Obstructive sleep apnea is a common disorder with important sequelae.1–7 Aging substantially increases the risk of obstructive apnea,8–11 although the mechanisms underlying this predisposition remain unclear.8,12–15 Most current evidence suggests that obstructive apnea results from an interaction of the anatomy of the upper airway with the control of pharyngeal dilator muscles.4 Afflicted patients have compromised pharyngeal anatomy with reduced airway lumen.16–19,20 Through reflex mechanisms that drive activation of dilator muscles, pharyngeal patency is well maintained during wakefulness.21–23 However, these protective reflexes are diminished during sleep, thereby leading to collapse of the pharyngeal airway in anatomically predisposed people.24 Thus, aging could predispose to apnea via changes in pharyngeal anatomy and biomechanics or via deterioration in the function of pharyngeal dilator muscles.25–31
By combining magnetic resonance imaging techniques with pharyngeal physiological assessments, we sought to determine the structural and functional basis for the increased propensity for airway collapse among older persons. We included normal and near-normal controls to avoid the confounding influences of repetitive pharyngeal collapse as might occur with sleep apnea.
METHODS
Subjects
Eighteen men and 20 women across a range of ages were enrolled (Table). The subjects were recruited using e-mail announcements and posters and through the Harvard Cooperative on Aging. Although some of our subjects had participated in prior studies, none of the aging-related findings of the present study have been previously reported. The women under 50 were premenopausal based on regular menstrual cycles, whereas women over age 50 were post-menopausal for at least 2 years. All were free from comorbid conditions, including snoring and were taking no medications, based on a thorough history and physical examination by a pulmonary specialist. All provided informed consent for the protocol, which was approved by the Brigham and Women’s Hospital Human Subjects’ Committee.
Table.
Characteristics of the participants
| Men
|
Women
|
Independent relation (P value) by analyses of variance
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Younger men (n = 8) | Older men (n = 10) | Younger women (n = 10) | Older women (n = 10) | Overall univariate R value (P value) with age | Univariate R value (P value) with age in men | Univariate R value (P value) with age in women | Age | Sex |
| Demographic | |||||||||
| Age (years) | 35.4 ± 9.5 | 65.3 ± 8.7 | 34.8 ± 7.8 | 65.3 ± 9.6 | |||||
| Body mass index (kg/m2) | 23.8 ± 2.1 | 24.4 ± 3.8 | 25.8 ± 4.5 | 26.1 ± 2.9 | 0.05 (.75) | 0.12 (.62) | 0.19 (.41) | 0.71 | 0.10 |
| Physiologic | |||||||||
| Apnea hypopnea index (/h) | 2.7 ± 2.1 | 5.0 ± 3.4 | 3.0 ± 3.7 | 4.2 ± 2.8 | 0.32 (.06) | 0.19 (.44) | 0.39 (.09) | 0.10 | 0.83 |
| Peak phasic GGEMG | 15.1 ± 9.0 | 15.1 ± 4.9 | 11.8 ± 7.6 | 9.7 ± 5.7 | −0.02 (.93) | 0.14 (.57) | −0.02 (.94) | 0.73 | 0.17 |
| Tonic GGEMG | 8.5 ± 5.1 | 8.3 ± 3.4 | 8.5 ± 4.2 | 8.3 ± 4.9 | 0.001 (.99) | 0.21 (.40) | 0.09 (.71) | 0.89 | 0.98 |
| Percent increase GGEMG with HNP | 101.6 ± 80.4 | 22.4 ± 24.3 | 76.1 ± 72.6 | 42.1 ± 49.5 | −0.55 (<.001) | −0.59 (.008) | −0.35 (.11) | 0.05 | 0.94 |
| Anatomic | |||||||||
| Soft palate length (mm) | 48.2 ± 5.8 | 55.2 ± 7.4 | 41.7 ± 3.1 | 48.1 ± 7.2 | 0.48 (.003) | 0.42 (.08) | 0.58 (.004) | 0.002 | 0.001 |
| Midsagittal tongue height (mm) | 68.9 ± 2.1 | 68.4 ± 6.6 | 61.9 ± 5.2 | 62.1 ± 6.5 | 0.02 (.88) | 0.08 (.76) | 0.05 (.83) | 0.93 | 0.001 |
| Pharyngeal airway length (mm) | 74.7 ± 3.2 | 78.9 ± 10.9 | 54.0 ± 6.4 | 62.0 ± 7.4 | 0.27 (.11) | 0.18 (.47) | 0.56 (.007) | 0.02 | 0.001 |
| Minimal axial airway area (mm2) | 822.3 ± 176 | 1066 ± 334.6 | 587 ± 228 | 755.6 ± 227 | 0.22 (.18) | 0.04 (.86) | 0.18 (.42) | 0.15 | 0.13 |
| Axial fat pad thickness (mm) | 14.1 ± 4.3 | 30.2 ± 15.8 | 15.5 ± 6.9 | 25.6 ± 8.3 | 0.59 (<.0001) | 0.58 (.008) | 0.65 (.001) | 0.001 | 0.63 |
| Airway volume (mm3) | 11318 ± 4319 | 14766 ± 5829 | 6561 ± 2129 | 8063 ± 4496 | 0.32 (.06) | 0.34 (.16) | 0.19 (.45) | 0.08 | 0.001 |
GGEMG = genioglossal electromyogram (% maximum); HNP = high negative pressure pulse with choanal pressure, −15cmH2O.
Equipment and Procedures
Overnight Polysomnography
Patients were monitored for a minimum of 7 hours of sleep by electroencephalography, electromyography, electrooculography, nasal pressure, nasal and oral airflow by thermister, chest and abdominal wall motion using piezo electrodes, electrocardiography, anterior tibialis electromyography, and arterial oxygen saturation using standard methods. Apneas and hypopneas were scored by a blinded registered polysomnographic technician using established criteria.32–35 Although 5 respiratory events per hour is traditionally considered normal, we prespecified a cutoff of 15 events per hour because the use of nasal pressure tends to increase the number of events scored, even in otherwise normal persons.36
Magnetic Resonance Imaging
All images were obtained with a 1.5 Tesla Magnetic Resonance Scanner (Signa Advantage; General Electric, Milwaukee, Wis). Sequential T1-weighted axial, sagittal, and coronal images were obtained in subjects while supine, with the head secured in the neutral anatomic Frankfort position. Using established techniques,20,37 we quantified the soft palate area, soft palate length, pharyngeal length (hard palate to epiglottis), tongue height, tongue width, and tongue area from the sagittal anatomy (Figure 1). From the minimal axial airway image (Figure 2), we examined airway cross-sectional area and dimensions, lateral pharyngeal wall thickness, intramandibular width, thickness of the pharyngeal fat pads, skeletal anteroposterior (mandible to vertebrae) and lateral (intramandibular) distances, as well as skeletal area. From the remaining axial images, volumetric analyses were performed to calculate the pharyngeal airway volume and the skeletal volume between the mandible and vertebrae. The images were transferred to a UNIX-based SUN Station where they were processed, analyzed, and interpreted. Edge detection algorithms using simple thresholding were used to avoid subjective bias in measurement.
Figure 1.
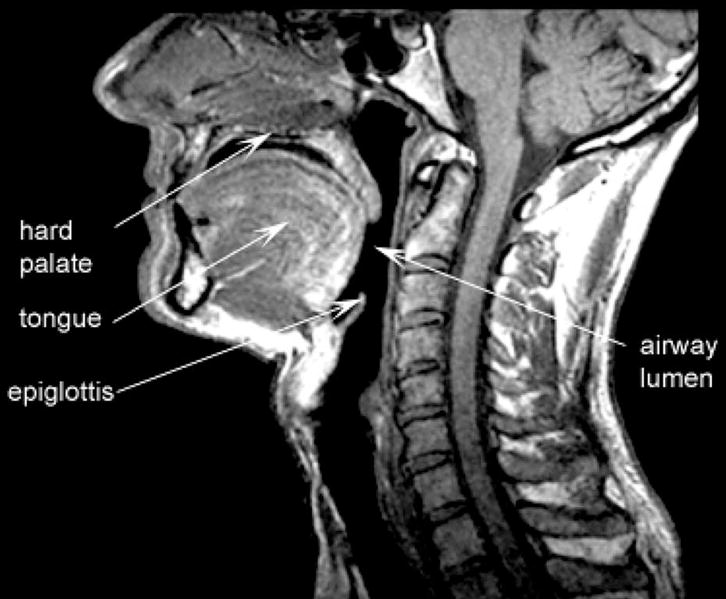
Midsagittal magnetic resonance image illustrating anatomical structures of interest.
Figure 2.
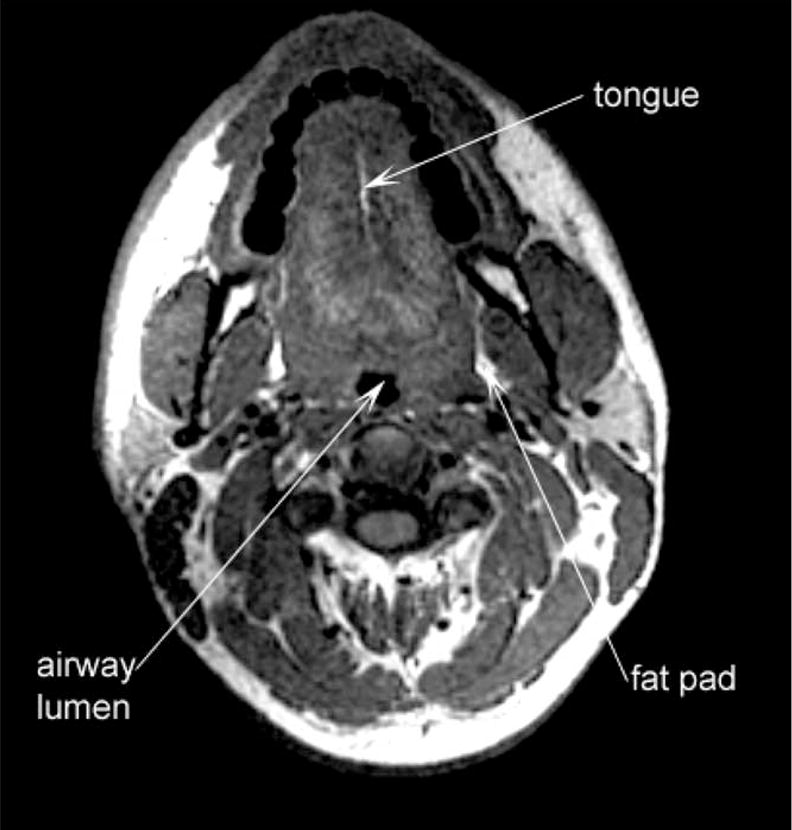
Axial magnetic resonance image illustrating structures relevant to pharyngeal collapse.
Upper Airway Physiology
The necessary equipment was used to monitor the following variables: intramuscular genioglossal electromyogram; nasal, pharyngeal and supraglottic resistance; and airway collapsibility. Each subject was fitted with a sealed nasal mask attached to a Fleisch pneumotachometer (measure airflow, OEM Medical, Richmond, Va), a Validyne mask pressure transducer (Validyne Engineering, Northridge, Calif), Millar pressure catheters (Millar Instruments, Inc., Houston, Tex) inserted intranasally to choanae and epiglottis (to measure the pressures at the top and bottom of the pharyngeal airway, respectively), and wire electrodes inserted into the genioglossus muscle. Genioglossus activity was quantified as a percent of maximum activity as generated by swallowing, maximum inspiratory pressure, or protrusion of the tongue. With this instrumentation in place, we assessed the mechanics of the pharyngeal airway, including the basal activity of the genioglossus muscle and its responsiveness to standard stimuli using negative pressure pulses. We recorded basal tidal breathing while the patient was awake to define genioglossal electromyogram tonic (baseline activity), phasic (increase in activity with each inspiration), and peak phasic activity (maximum activity during inspiration) and pharyngeal resistance at peak flow and at a flow of 0.2 liters per minute (ie, early inspiration). We recorded negative pressure stimulation (rapid pulse of negative pressure via nasal mask), which was measured after two sets of 35 pulses per set of negative pressure pulses delivered to achieve a choanal pressure of −10 cmH2O (defined as low negative pressure) and −15 cmH2O (defined as high negative pressure). The data were then signal averaged to assess the magnitude of the electromyographic response. Reflex responsiveness was quantified as the absolute increase in genioglossus electromyogram as a percentage of maximum activity and as the percentage increase in genioglossus electromyogram. All measurements and techniques were performed using reported methods.20,38
Protocol
Each subject underwent polysomnography, imaging, and physiology on separate days within 1 week of each other. Premenopausal women were studied in the follicular phase of the menstrual cycle.
Statistics
For aging effects, Pearson correlation coefficients were determined between age and normally distributed variables, and Spearman correlation coefficients were used for non-normally distributed variables. We performed 2-way analysis of variance to assess the effect of aging, the effect of sex, and their potential interaction (SigmaStat, SPSS Inc., Chicago, Ill). A value of P < .05 was used as the threshold for statistical significance.
RESULTS
We obtained full data sets in all 38 subjects (Table). There was a significant age-related decrease in the genioglossus response to negative pressure (R = −0.55; P <.005; Figure 3). This age-related decrease was statistically significant in men (R = −0.59, P = .008), and not quite significant in women (R = −0.35, P = .11; Table), but there were no significant differences in the effect of aging based on sex. No other important differences in upper airway physiology were observed as a function of aging (Table).
Figure 3.
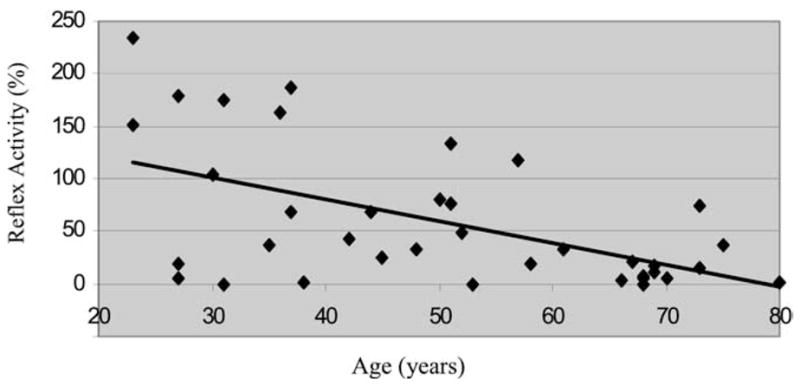
The decrease in negative pressure reflex with increasing age for the sample. With increasing age, there was a significant decrease in the genioglossal response to negative intrapharyngeal pressure pulses (R = −0.55; P < .001).
The bony shape (anteroposterior/lateral) surrounding the pharynx changed significantly with increasing age. The ratio of the anteroposterior to lateral dimension became progressively lower with increasing age (R = −0.51; P < .001), indicating a progressively more lateral skeleton around the airway. Soft palate length also increased progressively with aging (R = 0.48; P <.001, with the increase with age in women significantly more than the increase with age in men.
The length of the pharyngeal airway (the region susceptible to collapse; ie, hard palate to epiglottis) did not change significantly with aging overall (R = 0.27, P = .11; Table). However, a longer pharyngeal airway was seen with aging in women, in whom the lengthening with age was significantly greater than in men.
The parapharyngeal fat pads significantly increased in size with increasing age, independent of body mass index (Figure 4). This finding was true for the thickness of the parapharyngeal fat pads at the level of the minimum pharyngeal lumen (R = 0.69; P <.001), the area of the parapharyngeal fat pads at the level of the minimum pharyngeal lumen (R = 0.51; P <.001), and the fat pad volume measured on multiple axial slices (R = 0.30; P <.05). The age-related increase in fat-pad thickness was significant in both men and women (Table; R = 0.59), with no significant differences by sex after adjusting for age.
Figure 4.
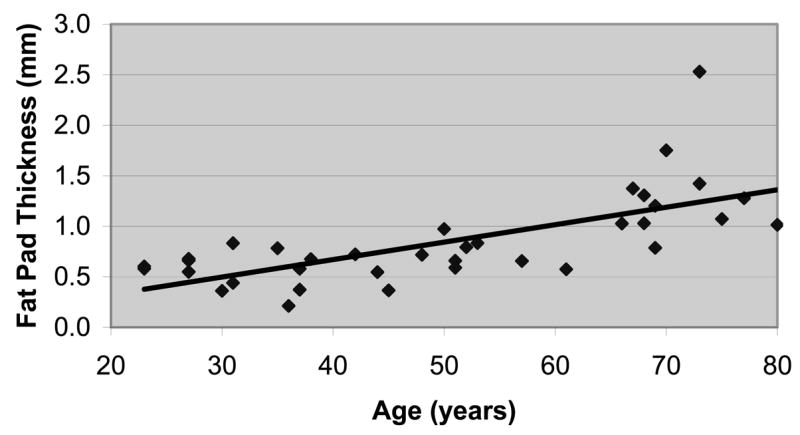
The graph shows a significant increase in the size of the fat pads with increasing age, independent of body mass index (R = 0.59; P <.005).
DISCUSSION
With aging, there was a significant decrease in the negative pressure reflex, particularly in men; increased deposition of parapharyngeal fat in both sexes; a lengthening of the soft palate, significantly in women; and a change in the bony shape surrounding the pharynx. In both sexes, the parapharyngeal fat pad size increased independent of body mass index. A longer pharyngeal airway was observed in association with aging in women but not in men. Thus, physiological and anatomical differences that could predispose to pharyngeal collapse are demonstrable.
The decrease in the negative pressure reflex associated with aging is consistent with other upper airway reflexes. Previous investigators have shown reduced responses to noxious stimuli in association with increasing age.39,40 As the negative pressure reflex allows the upper airway dilator muscles to compensate for a collapsing perturbation, this reflex is a primary mechanism whereby animals and humans maintain pharyngeal patency.23,41,42 The loss of this protective reflex with aging may therefore be a critical mechanism predisposing older persons to pharyngeal collapse.
The increase in parapharyngeal fat with aging is also potentially important. Although obesity is a major risk factor for obstructive apnea, this study suggests that the deposition of fat around the airway occurs independently of age-related changes in body fat. As the deposition of fat around the airway is associated with apnea predisposition, this age-related increase in parapharyngeal fat may be critical in the development of obstructive sleep apnea. The fact that pharyngeal lumen size did not decrease as a function of age is surprising, but may reflect ongoing compensatory reflex mechanisms present during wakefulness.
The increased pharyngeal airway length in older women may also predispose to pharyngeal collapse. Using a two-dimensional, finite element computational model of the pharyngeal airway, we have recently demonstrated that pharyngeal airway length importantly influences pharyngeal collapsibility.20 This model allows for the manipulation of specific anatomical and physiological variables to test their effect on pharyngeal mechanics (see prior publications for specific finite element model parameters). To assess this finding,20 we have taken an anatomically correct pharynx from a younger woman and manipulated pharyngeal airway length in proportion to our observed experimental differences (younger vs older). The pharyngeal airway of the older woman has a less negative closing pressure (ie, is more collapsible)43 than that of the younger woman, based solely on the observed differences in airway length. Thus, a greater length of the pharyngeal airway in older women may be one variable explaining the increased pharyngeal collapsibility.20 Our data are consistent with preliminary radiographic cephalometric data suggesting aging effects on pharyngeal length and an associated increased risk of sleep apnea.
The observed changes in bony shape with aging were not anticipated. Although retrognathia has traditionally been considered a congenital feature, an alternative school of thought suggests that chronic pharyngeal dilator muscle activity gradually leads to changes in bony shape, such that the anteroposterior dimension is reduced via remodeling. Lugaresi has reported that retrognathia may be acquired rather than congenital.44,45 Longitudinal prospective studies will be required to test whether this effect explains our observations.
This study has a number of limitations. First, the sample that we studied included normal controls and persons with minimal obstructive sleep apnea. We chose this sample to minimize the influence of repetitive pharyngeal collapse (seen in severe sleep apnea) on pharyngeal anatomy and physiology. We wanted to assess the effect of aging on normal anatomy and physiology, rather than having our findings confounded by disease. Second, some argue that a study of motor control during wakefulness says little about a disease that occurs during sleep. During sleep, the negative pressure reflex is substantially diminished in all subjects. Thus, comparisons of sleeping persons would be meaningless. Third, this study is cross-sectional in nature. Although our findings are correlative rather than causative, we believe that a more thorough understanding of the pathogenesis of sleep apnea can evolve from these observations. Ultimately, longitudinal studies would be useful to define the natural history of pharyngeal structure and function.
Our observations suggest important mechanisms by which aging may increase the propensity for pharyngeal collapse. New targets for the prevention and treatment of pharyngeal collapse would certainly be welcome.43
CLINICAL SIGNIFICANCE.
Sleep apnea in the elderly has attributable effects on brain function and associated risk of cardiovascular diseases including stroke.
Our studies demonstrate impairments in protective pharyngeal reflexes with normal aging. These impairments may be important in predisposing older individuals to upper airway collapse.
With aging, preferential deposition of fat around the upper airway occurs suggesting changes in fat distribution may compromise airway mechanics, independent of overall body fat.
Acknowledgments
This work was supported by NIH NCRR GCRC MO1 RR02635, 1 P50 HL60292, RO1 HL48531 and T32 HL07633. Dr Malhotra has received grants from the National Institute of Aging (Beeson Award, AG024837-01), Medical Research Council of Canada, and the American Heart Association. Dr Pillar has received a Fulbright scholarship for his research. The Harvard Cooperative on Aging helped recruit some of the subjects. The funding sources had no role in the design of the study or data analysis or interpretation.
References
- 1.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet. 2002;359:204–210. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 2.Lavie P, Here P, Hoffstein V. Obstructive sleep apnea syndrome as a risk factor for hypertension. BMJ. 2000;320:479–482. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peppard P, Young T, Palta M, Skatrud J. Prospective study of the association between sleep disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 4.Malhotra A, White D. Seminar: obstructive sleep apnoea. Lancet. 2002;360:237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 5.Jenkinson C, Davies RJ, Mullins R, Stradling JR. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999;353:2100–2105. doi: 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- 6.Nieto F, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 7.Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;163:344–348. doi: 10.1164/ajrccm.163.2.2005037. [DOI] [PubMed] [Google Scholar]
- 8.Young T, Peppard P, Gottlieb D. The epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 9.Shahar E, Redline S, Young T, et al. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med. 2003;167:1186–1192. doi: 10.1164/rccm.200210-1238OC. [DOI] [PubMed] [Google Scholar]
- 10.Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167:1181–1185. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- 11.Dancey D, Hanly P, Soong C, et al. Impact of menopause on the prevalence and severity of sleep apnea. Chest. 2001;120:151–155. doi: 10.1378/chest.120.1.151. [DOI] [PubMed] [Google Scholar]
- 12.Young T. Menopause, hormone replacement therapy, and sleep-disordered breathing: are we ready for the heat? Am J Respir Crit Care Med. 2001;163:597–598. doi: 10.1164/ajrccm.163.3.ed09-01a. [DOI] [PubMed] [Google Scholar]
- 13.Mooe T, Rabben T, Wiklund U, et al. Sleep-disordered breathing in women: occurrence and association with coronary artery disease. Am J Med. 1996;101:251–256. doi: 10.1016/S0002-9343(96)00122-2. [DOI] [PubMed] [Google Scholar]
- 14.Ancoli-Israel S. Sleep problems in older adults: putting myths to bed. Geriatrics. 1997;52:20–30. [PubMed] [Google Scholar]
- 15.Ancoli-Israel S, Kripke DF. Prevalent sleep problems in the aged. Biofeedback Self Regul. 1991;16:349–359. doi: 10.1007/BF00999989. [DOI] [PubMed] [Google Scholar]
- 16.Haponik E, Smith P, Bohlman M, et al. Computerized tomography in obstructive sleep apnea: correlation of airway size with physiology during sleep and wakefulness. Am Rev Respir Dis. 1983;127:221–226. doi: 10.1164/arrd.1983.127.2.221. [DOI] [PubMed] [Google Scholar]
- 17.Suratt PM, McTier RF, Findley LJ, et al. Changes in breathing and the pharynx after weight loss in obstructive sleep apnea. Chest. 1987;92:631–637. doi: 10.1378/chest.92.4.631. [DOI] [PubMed] [Google Scholar]
- 18.Schwab RJ, Gupta KB, Gefter WB, et al. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995;152:1673–1689. doi: 10.1164/ajrccm.152.5.7582313. [DOI] [PubMed] [Google Scholar]
- 19.Schwab R, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–530. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 20.Malhotra A, Huang Y, Fogel R, et al. The male predisposition to pharyngeal collapse: the importance of airway length. Am J Resp Crit Care Med. 2002;166:1388–1395. doi: 10.1164/rccm.2112072. [DOI] [PubMed] [Google Scholar]
- 21.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal EMG in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanisms) J Clin Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fogel R, Malhotra A, Pillar G, et al. Genioglossal activation in patients with obstructive sleep apnea versus control subjects. Mechanisms of muscle control. Am J Respir Crit Care Med. 2001;164:2025–2030. doi: 10.1164/ajrccm.164.11.2102048. [DOI] [PubMed] [Google Scholar]
- 23.Malhotra A, Pillar G, Fogel RB, et al. Pharyngeal pressure and flow effects on genioglossus activation in normal subjects. Am J Respir Crit Care Med. 2002;165:71–77. doi: 10.1164/ajrccm.165.1.2011065. [DOI] [PubMed] [Google Scholar]
- 24.Mezzanotte WS, Tangel DJ, White DP. Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am J Respir Crit Care Med. 1996;153:1880–1887. doi: 10.1164/ajrccm.153.6.8665050. [DOI] [PubMed] [Google Scholar]
- 25.Mortimore IL, Bennett SP, Douglas NJ. Tongue protrusion strength and fatiguability: relationship to apnoea/hypopnoea index and age. J Sleep Res. 2000;9:389–393. doi: 10.1046/j.1365-2869.2000.00222.x. [DOI] [PubMed] [Google Scholar]
- 26.Martin SE, Mathur R, Marshall I, Douglas NJ. The effect of age, sex, obesity and posture on upper airway size. Eur Respir J. 1997;10:2087–2090. doi: 10.1183/09031936.97.10092087. [DOI] [PubMed] [Google Scholar]
- 27.Thurnheer R, Wraith PK, Douglas NJ. Influence of age and gender on upper airway resistance in NREM and REM sleep. J Appl Physiol. 2001;90:981–988. doi: 10.1152/jappl.2001.90.3.981. [DOI] [PubMed] [Google Scholar]
- 28.Worsnop C, Kay A, Kim Y, et al. Effect of age on sleep onset-related changes in respiratory pump and upper airway muscle function. J Appl Physiol. 2000;88:1831–1839. doi: 10.1152/jappl.2000.88.5.1831. [DOI] [PubMed] [Google Scholar]
- 29.Behan M, Brownfield MS. Age-related changes in serotonin in the hypoglossal nucleus of rat: implications for sleep-disordered breathing. Neurosci Lett. 1999;267:133–136. doi: 10.1016/s0304-3940(99)00337-7. [DOI] [PubMed] [Google Scholar]
- 30.Browne H, Adams L, Simonds A, Morrell M. Ageing does not influence the sleep-related decrease in the hypercapnic ventilatory response. Eur Respir J. 2003;21:523–529. doi: 10.1183/09031936.03.00039002. [DOI] [PubMed] [Google Scholar]
- 31.Shochat T, Loredo J, Ancoli-Israel S. Sleep disorders in the elderly. Curr Treat Options Neurol. 2001;3:19–36. doi: 10.1007/s11940-001-0021-x. [DOI] [PubMed] [Google Scholar]
- 32.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles, CA: NIH Publication; 1968. p. 204. [DOI] [PubMed] [Google Scholar]
- 33.The Report of an American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in adults. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 34.Malhotra A, Pillar G, Fogel RB, et al. Genioglossal but not palatal muscle activity relates closely to pharyngeal pressure. Am J Respir Crit Care Med. 2000;162:1058–1062. doi: 10.1164/ajrccm.162.3.9912067. [DOI] [PubMed] [Google Scholar]
- 35.Malhotra A, Fogel R, Edwards J, et al. Local mechanisms drive genioglossus muscle activation in obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1746–1749. doi: 10.1164/ajrccm.161.5.9907109. [DOI] [PubMed] [Google Scholar]
- 36.Hosselet JJ, Norman RG, Ayappa I, Rapoport DM. Detection of flow limitation with a nasal cannula/pressure transducer. Am J Respir Crit Care Med. 1998;157:1461–1467. doi: 10.1164/ajrccm.157.5.9708008. [DOI] [PubMed] [Google Scholar]
- 37.Welch K, Foster G, Ritter C, et al. A novel volumetric magnetic resonance imaging paradigm to study upper airway anatomy. Sleep. 2002;25:532–542. [PubMed] [Google Scholar]
- 38.Pillar G, Fogel RB, Malhotra A, et al. Genioglossal inspiratory activtion: central respiratory vs mechanoreceptor influences. Respir Physiol. 2001;127:23–38. doi: 10.1016/s0034-5687(01)00230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heft M, Cooper B, O’Brien K, et al. Aging effects on the perception of noxious and non-noxious thermal stimuli applied to the face. Aging. 1996;8:35–41. doi: 10.1007/BF03340113. [DOI] [PubMed] [Google Scholar]
- 40.Erskine R, Murphy P, Langton J, Smith G. Effect of age on the sensitivity of upper airway reflexes. Br J Anaesth. 1993;70:574–575. doi: 10.1093/bja/70.5.574. [DOI] [PubMed] [Google Scholar]
- 41.Mathew OP, Abu-Osba YK, Thach BT. Genioglossus muscle response to upper airway pressure changes: afferent pathways. J Appl Physiol. 1982;52:445–450. doi: 10.1152/jappl.1982.52.2.445. [DOI] [PubMed] [Google Scholar]
- 42.Horner RL, Innes JA, Murphy K, Guz A. Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J Physiol (Lond) 1991;436:15–29. doi: 10.1113/jphysiol.1991.sp018536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz AR, Smith PL, Wise RA, et al. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol. 1988;64:535–542. doi: 10.1152/jappl.1988.64.2.535. [DOI] [PubMed] [Google Scholar]
- 44.Sforza E, Addati G, Cirignotta F, Lugaresi E. Natural evolution of sleep apnoea syndrome: a five year longitudinal study. Eur Respir J. 1994;7:1765–1770. doi: 10.1183/09031936.94.07101765. [see comments] [DOI] [PubMed] [Google Scholar]
- 45.Lugaresi E, Vela-Bueno A. Sleep-related respiratory disorders. Semin Neurol. 1987;7:259–268. doi: 10.1055/s-2008-1041426. [DOI] [PubMed] [Google Scholar]


