients also had enhanced error rates in OA, they were not statistically different from comparison subjects (see Table 1 and panel B of Figure 3).
The presence of focal cortical contusions affected performance in the SIT, F[2,80]= 9.2, p<0.001, but not in OA or the IGT. Patients in the focal lesion group scored lower in the SIT than patients in the diffuse injury group, T[56]= −2.35, p<0.05, while both groups were significantly impaired in contrast to the comparison subjects, T[24.31]= −4.24, p<0.001 and T[63]= −2.4, p<0.05 for focal lesion and diffuse injury versus comparison subjects, respectively. When TBI severity was equated across groups, patients in the focal lesions and without lesions did not differ significantly from each other in any of the three measures. Thus, in our sample of TBI patients, the presence of focal cortical contusions did not have a specific effect on test performance over and above that of TBI severity alone.
In summary, all three behavioral measures were sensitive to general effects of TBI. Within the patient group, the SIT compared to OA and the IGT was more sensitive to both effects of injury severity and presence of focal cortical contusions.
PLS results
Focal lesion group
The objective of the PLS analyses is to identify patterns of brain regions that explain the variability in the behavioral data. Whereas a principal component analysis computes the pairwise covariance between the dependent variables (i.e., intercorrelations between brain volumes, or, between behavioral measures), PLS determines the covariance between brain volumes and behavioral measures. Thus, PLS identifies not simply patterns of brain volumes that covary together across subjects, or sets of behavioral measures that covary across subjects, but rather brain volume-behavior pairings that covary together. We identified significant latent variables between behavioral performance and regionally-specific brain volumes for gray matter and white matter in the focal lesion group, each accounting for a large portion of the brain-behavior covariance (covariance explained: gray matter: 82.67%, white matter: 72.01%).
As expected given the lesion location, the right ventral frontal sector was strongly associated with behavior, but regional effects were not restricted to this region. Additionally, there was evidence of specificity within the three behavioral measures; not all measures contributed significantly to the overall covariance with brain volumes.
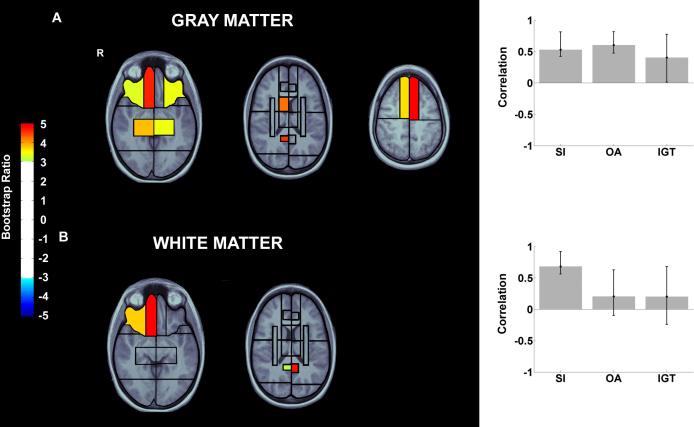
As illustrated in Figure 4 (panel A), performance on all three tests significantly covaried with gray matter volume loss in the frontal cortex, including the right medial ventral sector, the lateral ventral sectors bilaterally, and the medial superior sector bilaterally. Significant effects, however, were not restricted to the frontal lobes. Additional regions contributing to this pattern included the bilateral medial temporal lobes, right posterior cingulate gyrus/retrosplenial cortex, and right anterior basal ganglia. In the analysis of white matter loss, only the SIT contributed reliably to the behavioral pattern. Reliable white matter volume loss in the right lateral and medial ventral frontal cortex was associated with SIT performance. Furthermore, white matter volumes in bilateral posterior cingulate gyrus/retrosplenial cortex were associated with behavior (see Figure 4, panel B).
Figure 4.
Latent variable from PLS analyses of brain volumes and performance in Smell Identification Test (SIT), Object Alternation (OA), Iowa Gambling Task (IGT) in 18 TBI patients with focal lesions. The pattern of test performance associated with the latent variable, expressed as correlations between test scores with the pattern of volume changes, is presented on the right side. Error bars represent 95% confidence intervals. Tests with error bars crossing the horizontal axis did not significantly contribute to the latent variable. On the left are regional plots of boostrap ratios indicating pattern of gray and white matter volume changes associated with the pattern of test scores depicted on the right. The color bar indicates the coding scheme according to the level of the bootstrap ratio, interpreted similar to a Z-score. Not all 38 regions and only reliable salience-to-SE ratios are shown (assessed by bootstrap resampling, p<0.01). The anatomical underlay is derived from a template of 12 healthy control subjects' T1-weighted images. Images are displayed in radiological convention (right hemisphere displayed on left side of image). A: PLS analysis of gray matter volumes and behavior (LV1: singular value= 3.32, p<0.01). Reliable peaks were observed in bilateral ventral lateral and right ventral medial frontal, bilateral superior medial frontal, bilateral medial temporal, right anterior basal ganglia and right posterior cingulate gyrus/retrosplenial cortex gray matter. B: PLS analysis of white matter volumes and behavior (LV1: singular value= 2.81, p<0.01). Reliable peaks were observed in right lateral and medial ventral frontal and bilateral posterior cingulate gyrus/retrosplenial cortex white matter. (R= right hemisphere).
In order to rule out potential contamination of our results by two of the focal lesion patients who had suspected unilateral olfactory bulb/tract damage, we performed additional PLS analyses excluding these patients. The results of these analyses were highly similar to the ones reported above. Consistently, we found covariance of test performance with gray matter loss in bilateral frontal cortex, including right medial and lateral ventral frontal cortex, and in posterior cingulate gyrus/retrosplenial cortex. The medial temporal lobe involvement fell short of significance in this analysis. Instead we now found anterior and posterior temporal lobe gray matter loss related to behavior. As for white matter loss, again, right medial and lateral ventral frontal cortex as well as posterior cingulate gyrus/retrosplenial cortex damage was related to smell identification performance only. Additionally, we now found right medial temporal lobe white matter loss related to smell identification.
Diffuse injury group
In the diffuse injury group, there was a significant latent variable identified for gray matter (covariance explained: 68.9%), but not white matter. A latent variable in the analysis of CSF volumes approached significance (p=0.077; covariance explained: 62.8%).
In contrast to the focal lesion group, the IGT did not contribute significantly to the brain-behavior covariance the diffuse injury group. SIT and OA performance was associated with higher gray matter volumes in bilateral posterior temporal lobes (see Figure 5, panel A). None of the other regional gray matter volumes was reliably related to behavior. As for CSF volumes, we observed reliable covariance of behavior on the SIT only and volume loss in left ventral medial frontal cortex (see Figure 5, panel B) indicating regional specificity of smell identification performance to ventral frontal cortex integrity in the absence of lesions. We may have seen a left-lateralization of this effect in the diffuse injury group because patients in our sample with greater right-lateralized ventral frontal damage were included in the focal lesion group.
Figure 5.
Latent variable 1 from PLS analyses of brain volumes and performance in Smell Identification Test (SIT), Object Alternation (OA), Iowa Gambling Task (IGT) in 40 TBI patients with diffuse brain injury. The pattern of test performance associated with the latent variable, expressed as correlations between test scores with the pattern of volume changes, is presented on the right side. Error bars represent 95% confidence intervals. Tests with error bars crossing the horizontal axis did not significantly contribute to the latent variable. On the left are regional plots of boostrap ratios indicating pattern of gray and white matter volume changes associated with the pattern of test scores depicted on the right. The color bar indicates the coding scheme according to the level of the bootstrap ratio, interpreted similar to a Z-score. Not all 38 regions and only reliable salience-to-SE ratios are shown (assessed by bootstrap resampling, p<0.01). The anatomical underlay is derived from a template of 12 healthy control subjects' T1-weighted images. Images are displayed in radiological convention (right hemisphere displayed on left side of image). A: PLS analysis of gray matter volumes and behavior (LV1: singular value= 3.32, p<0.01). Reliable peaks were observed in bilateral ventral lateral and right ventral medial frontal, bilateral superior medial frontal, bilateral medial temporal, right anterior basal ganglia and right posterior cingulate gyrus/retrosplenial cortex gray matter. B: PLS analysis of white matter volumes and behavior (LV1: singular value= 2.81, p<0.01). Reliable peaks were observed in right lateral and medial ventral frontal and bilateral posterior cingulate gyrus/retrosplenial cortex white matter. (R= right hemisphere).
Post-hoc tests of the ROIs identified by PLS
The multivariate PLS analyses allowed us to identify the image-wide distribution of brain regions related to performance measures. To verify the effects in the ROIs observed in the PLS analyses by conventional methods, we assessed correlations between the single behavioral measures and the identified ROIs, much the same way post-hoc univariate tests would be applied to omnibus findings in ANOVA. Broadly speaking, the results of these follow-up tests confirmed the findings from the PLS analyses (see Table 2).
Table 2.
Pearson correlations between test behavior and ROIs identified by the PLS analyses in patients with focal lesions and diffuse injury
| Test: | SIT | OA | IGT | |
|---|---|---|---|---|
|
Focal Lesion Group (N=18) | ||||
| Gray matter | Left medial superior frontal lobe | 0.38 | 0.58* | 0.53* |
| Right medial superior frontal lobe | 0.40 | 0.54* | 0.45 | |
| Left lateral ventral frontal lobe | 0.58* | 0.28 | 0.46 | |
| Right lateral ventral frontal lobe | 0.36 | 0.35 | 0.39 | |
| Right medial ventral frontal lobe | 0.50* | 0.34 | 0.34 | |
| Left medial temporal lobe | 0.36 | 0.58* | 0.34 | |
| Right medial temporal lobe | 0.54* | 0.41 | 0.46 | |
| Right posterior cingulate gyrus/retrosplenial cortex | 0.71** | 0.61** | 0.22 | |
| Right anterior basal ganglia | 0.69** | 0.34 | 0.47 | |
| White matter | Right lateral ventral frontal lobe | 0.61** | 0.13 | 0.35 |
| Right medial ventral frontal lobe | 0.70** | 0.14 | 0.37 | |
| Left posterior cingulate gyrus/retrosplenial cortex | 0.58* | 0.51** | −0.06 | |
| |
Right posterior cingulate gyrus retrosplenial cortex |
0.65** |
0.21 |
−0.21 |
|
Diffuse Injury Group (N=40) | ||||
| Gray matter | Left posterior temporal lobe | 0.39* | 0.24 | 0.08 |
| Right posterior temporal lobe | 0.48** | 0.10 | 0.08 | |
| CSF | Left medial ventral frontal lobe | −0.32* | −0.05 | 0.25 |
p<0.05
p<0.01; SIT: Smell Identification Test; OA: Object Alternation, inverted number of total errors; IGT: Iowa Gambling Task; CSF: cerebrospinal fluid
As shown in Table 2, in the focal lesion group, the SIT was the only measure significantly correlated with ventral frontal cortex gray and white matter volumes, whereas OA and IGT performance correlated with gray matter volumes in superior frontal lobe. Furthermore, posterior cingulate gyrus/retrosplenial cortex gray and white matter integrity was related to the SIT and OA. Thus, although all three behavioral measures contributed significantly to the regional pattern of gray matter loss in the multivariate analysis of focal injury patients, the ventral frontal part of this pattern was the only one related to SIT performance as seen in the correlations.
In the diffuse injury group, correlations between performance on the SIT and left ventral medial CSF and bilateral posterior temporal gray matter volumes were consistent with the results of the PLS analyses. Although the PLS analysis suggested OA performance contributed to the gray matter findings (albeit to a lesser degree than the SIT), this finding was not confirmed in the correlational analysis.
DISCUSSION
This is the first study of brain-behavior relationships in a large TBI sample to combine tissue segmentation, regional brain parcellation, and lesion location analysis. Our focus was on neuropsychological functions sensitive to damage in the ventral frontal cortex, a brain region commonly implicated in TBI. We observed two primary results: Firstly, structural integrity of the ventral frontal cortex was reliably related to smell identification (the SIT), but less reliably so to object alternation (OA) or gambling (IGT) performance. Even in patients with lesions, OA and IGT performance showed stronger associations with residual tissue volumes outside the ventral frontal cortex, including the superior medial frontal lobes, sectors of the temporal lobes, and the posterior cingulate gyrus/retrosplenial cortex. Secondly, although most of our brain-behavior correlations were seen in patients with lesions, we still found smell identification associated with parenchymal integrity in medial ventral frontal cortex without lesions. Thus, extending conventional lesion analysis, our approach suggests some ventral frontal cortex functions may covary with ventral frontal cortex damage caused by diffuse injury in TBI. Importantly, the overall brain-behavior patterns in TBI patients with and without lesions were not only quantitatively but also qualitatively different, underlining the necessity to consider these two types of TBI neuropathology separately.
Previous studies in TBI found associations of neuropsychological dysfunctions and atrophy in regions selected for ease of visualization on imaging or for theoretical reasons, such as corpus callosum, cingulate gyrus or fornix (Gale, Johnson, Bigler, & Blatter, 1995; Tomaiuolo et al., 2004; Verger et al., 2001). However, as demonstrated here, regions outside such pre-determined ROIs may be implicated in neuropsychological test performance. Whole-brain approaches offer an unbiased way of assessing structure-function relationships in this population. Furthermore, sampling across tissue compartments reveals the contribution of cortical volume loss to behavior, in contrast to previous studies where analysis was restricted to measures of white matter loss (Bigler, Anderson, & Blatter, 2002; Levin et al., 1990; Serra-Grabulosa et al., 2005; Wilde et al., 2005). For instance, we found no significant correlations between behavior and white matter volumes in the diffuse injury group, suggesting that gray matter volume loss is more important to behavior than white matter volume loss in the absence of larger focal lesions. For a similar finding in relation to standard neuropsychological tests, see Levine et al. (submitted-b).
Our results were dominated by effects of smell identification performance, which in the diffuse injury group was the only test significantly associated with brain volume loss. Beside the high frequency of olfactory impairment after TBI (Callahan & Hinkebein, 1999; Doty et al., 1997; Levin, High, & Eisenberg, 1985), smell identification problems are strongly associated with the presence of clinically diagnosed lesions in TBI measured with conventional imaging techniques. In the current study, we additionally confirm a quantitative relationship of smell identification problems and degree of radiological abnormalities even without large focal lesions.
Previous investigations have found high incidences of olfactory bulb and tract damage associated with olfactory deficits after TBI (Yousem et al., 1999; Yousem, et al., 1996). As noted above, our imaging parameters were not optimized to the detection of extraparenchymal damage in the olfactory system, so we were unable to empirically assess the contribution of such damage to our results. At minimum, total anosmia due to transection of cranial nerve I (the olfactory tracts) did not contribute to our results, in that patients with self-reported anosmia were excluded from this study; the vast majority of the remaining patients, recruited from consecutive admissions, performed above chance on the SIT, confirming residually intact sensory thresholds. This is contrasted to the previous large-scale study of olfaction and imaging in TBI of patients recruited after presentation to a smell and taste clinic, were 22% performed at or below chance levels on the SIT and 44% were anosmic (Yousem et al., 1999). In that study, olfactory tract and bulb volumes were correlated with SIT performance only among the anosmic patients; cortical temporal volumes were uncorrelated with performance and frontal volumes were not assessed.
Furthermore, only two of our 18 focal lesion patients scored in the anosmic range on the SIT. Although there was evidence of olfactory tract damage in these patients upon inspection by a neuroradiologist (F.G.), it was restricted to the left hemisphere in both patients, contralateral to the side of the right-hemispheric effects in the PLS and the brain lesion location. Due to the heavily ipsilateral nature of olfactory projections, and the right-sided nature of the ventral frontal cortex involvement, a left olfactory tract lesion is unlikely to be the sole source of an olfactory deficit (cf. Eslinger, Damasio, & Van Hoesen, 1982). Accordingly, when these two patients were excluded from our analyses the critical findings concerning ventral frontal cortex held.
We acknowledge however, that TBI forces sufficient to damage the ventral medial prefrontal cortex may also cause olfactory tract or nerve shearing in the nasal cavity. Subtle olfactory tract or bulb damage cannot be ruled out as contributing to our results as the integrity of these extraparenchymal stuctures was not empirically assessed. Our finding of superior sensitivity of SIT to ventral medial prefrontal cortex damage may therefore be caused by the fact that in TBI, unlike in other patient populations with exclusively focal lesions, ventral medial prefrontal damage is necessarily correlated with extraparenchymal, primary sensory damage. Nonetheless, our findings replicate previous research on patients with ventral frontal damage in the absence of olfactory tract or bulb damage (Jones-Gotman et al., 1997) as well as recent findings from our own laboratory in a sample of 32 patients with focal lesions due to strokes or tumors, where SIT performance was related to bilateral ventral frontal and right dorsolateral damage (Wiederkehr et al., in preparation).
Smell identification performance closely paralleled the location of focal lesions. It was further associated with residual integrity of a predominantly right-hemispheric combination of brain regions composed of ventral frontal cortex, medial temporal lobes and posterior cingulate gyrus/retrosplenial cortex. The primary olfactory cortex on the medial surface of the temporal lobe receives input from the ipsilateral olfactory bulb. Via thalamic, perirhinal and entorhinal regions, the ventral prefrontal cortex receives secondary and tertiary olfactory projections (Rolls, 2004). Furthermore, the cingulate gyrus, particularly vulnerable to TBI (Gale, Baxter, Roundy, & Johnson, 2005; Levine et al., submitted-b; Yount et al., 2002), provides a functional contribution to many tasks (Levine et al., submitted-a). In our case, the one may argue that damage to the posterior cingulate gyrus/retrosplenial cortex, intricately connected to medial frontal cortex (Cavada, Company, Tejedor, Cruz-Rizzolo, & Reinoso-Suarez, 2000) may have contributed to the smell deficits. Thus, the localization of smell identification problems in TBI patients with focal cortical contusions can be considered in good accordance to previous studies of olfactory problems after ventral prefrontal or medial temporal lobe lesions (Jones-Gotman et al., 1997; Zatorre & Jones-Gotman, 1991), functional neuroimaging studies (Dade, Zatorre, & Jones-Gotman, 2002; Wang, Eslinger, Smith, & Yang, 2005), and findings of smell loss as reported in other samples of patients with TBI (Green, Rohling, Iverson, & Gervais, 2003; Levin et al., 1985; Yousem et al., 1996). While this lesion-behavior relationship was expected, we showed gradual ventral frontal tissue loss in the absence of focal cortical contusions was likewise associated with smell identification problems following TBI.
An interesting additional finding in the diffuse injury group of patients was the association of smell identification problems to gray matter loss in lateral posterior temporal lobe regions. The SIT requires not only sensory detection of odors but also their classification to semantic verbal labels. The posterior temporal lobes are activated in functional neuroimaging studies involving integration of semantic and multisensory information (Beauchamp, 2005; Büchel, Price, & Friston, 1998). Lesions of posterior temporal lobe regions within the ventral visual pathway correspond with difficulties in connecting categorical semantic knowledge to sensory information (Damasio, Tranel, Grabowski, Adolphs, & Damasio, 2004; Gainotti, Silveri, Daniele, & Giustolisi, 1995; Humphreys & Forde, 2001). Reduced lateral posterior temporal lobe gray matter in our diffuse injury group may therefore have reflected inefficient connection of conceptual knowledge about the odor descriptions to the sensory features of the odors themselves (see also: Jones-Gotman & Zatorre [1988]).
The OA task was only reliably related to regional tissue volumes outside the ventral frontal cortex, including the medial temporal lobes, posterior cingulate gyrus/retrosplenial cortex and superior medial frontal cortex. These results contrast with studies implicating the importance of the ventral frontal regions to OA and other reward alternation performance in humans (Kringelbach & Rolls, 2004; Rolls, 2000) and non-human primates (Mishkin, Vest, Waxler, & Rosvold, 1969; Pribram & Mishkin, 1956). The medial temporal lobe and posterior cingulate gyrus/retrosplenial cortex may mediate mnemonic components of OA, such as associative memory for relationships between information presented at different times (Cohen & Eichenbaum, 1993). OA requires flexible learning and unlearning of correct responses in each trial, whereas other tasks used in patient studies involved a slower rate of reward acquisition and switching (Fellows & Farah, 2003; Hornak et al., 2004). Thus OA may be more sensitive to functions associated with dorsal prefrontal regions, such as resolution of response conflict (de Wit, Kosaki, Balleine, & Dickinson, 2006) and selective attention and working memory (Faw, 2003) may have had a larger contribution to OA performance than processing of the emotional aspects of this task (reward representation and changing response patterns to obtain reward) considered functions of the ventral frontal cortex (Kringelbach & Rolls, 2004). Our study is not alone in this respect. Lesions in previous patient studies of reward alternation behavior have not been strictly confined to ventral frontal lobes; they have encroached well into more superior medial brain areas (Fellows & Farah, 2003; Freedman et al., 1998; Hornak et al., 2004). Results from functional neuroimaging studies of object alternation are thus far inconsistent. Whereas some point to a particular involvement of ventral medial frontal cortex regions (Gold, Berman, Randolph, Goldberg, & Weinberger, 1996; O'Doherty, Kringelbach, Rolls, Hornak, & Andrews, 2001; Zald, Curtis, Folley, & Pardo, 2002), other studies – consistent with our findings – show additional, more dorsal frontal regions critically involved (Zald, Curtis, Chernitsky, & Pardo, 2005), with a particular importance of medial polar frontal cortex (Turner & Levine, 2006).
Finally, we failed to confirm the specificity of the Iowa Gambling Task to ventral frontal brain regions. Indeed, IGT performance was not significantly related to structural integrity of most of the assessed regional brain volumes, even in the presence of lesions. Some previous patient studies showed decision-making problems following ventromedial prefrontal cortex lesions, especially if damage was sustained to the right hemisphere (Bechara, et al., 2000; Tranel, Bechara, & Denburg, 2002). Such an association might be expected in the present study given the predominance of right ventral frontal lesions. Instead we found, similar to our results with the OA task, the only significant correlation to gambling performance was in the focal lesion group and confined to gray matter volumes in superior medial frontal cortex. The specificity of the Iowa Gambling Task to ventral frontal cortex damage has been challenged (Clark, Manes, Antoun, Sahakian, & Robbins, 2003; Manes et al., 2002). For instance, Manes and colleagues (Manes et al., 2002) reported patients with focal ventral frontal cortex damage performed similar to controls, whereas patients with damage to either dorsolateral, dorsomedial or more extended frontal lobe regions were impaired. We have also found that the IGT is unrelated to focal frontal damage in our sample of 32 patients (Wiederkehr et al., in preparation).
A composite IGT score could be regarded as a limitation since effects involving acquisition slopes may be obscured. The first two blocks of the IGT were differentially influenced in the TBI patients compared to comparison subjects. Comparison subjects started out with lower difference scores in block 1 and TBI patients scored lower in block 2, while all other blocks, when analyzed separately, were statistically identical between patients and comparison subjects. Owing to this result of differential performance patterns across IGT blocks (see also Brand, Recknor, Grabenhorst, & Bechara [2007] for healthy individuals' differential performance during IGT blocks), we performed two additional PLS analyses using brain volumes and SIT, OA and separate blocks of the IGT in focal lesion patients and diffuse injury patients. None of the analyses from focal lesion patients returned significant LVs, due to the greater variability among now seven instead of three behavioral measures in this relatively small sample. In the diffuse injury group, none of the single IGT blocks was significantly related to brain volumes. Furthermore, conventional correlations between separate IGT blocks and ventral frontal cortex volumes in either focal or diffuse injury group did not reveal any significant results (all p's> 0.05). Finally, also re-analyzing our results by excluding the first two IGT blocks due to their less reliable contribution to the overall IGT outcome (see also: Levine et al., [2005], Dunn et al. [2006]), to rule out contamination of the composite score by these trials did not qualitatively change our results. Thus, it appears safe to say that, at least in our form of analysis, IGT blocks did not covary differentially with brain volumes in our patient group.
CONCLUSION
We used a unique combination of methods to determine brain-behavior covariations in a large sample of TBI patients. Combining tissue segmentation and regional brain parcellation with a multivariate analysis technique guided our unbiased selection of ROIs with the most important behavioral impact. OA and IGT, tests at the intersection of cognition and reward/risk tolerance, were impaired by TBI, but they were most sensitive to superior medial frontal lesions, possibly due to cognitive demands such as response conflict resolution, working memory and attentional functions. The one test involving the most definite ventral frontal cortical anatomy, the SIT, was also impaired, but here the impairment coincided with focal ventral frontal lobe damage. The association between the SIT and the integrity of the ventral frontal cortex held even in TBI patients without focal lesions. Performance in these patients was additionally associated with the integrity of temporal association areas, possibly reflecting semantic processing.
Human research on complex tasks associated with the ventral frontal cortex has often used patients with focal lesions restricted to these regions (Bechara et al., 2000). Subsequent studies incorporating a wider range of lesion locations have questioned the specificity of these lesion-behavior relationships (Manes et al., 2002). Research in patients with TBI can augment such analyses by incorporating both diffuse and focal lesion effects. Our results are consistent with the notion that complex cognitive tasks (such as the object alternation and gambling tests) do not consistently localize to a single region of association cortex (Mesulam, 1998). Smell identification, although more specifically associated with ventral frontal regions, was also affected by distributed damage.
ACKNOWLEDGEMENTS
We gratefully acknowledge the technical assistance of Ann Campbell, Tharshini Chandra, Catherine Hynes, Sabitha Kanagasabai, Elena Irinia Nica, Marina Mandic, Colleen O'Toole, Karen Philp, Joel Ramirez, Adrianna Restagno, Jovanka Skocic, and Gary Turner. Simon Graham, Nancy Lobaugh, Natasa Kovacevic, Jeremy B. Caplan and Randy McIntosh are thanked for assistance with image analysis. We also thank the participants for volunteering their time to participate in this research. This research was supported by grants from the Canadian Institutes of Health Research (Grant #'s MT-14744, MOP-37535, and MOP-108540), and the NIH-NICHD (Grant # HD42385-01) to B.L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arndt S, Cohen G, Alliger RJ, Swayze VW, II, Andreasen NC. Problems with ratio and proportion measures of imaged cerebral structures. Psychiatry Research. 1991;40:79–89. doi: 10.1016/0925-4927(91)90031-k. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS. See me, hear me, touch me: multisensory integration in lateral occipital-temporal cortex. Current Opinion in Neurobiology. 2005;15:145–153. doi: 10.1016/j.conb.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123:2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Anderson CV, Blatter DD. Temporal lobe morphology in normal aging and traumatic brain injury. American Journal of Neuroradiology. 2002;23:255–266. [PMC free article] [PubMed] [Google Scholar]
- Blatter DD, Bigler ED, Gale SD, Johnson SC, Anderson CV, Burnett BM, et al. MR-based brain and cerebrospinal fluid measurement after traumatic brain injury: correlation with neuropsychological outcome. American Journal of Neuroradiology. 1997;18:1–10. [PMC free article] [PubMed] [Google Scholar]
- Bocti C, Rockel C, Roy P, Gao F, Black SE. Topographical patterns of lobar atrophy in frontotemporal dementia and Alzheimer's disease. Dementia and Geriatric Cognitive Disorders. 2006;21:364–372. doi: 10.1159/000091838. [DOI] [PubMed] [Google Scholar]
- Brand M, Recknor EC, Grabenhorst F, Bechara A. Decisions under ambiguity and decisions under risk: correlations with executive functions and comparisons of two different gambling tasks with implicit and explicit rules. Journal of Clinical and Experimental Neuropsycholy. 2007;29:86–99. doi: 10.1080/13803390500507196. [DOI] [PubMed] [Google Scholar]
- Büchel C, Price C, Friston K. A multimodal language region in the ventral visual pathway. Nature. 1998;394:274–277. doi: 10.1038/28389. [DOI] [PubMed] [Google Scholar]
- Callahan CD, Hinkebein J. Neuropsychological significance of anosmia following traumatic brain injury. Journal of Head Trauma Rehabilitation. 1999;14:581–587. doi: 10.1097/00001199-199912000-00006. [DOI] [PubMed] [Google Scholar]
- Carone DA, Benedict RH, Dwyer MG, Cookfair DL, Srinivasaraghavan B, Tjoa CW, Zivadinov R. Semi-automatic brain region extraction (SABRE) reveals superior cortical and deep gray matter atrophy in MS. Neuroimage. 2006;29:505–514. doi: 10.1016/j.neuroimage.2005.07.053. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cerebral Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Cicerone KD, Tanenbaum LN. Disturbance of social cognition after traumatic orbitofrontal brain injury. Archives of Clinical Neuropsychology. 1997;12:173–188. [PubMed] [Google Scholar]
- Clark L, Manes F, Antoun N, Sahakian BJ, Robbins TW. The contributions of lesion laterality and lesion volume to decision-making impairment following frontal lobe damage. Neuropsychologia. 2003;41:1474–1483. doi: 10.1016/s0028-3932(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia, and the hippocampal system. MIT Press; Boston, Massachusetts: 1993. [Google Scholar]
- Collins DL, Evans AC. ANIMAL: Validation and applications of non-linear registration based segmentation. International Journal of Pattern Recognition. 1997;11:1271–1294. [Google Scholar]
- Dade LA, Gao FQ, Kovacevic N, Roy P, Rockel C, O'Toole CM, et al. Semiautomatic brain region extraction: a method of parcellating brain regions from structural magnetic resonance images. Neuroimage. 2004;22:1492–1502. doi: 10.1016/j.neuroimage.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Dade LA, Zatorre RJ, Jones-Gotman M. Olfactory learning: convergent findings from lesion and brain imaging studies in humans. Brain. 2002;125:86–101. doi: 10.1093/brain/awf003. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- de Wit S, Kosaki Y, Balleine BW, Dickinson A. Dorsomedial prefrontal cortex resolves response conflict in rats. Journal of Neuroscience. 2006;26:5224–5229. doi: 10.1523/JNEUROSCI.5175-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL. Smell Identification Test SIT™. Administration Manual. 3rd ed. Sensonics, Inc; Haddon Heights, NJ: 1995. [Google Scholar]
- Doty RL, Yousem DM, Pham LT, Kreshak AA, Geckle R, Lee WW. Olfactory dysfunction in patients with head trauma. Archives of Neurology. 1997;54:1131–1140. doi: 10.1001/archneur.1997.00550210061014. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Dalgleish T, Lawrence AD. The somatic marker hypothesis: a critical evaluation. Neuroscience and Biobehavioral Reviews. 2006;30:239–271. doi: 10.1016/j.neubiorev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Edgington ES. Randomization tests. New York: Marcel Dekker. 1980 [Google Scholar]
- Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals and other measures of statistical accuracy. Statistical Sciences. 1986;1:54–77. [Google Scholar]
- Eslinger PJ, Damasio AR, Van Hoesen GW. Olfactory dysfunction in man: anatomical and behavioral aspects. Brain and Cognition. 1982;1:259–285. doi: 10.1016/0278-2626(82)90028-8. [DOI] [PubMed] [Google Scholar]
- Faw B. Pre-frontal executive committee for perception, working memory, attention, long-term memory, motor control, and thinking: a tutorial review. Consciousness and Cognition. 2003;12:83–139. doi: 10.1016/s1053-8100(02)00030-2. [DOI] [PubMed] [Google Scholar]
- Feinstein A, Roy P, Lobaugh N, Feinstein K, O'Connor P, Black S. Structural brain abnormalities in multiple sclerosis patients with major depression. Neurology. 2004;62:586–590. doi: 10.1212/01.wnl.0000110316.12086.0c. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Fontaine A, Azouvi P, Remy P, Bussel B, Samson Y. Functional anatomy of neuropsychological deficits after severe traumatic brain injury. Neurology. 1999;53:1963–1968. doi: 10.1212/wnl.53.9.1963. [DOI] [PubMed] [Google Scholar]
- Freedman M, Black S, Ebert P, Binns M. Orbitofrontal function, object alternation and perseveration. Cerebral Cortex. 1998;8:18–27. doi: 10.1093/cercor/8.1.18. [DOI] [PubMed] [Google Scholar]
- Gainotti G, Silveri MC, Daniele A, Giustolisi L. Neuroanatomical correlates of category-specific semantic disorders: a critical survey. Memory. 1995;3:247–264. doi: 10.1080/09658219508253153. [DOI] [PubMed] [Google Scholar]
- Gale SD, Baxter L, Roundy N, Johnson SC. Traumatic brain injury and grey matter concentration: a preliminary voxel based morphometry study. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76:984–988. doi: 10.1136/jnnp.2004.036210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale SD, Johnson SC, Bigler ED, Blatter DD. Nonspecific white matter degeneration following traumatic brain injury. Journal of the International Neuropsychological Society. 1995;1:17–28. doi: 10.1017/s1355617700000060. [DOI] [PubMed] [Google Scholar]
- Gansler DA, Covall S, McGrath N, Oscar-Berman M. Measures of prefrontal dysfunction after closed head injury. Brain and Cognition. 1996;30:194–204. doi: 10.1006/brcg.1996.0012. [DOI] [PubMed] [Google Scholar]
- Gentry LR, Godersky JC, Thompson B. MR imaging of head trauma: review of the distribution and radiopathologic features of traumatic lesions. American Journal of Roentgenology. 1988;150:663–672. doi: 10.2214/ajr.150.3.663. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Ramirez J, Kohler S, Westmacott R, Black SE, Moscovitch M. Retrieval of autobiographical memory in Alzheimer's disease: relation to volumes of medial temporal lobe and other structures. Hippocampus. 2005;15:535–550. doi: 10.1002/hipo.20090. [DOI] [PubMed] [Google Scholar]
- Gold JM, Berman KF, Randolph C, Goldberg TE, Weinberger DR. PET validation of a novel prefrontal task: delayed response alternation. Neuropsychology. 1996;10:3–10. [Google Scholar]
- Green P, Allen LMI, Astner K. The Word Memory Test. CogniSyst, Inc; Durham, NC: 1996. [Google Scholar]
- Green P, Rohling ML, Iverson GL, Gervais RO. Relationships between olfactory discrimination and head injury severity. Brain Injury. 2003;17:479–496. doi: 10.1080/0269905031000070242. [DOI] [PubMed] [Google Scholar]
- Holbourn AHS. Mechanics of head injury. Lancet. 1943;242:438–441. [Google Scholar]
- Hornak J, O'Doherty J, Bramham J, Rolls ET, Morris RG, Bullock PR, et al. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. Journal of Cognitive Neuroscience. 2004;16:463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Hijman R, Tulleken CA, Heeren TJ, Schneider N, van Ree JM. Odor discrimination in patients with frontal lobe damage and Korsakoff's syndrome. Neuropsychologia. 2002;40:888–891. doi: 10.1016/s0028-3932(01)00167-1. [DOI] [PubMed] [Google Scholar]
- Humphreys GW, Forde EM. Hierarchies, similarity, and interactivity in object recognition: “category-specific” neuropsychological deficits. Behavioral and Brain Sciences. 2001;24:453–476. [PubMed] [Google Scholar]
- Jones-Gotman M, Zatorre RJ. Olfactory identification deficits in patients with focal cerebral excision. Neuropsychologia. 1988;26:387–400. doi: 10.1016/0028-3932(88)90093-0. [DOI] [PubMed] [Google Scholar]
- Jones-Gotman M, Zatorre RJ, Cendes F, Olivier A, Andermann F, McMackin D, et al. Contribution of medial versus lateral temporal-lobe structures to human odour identification. Brain. 1997;120:1845–1856. doi: 10.1093/brain/120.10.1845. [DOI] [PubMed] [Google Scholar]
- Kovacevic N, Henderson JT, Chan E, Lifshitz N, Bishop J, Evans AC, et al. A three-dimensional MRI atlas of the mouse brain with estimates of the average and variability. Cerebral Cortex. 2005;15:639–645. doi: 10.1093/cercor/bhh165. [DOI] [PubMed] [Google Scholar]
- Kovacevic N, Lobaugh NJ, Bronskill MJ, Levine B, Feinstein A, Black SE. A robust method for extraction and automatic segmentation of brain images. Neuroimage. 2002;17:1087–1100. doi: 10.1006/nimg.2002.1221. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72:341–332. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Levin HS. Cognitive function outcomes after traumatic brain injury. Current Opinion in Neurology. 1998;11:643–646. doi: 10.1097/00019052-199812000-00006. [DOI] [PubMed] [Google Scholar]
- Levin HS, Goldstein FC. Organization of verbal memory after severe closed-head injury. Journal of Clinical and Experimental Neuropsychology. 1986;8:643–656. doi: 10.1080/01688638608405185. [DOI] [PubMed] [Google Scholar]
- Levin HS, High WM, Eisenberg HM. Impairment of olfactory recognition after closed head injury. Brain. 1985;108:579–591. doi: 10.1093/brain/108.3.579. [DOI] [PubMed] [Google Scholar]
- Levin HS, Williams DH, Valastro M, Eisenberg HM, Crofford MJ, Handel SF. Corpus callosal atrophy following closed head injury: detection with magnetic resonance imaging. Journal of Neurosurgery. 1990;73:77–81. doi: 10.3171/jns.1990.73.1.0077. [DOI] [PubMed] [Google Scholar]
- Levine B, Black SE, Cheung G, Campbell A, O'Toole C, Schwartz ML. Gambling task performance in traumatic brain injury: relationships to injury severity, atrophy, lesion location, and cognitive and psychosocial outcome. Cognitive and Behavioral Neurology. 2005;18:45–54. [PubMed] [Google Scholar]
- Levine B, Fujiwara E, O'Connor C, Richard N, Kovacevic N, Mandic M, et al. In vivo characterization of traumatic brain injury neuropathology with structural and functional neuroimaging. Journal of Neurotrauma. 2006;23:1396–1411. doi: 10.1089/neu.2006.23.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Kovacevic N, Nica I, Cheung G, Schwartz ML, Black SE. The Toronto traumatic brain injury study II: Quantified MRI and cognition. (submitted-a) [DOI] [PubMed]
- Levine B, Kovacevic N, Nica I, Cheung G, Schwartz ML, Black SE. The Toronto traumatic brain injury study II: Quantified MRI and cognition. (submitted-b) [DOI] [PubMed]
- MacKenzie JD, Siddiqi F, Babb JS, Bagley LJ, Mannon LJ, Sinson GP, et al. Brain atrophy in mild or moderate traumatic brain injury: a longitudinal quantitative analysis. American Journal of Neuroradiology. 2002;23:1509–1515. [PMC free article] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, et al. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125:624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Bookstein FL, Haxby JV, Grady CL. Spatial pattern analysis of functional brain images using partial least squares. Neuroimage. 1996;3:143–157. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Lobaugh NJ. Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage. 2004;23(Suppl 1):S250–263. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Vest B, Waxler M, Rosvold E. A re-examination of the effects of frontal lesions on object alternation. Neuropsychologia. 1969;7:357–363. [Google Scholar]
- O'Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Barnes CL. Architecture and connections of the frontal lobe. In: Perecman E, editor. The Frontal Lobes Revisited. IRBN Press; New York: 1987. [Google Scholar]
- Potter H, Butters N. An assessment of olfactory deficits in patients with damage to prefrontal cortex. Neuropsychologia. 1980;18:621–628. doi: 10.1016/0028-3932(80)90101-3. [DOI] [PubMed] [Google Scholar]
- Pribram KH, Mishkin M. Analysis of the effects of frontal lesions in monkey. III. Object alternation. Journal of Comparative and Physiological Psychology. 1956;49:41–45. doi: 10.1037/h0046248. [DOI] [PubMed] [Google Scholar]
- Prigatano GP. Personality disturbances associated with traumatic brain injury. Journal of Consulting and Clinical Psychology. 1992;60:360–368. doi: 10.1037//0022-006x.60.3.360. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cerebral Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. Brain and Cognition. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Serra-Grabulosa JM, Junque C, Verger K, Salgado-Pineda P, Maneru C, Mercader JM. Cerebral correlates of declarative memory dysfunctions in early traumatic brain injury. Journal of Neurology, Neurosurgery and Psychiatry. 2005;76:129–131. doi: 10.1136/jnnp.2004.027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Gow CA. “Frontal dysfunction” after traumatic brain injury. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1992;5:272–282. [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotactic atlas of the human brain. Stuttgart: Thieme. 1988 [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;304:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Tomaiuolo F, Carlesimo GA, Di Paola M, Petrides M, Fera F, Bonanni R, et al. Gross morphology and morphometric sequelae in the hippocampus, fornix, and corpus callosum of patients with severe non-missile traumatic brain injury without macroscopically detectable lesions: a T1 weighted MRI study. Journal of Neurology, Neurosurgery and Psychiatry. 2004;75:1314–1322. doi: 10.1136/jnnp.2003.017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Bechara A, Denburg NL. Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex. 2002;38:589–612. doi: 10.1016/s0010-9452(08)70024-8. [DOI] [PubMed] [Google Scholar]
- Turner GR, Levine B. The functional neuroanatomy of classic delayed response tasks in humans and the limitations of cross-method convergence in prefrontal function. Neuroscience. 2006;139:327–337. doi: 10.1016/j.neuroscience.2005.08.067. [DOI] [PubMed] [Google Scholar]
- Verger K, Junque C, Levin HS, Jurado MA, Perez-Gomez M, Bartres-Faz D, et al. Correlation of atrophy measures on MRI with neuropsychological sequelae in children and adolescents with traumatic brain injury. Brain Injury. 2001;15:211–221. doi: 10.1080/02699050010004059. [DOI] [PubMed] [Google Scholar]
- Wallesch CW, Curio N, Galazky I, Jost S, Synowitz H. The neuropsychology of blunt head injury in the early postacute stage: effects of focal lesions and diffuse axonal injury. Journal of Neurotrauma. 2001;18:11–20. doi: 10.1089/089771501750055730. [DOI] [PubMed] [Google Scholar]
- Wang J, Eslinger PJ, Smith MB, Yang QX. Functional magnetic resonance imaging study of human olfaction and normal aging. The Journals of Gerontology; Series A; Biological sciences and medical sciences. 2005;60:510–514. doi: 10.1093/gerona/60.4.510. [DOI] [PubMed] [Google Scholar]
- Wasserman S, Bockenholt U. Bootstrapping: applications to psychophysiology. Psychophysiology. 1989;26:208–221. doi: 10.1111/j.1469-8986.1989.tb03159.x. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Hunter JV, Newsome MR, Scheibel RS, Bigler ED, Johnson JL, et al. Frontal and temporal morphometric findings on MRI in children after moderate to severe traumatic brain injury. Journal of Neurotrauma. 2005;22:333–344. doi: 10.1089/neu.2005.22.333. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Watson JD, Sicotte NL, Mazziotta JC. Automated image registration: II. Intersubject validation of linear and nonlinear models. Journal of Computer Assisted Tomography. 1998;22:153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- Yount R, Raschke KA, Biru M, Tate DF, Miller MJ, Abildskov T, et al. Traumatic brain injury and atrophy of the cingulate gyrus. Journal of Neuropsychiatry and Clinical Neurosciences. 2002;14:416–423. doi: 10.1176/jnp.14.4.416. [DOI] [PubMed] [Google Scholar]
- Yousem DM, Geckle RJ, Bilker WB, Kroger H, Doty RL. Posttraumatic smell loss: relationship of psychophysical tests and volumes of the olfactory bulbs and tracts and the temporal lobes. Academic Radiology. 1999;6:264–272. doi: 10.1016/s1076-6332(99)80449-8. [DOI] [PubMed] [Google Scholar]
- Yousem DM, Geckle RJ, Bilker WB, McKeown DA, Doty RL. Posttraumatic olfactory dysfunction: MR and clinical evaluation. American Journal of Neuroradiology. 1996;17:1171–1179. [PMC free article] [PubMed] [Google Scholar]
- Zachary RA. Shipley Institute of Living Scale. Revised manual. Los Angeles: Western Psychological Services. 1986 [Google Scholar]
- Zahn TP, Mirsky AF. Reaction time indicators of attention deficits in closed head injury. Journal of Clinical and Experimental Neuropsychology. 1999;21:352–367. doi: 10.1076/jcen.21.3.352.924. [DOI] [PubMed] [Google Scholar]
- Zald DH, Curtis C, Chernitsky LA, Pardo JV. Frontal lobe activation during object alternation acquisition. Neuropsychology. 2005;19:97–105. doi: 10.1037/0894-4105.19.1.97. [DOI] [PubMed] [Google Scholar]
- Zald DH, Curtis C, Folley BS, Pardo JV. Prefrontal contributions to delayed spatial and object alternation: a positron emission tomography study. Neuropsychology. 2002;16:182–189. [PubMed] [Google Scholar]
- Zatorre RJ, Jones-Gotman M. Human olfactory discrimination after unilateral frontal or temporal lobectomy. Brain. 1991;114:71–84. [PubMed] [Google Scholar]