Abstract
Background
Cardiac resynchronization therapy (CRT) has been shown to improve cardiac function and reduce Cheyne-Stokes respiration but has not been evaluated in patients with obstructive sleep apnea (OSA). In this pilot study, we investigated the impact of both CRT and CRT plus increased rate pacing in heart failure (ie, congestive heart failure [CHF]) patients with OSA. We hypothesized that through increased cardiac output CRT/pacing would reduce obstructive events and daytime symptoms of sleepiness.
Methods
Full polysomnograms were performed on CHF patients who were scheduled for CRT, and those patients with an apnea-hypopnea index (AHI) of > 5 events per hour were approached about study enrollment. Patients had a pre-CRT implant baseline echocardiogram and an echocardiogram a mean (± SEM) duration of 6.6 ± 1.4 months post-CRT implant; polysomnography; and responded to the Minnesota Living with Heart Failure questionnaire, the Epworth sleepiness scale, and the Functional Outcomes of Sleep Questionnaire. An additional third polysomnography was performed combining CRT with a pacing rate of 15 beats/min above the baseline sleeping heart rate within 1 week of the second polysomnography. Assessments for the change in cardiac output during the polysomnography were performed using circulation time to pulse oximeter as a surrogate.
Results
Twenty-four patients were screened, and 13 patients (mean age, 68.6 years; body mass index, 28.7 kg/m2) had evidence of OSA. The mean AHI decreased from 40.9 ± 6.4 to 29.5 ± 5.9 events per hour with CRT (p = 0.04). The mean baseline ejection fraction was 22 ± 1.7% and increased post-CRT to 33.6 ± 2.0% (p < 0.05). The reduction in AHI with CRT closely correlated with a decrease in circulation time (r = 0.89; p < 0.001) with CRT. Increased rate pacing made no additional impact on the AHI or circulation time. CRT had a limited impact on sleep architecture or daytime symptom scores.
Conclusions
CRT improved cardiac function and reduced the AHI. Reduced circulatory delay likely stabilized ventilatory control systems and may represent a new therapeutic target in OSA.
Keywords: cardiac pacing, congestive heart failure, obstructive sleep apnea, resynchronization
Both sleep disorders and cardiovascular diseases are receiving increasing attention because of their high prevalence and their potential interactions. Previous reports1,2 have suggested that sleep apnea can contribute to the development of congestive heart failure (CHF), while, conversely, impairments in cardiac function can lead to breathing instability. Thus, some authors have suggested a potential cycle whereby impaired cardiac function leads to breathing instability, yielding further deterioration in cardiac function. Substantial attention has focused on the role of treating sleep apnea with continuous positive airway pressure in an attempt to improve cardiovascular disease and clinical outcome. Although these data are still evolving, there are some data suggesting that the treatment of obstructive sleep apnea (OSA) and central sleep apnea (CSA) can improve left ventricular ejection fraction (LVEF).3,4
Methods for improving cardiac function, which also impact sleep-disordered breathing, have been somewhat more difficult to identify. One previous report5 has suggested that invasive hemodynamic monitoring to optimize medical therapy yields improvements in CSA. In addition, cardiac resynchronization therapy (CRT) has been used to treat CHF patients with ventricular dyssynchrony, with reports6 of marked improvements in central apnea in association with improved cardiac function. Some investigations7–9 into sleep apnea pathogenesis have demonstrated some common underlying mechanisms in OSA and CSA, one of which is that OSA and CSA are likely associated with increased intrinsic breathing instability (so-called ventilatory loop gain).
The existing data regarding the treatment of OSA using cardiac pacemakers and/or CRT are rather sparse. In the study by Garrigue et al,10 significant reductions in apnea-hypopnea index (AHI) were observed with overdrive pacing in a population of patients with both OSA and CSA who were using pacemakers. Subsequent attempts to reproduce these findings in OSA patients have been largely unsuccessful.11,12 Thus, enthusiasm for cardiac pacing as a viable therapy for the treatment of OSA has diminished considerably. On the other hand, CRT, which has been shown to improve ejection fraction, reduce mortality,13,14 and eliminate CSA, has not been investigated as a treatment of OSA, to our knowledge.
Based on some of the common mechanisms underlying OSA and CSA, we hypothesized that CRT could lead to improvements in AHI in patients with predominant OSA. We further sought to identify whether any observed changes in cardiac function (as assessed by circulation time) were important in mediating the improvement in sleep apnea severity.
Materials And Methods
Patients/Sleep Studies
Patients for this study were selected from the Arrhythmia Service at Brown Medical School and Rhode Island Hospital. All patients had evidence of an LVEF of < 35% by echocardiogram, New York Heart Association class II-III symptoms, and a QRS duration of > 120 ms. Patients were referred to the Arrhythmia Service from their primary cardiologist for symptoms of ongoing CHF, including dyspnea on exertion, fatigue, or limited exercise intolerance. All patients being considered for CRT were approached about participation in the study. After informed consent was given, patients underwent full in-home, screening, overnight polysomnogram (PSG) with EEG, electrooculogram, chin electromyogram, measurement of airflow by nasal pressure and oronasal thermistor, measurement of chest/abdominal wall effort with piezo-belts, and continuous oximetry (Siesta; Compu-medics; Sydney, NSW, Australia). Sleep studies were scored by a registered polysomnography technician, who was blinded to the study hypothesis, and a sleep physician, and were reviewed by a third accredited sleep physician according to the rules set forth by Rechtshaffen and Kales15 and arousals by the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association.16 Obstructive and central apneic events were scored according to standard criteria17 by the three persons noted above. An AHI of 5 events per hour of sleep was required for further participation in this research. Lung-to-finger circulation time (LFCT), a modification of the previously reported lung-to-ear circulation time (LECT),18,19 was recorded as an estimate of circulatory delay. The LECT has been previously validated18 against cardiac output in patients with OSA and CHF. In this study, measures of LFCT were obtained in a repeated fashion using the same finger in all individuals during consecutive studies. Briefly, the LFCT was recorded as the interval (with time measured in seconds) from the beginning of the first postapnea breath (recorded from the nasal pressure transducer signal) to the nadir of the dip in arterial oxygen saturation (SaO2) seen on the SaO2 tracing following the apnea. As previously described,18 10 consecutive apnea-hyperpnea cycles from stage 2 sleep were analyzed for each individual with the SaO2 probe on the index finger. Exclusion criteria included previous pacemaker placement or active cardiac ischemic symptoms. This study was approved by the Lifespan Academic Medical Center Institutional Review Board.
Protocol
At the time of the screening polysomnography (baseline), patients completed the Epworth sleepiness scale, Functional Outcomes of Sleep (FOSQ), and Minnesota Living with Heart Failure questionnaires. Within 2 weeks of the initial polysomnography, patients identified with sleep-disordered breathing (AHI, > 5 events per hour of sleep) underwent an echocardiogram and subsequently underwent placement of a biventricular defibrillator (Atlas HF device or Epic HF device; St Jude Medical Inc; St. Paul, MN). Standard right atrial and right ventricular leads were placed. A left ventricular (LV) lead was placed into a lateral branch of the coronary sinus. The majority of the LV leads were situated in the basal one third segment of the ventricle. Patients underwent outpatient follow-up and device interrogations as recommended by their attending cardiologist. The CRT defibrillator was set to DDD mode and a base rate of 60 beats/min.
After CRT pacing for a mean (± SEM) duration of 6.6 ± 1.4 months, all subjects underwent a repeat polysomnography (ie, CRT with repeat polysomnography [CRT-P]) and repeated the Epworth sleepiness scale, FOSQ, and Minnesota Living with Heart Failure questionnaires. Body mass index (BMI) and cardiac medications were also recorded. Follow-up echocardiograms were completed within 2 weeks of the CRT-P. Next, subjects were seen in the device clinic at Rhode Island Hospital and had their device base rate increased by 15 beats/min over their baseline mean sleeping heart rate (mean rate for group, 76 ± 4 beats/min). A third full in-home polysomnography was performed at the elevated pacing rate the night following the base rate change (overdrive CRT-P) within 1 week of the second polysomnography. Patients then returned to the device clinic and had their base rate returned to an appropriate level. No patient received treatment with continuous positive airway pressure during the 6 months between the baseline and CRT-P studies.
Statistical Analysis
Polysomnographic variables were compared across the three time points using analysis of variance (ANOVA) with Tukey post hoc tests. Demographic, echocardiographic, and questionnaire data were compared using paired t test or Fisher exact test. Linear regression was used to compare sleep apnea severity and the LFCTs. α values of p < 0.05 were considered to be significant.
Results
Between April 2004 and August 2005, 65 patients who were being considered for implantation of CRT devices were approached about participation in this study. Of these, 36 patients declined to participate, 3 patients did not ultimately require CRT, decompen-sated CHF requiring hospitalization developed in 1 patient prior to study enrollment, 1 patient left the state prior to study enrollment, and 2 patients were found to have OSA that had been previously treated (ie, prior to study enrollment). The remaining 24 patients were screened, and 13 patients (54%) met the inclusion criteria, including an AHI of > 5 events per hour of sleep. Demographics and patient cardiac medications from baseline and after 6 months of CRT are presented in Tables 1 and 2. OSA was predominant among all enrolled patients. One patient also had a Cheyne-Stokes respiration (CSR) pattern as well as a predominance of obstructive events. Six patients had coronary artery disease, and seven patients had cardiomyopathy of nonischemic origin. After a mean (± SEM) duration of 6.6 ± 1.4 months of CRT, there was improvement in the mean LVEF (baseline, 22.5 ± 1.7%; post-CRT, 33.6 ± 2.0%; p < 0.001) [Table 2]. The mean heart rate during sleep was 60.5 ± 1.2 beats/min at baseline, 64.4 ± 1.1 beats/min during the CRT-P, and 76.0 ± 1.3 beats/min during the overdrive CRT-P (p < 0.001 [for baseline and CRT-P compared to overdrive CRT-P]).
Table 1.
Baseline Characteristics/Demographics*
| Demographics | Values (n = 13) |
|---|---|
| Age, yr | 68.6 ± 3.7 |
| Gender | |
| Male | 11 |
| Female | 2 |
| CMP | |
| Ischemic | 6 |
| Nonischemic | 7 |
Values are given as the mean ± SEM or No. of patients. CMP = cardiomyopathy.
Table 2.
Patient Demographics, Cardiac Function, Medications, and Questionnaire Data From Baseline to Post-CRT*
| Variables | Baseline | CRT | p Value |
|---|---|---|---|
| BMI, kg/m2 | 28.7 ± 1.5 | 29.2 ± 1.5 | 0.48 |
| QRS, ms | 160 ± 5 | 120 ± 4 | < 0.01 |
| LVEF, % | 22.5 ± 1.7 | 33.6 ± 2.0 | < 0.001 |
| Diuretics | 12 | 12 | NS |
| β-Blockers | 13 | 13 | NS |
| ACE inhibitors | 12 | 12 | NS |
| Spironolactone | 2 | 1 | NS |
| Digoxin | 6 | 6 | NS |
| ESS score | 9.6 ± 1.5 | 8.0 ± 1.4 | 0.39 |
| FOSQ score | 11.4 ± 1.2 | 13.5 ± 0.8 | 0.30 |
| Minnesota LHFQ score | 47.0 ± 9.4 | 34.8 ± 7.5 | 0.39 |
Values are given as the mean ± SEM or No. of patients, unless otherwise indicated. ESS = Epworth sleepiness scale; LHFQ = Living with Heart Failure questionnaire; NS = not statistically significant; ACE = angiotensin-converting enzyme.
Sleep architecture showed no significant changes between baseline and the CRT night. However, there was a trend for an increase in slow-wave sleep noted in the overdrive CRT group, but the large variability in the measurements did not allow the increase to be statistically significant. The AHI for each individual for each of the three PSGs is shown in Figure 1, and, as can be seen, there was some improvement in the AHI in 9 of 13 patients. CRT-associated reductions in AHI for the group were characterized by an improvement in both CSAs and OSAs (Table 3). Hypopneas did not substantially change during the CRT-P night (Table 3). Mixed apneas were limited in number and did not change during the CRT-P or overdrive CRT-P. The mean spontaneous arousal index remained unchanged with CRT or overdrive pacing (Table 3). The mean percentage of supine sleep was not increased from the baseline to CRT-P or overdrive CRT-P night. The FOSQ increased insignificantly (11.4 ± 1.2 vs 13.5 ± 0.8, respectively; p = 0.30), and a small insignificant decrease was noted in Minnesota Living with Heart Failure scores with CRT (47.0 ± 9.4 vs 34.8 ± 7.5, respectively; p = 0.39). Epworth sleepiness scale scores were not abnormal at baseline and were not substantially impacted by CRT (9.6 ± 1.5 vs 8.0 ± 1.4, respectively) [Table 2]. Mean LFCT decreased from baseline of 22.5 ± 1.5 to 19.6 ± 1.5 s with CRT (p = 0.01 [ANOVA]) but did not further decease with overdrive CRT (19.1 ± 1.4 s; p = 0.91 [ANOVA]) [Table 3]. Linear regression analysis revealed a strong relationship between change in AHI and the change in LFCT with CRT (r = 0.89; p < 0.001) [Fig 2] but did not correlate as well with the change in LVEF (r = 0.46; p = 0.16). Last, in the six patients who had the greatest improvements in AHI with pacing, compared to the whole group there were not significant differences in their BMI, LVEF, or sleep architecture.
Figure 1.
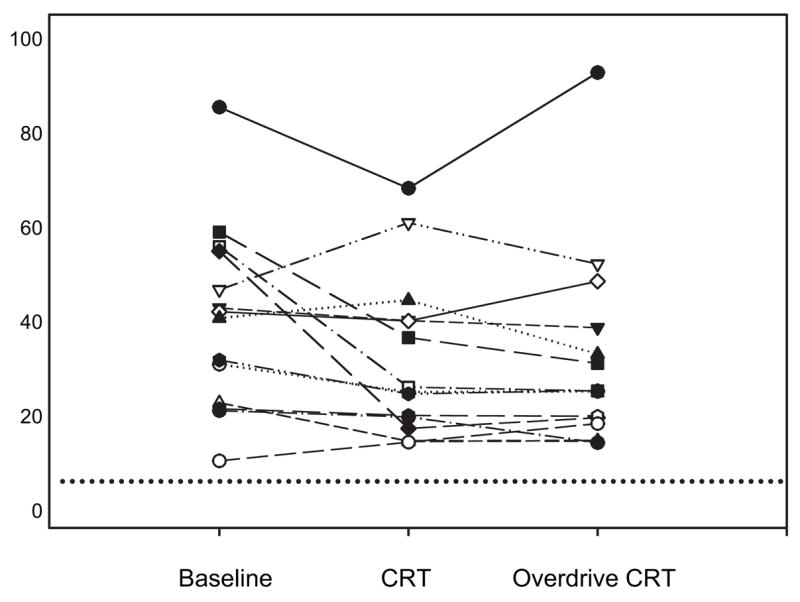
Individual values for AHI in the 13 patients over the baseline, after > 6 months of CRT (biventricular pacing synchronous with the patient’s intrinsic sinus rhythm rate) and overdrive CRT (single night of atrial biventricular pacing at a rate of 15 beats/min above the patient’s intrinsic sleeping heart rate). Dashed line = AHI of 5.0 events per hour of sleep.
Table 3.
Sleep Architecture, Sleep-Disordered Breathing Measures, Mean Sleeping Heart Rate, and LFCT Across All Conditions*
| Variables | Baseline | CRT | Overdrive CRT | p Value |
|---|---|---|---|---|
| Sleep architecture | ||||
| Total sleep time, h | 4.8 ± 0.4 | 4.7 ± 0.4 | 5.2 ± 0.5 | 0.42 |
| Sleep stage, % | ||||
| 1/2 | 72.4 ± 3.9 | 71.3 ± 3.7 | 63.1 ± 5.9 | 0.35 |
| 3/4 | 12.6 ± 2.6 | 14.5 ± 3.4 | 23.5 ± 5.6 | 0.21 |
| REM | 14.9 ± 2.8 | 14.6 ± 2.7 | 14.5 ± 2.4 | 0.99 |
| Arousal index | 9.0 ± 4.3 | 7.1 ± 1.0 | 7.2 ± 1.2 | 0.21 |
| Respiratory measures | ||||
| AHI | 40.9 ± 6.4 | 29.5 ± 5.9 | 31.1 ± 7.8 | 0.02 |
| OSA | 24.1 ± 7.0 | 12.1 ± 5.0 | 14.3 ± 7.1 | 0.001 |
| CSA | 2.0 ± 1.0 | 0.3 ± 0.1 | 0.6 ± 0.4 | 0.15 |
| Hypopnea index | 14.8 ± 3.7 | 16.1 ± 3.9 | 16.0 ± 4.0 | 0.95 |
| SaO2 nadir, % | 82.3 ± 1.7 | 82.1 ± 1.7 | 83.3 ± 1.7 | 0.61 |
| Cardiac parameters | ||||
| MSHR, beats/min | 60.5 ± 1.2 | 64.4 ± 1.1 | 76.0 ± 1.3 | < 0.001 |
| LFCT, s | 22.5 ± 1.5 | 19.6 ± 1.5 | 19.1 ± 1.4 | 0.01 |
Values are given as the mean ± SEM, unless otherwise indicated. MSHR = mean sleeping heart rate; REM = rapid eye movement.
Figure 2.
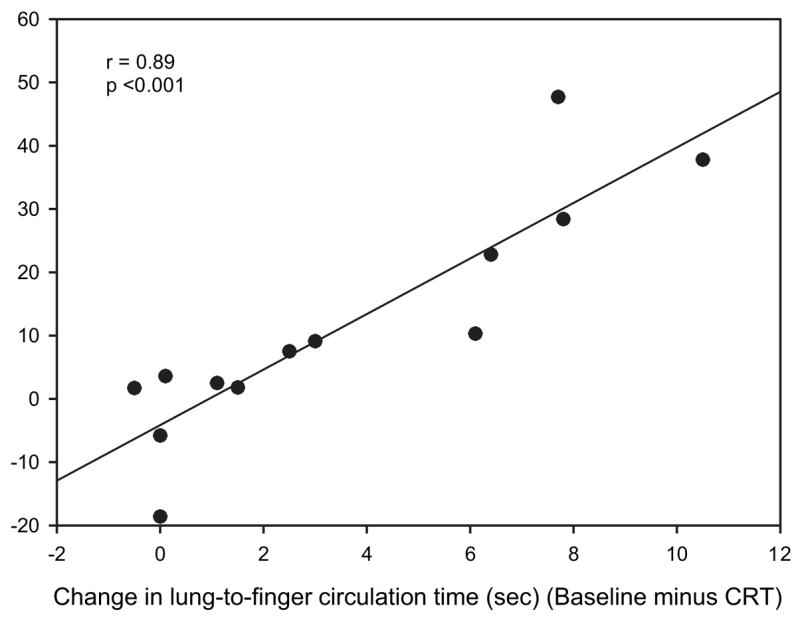
Regression analysis comparing the change in AHI from baseline to after CRT to the change in LFCT (in seconds) from baseline to after CRT.
Discussion
In the present study, we observed in a group of CHF patients undergoing CRT that the prevalence of newly discovered OSA was 54%, which is consistent with previous reports, and that with improvements in cardiac function and sleep-disordered breathing improved in most patients. The improvement in obstructive apneas correlated closely to the change in circulation time, which is an indirect measure of cardiac output. Similar to studies with traditional (ie, non-CRT) pacing, CRT pacing with an increased rate did not further eliminate apneas and hypopneas. Heart failure symptoms tended toward improvement, but daytime sleepiness did not. As others have noted, patients with CHF and incidentally diagnosed OSA were not particularly sleepy at trial entry, and there was limited change in these symptoms despite improvements in AHI.
The impact of overdrive pacing on sleep-disordered breathing has been highlighted in a series of articles with conflicting results. Garrigue and colleagues10 revealed that in bradycardic patients with implanted right atrial-synchronous ventricular pacemakers and concomitant OSA/CSA atrial pacing at a rate 15 beats/min faster than the mean nocturnal heart rate led to a 60% reduction in AHI. These authors observed similar improvements in both OSAs and CSAs. However, two other studies failed to reproduce these results in patients with predominantly obstructive apnea. Luthje and colleagues12 reported that, in a population of patients with dual-chamber pacemakers or implantable defibrillators, increased-rate pacing had no impact on AHI, brain natriuretic peptide (BNP) levels, or urinary catechol-amine levels. Similarly, Simantirakis et al11 reported that neither 1 night nor up to 1 month of atrial increased-rate pacing impacted AHI. In the present study, we obtained significant reductions in AHI with CRT, but additional increased-rate pacing similarly made no significant impact on AHI. The difference between studies likely relates to the impact of the different interventions on cardiac output. In all of the patients in the study by Garrigue et al,10 the pacemaker was placed for symptomatic bradycardia. Thus, with increased-rate pacing, greater improvements in cardiac function20 might be expected compared with those patients in the study by Simantirakis et al,11 in whom only a defibrillator was placed or who had no bradycardia. In addition, a very slightly reduced ejection fraction (54 ± 11%) was observed in the study by Garrigue et al,10 but a more pronounced impairment in LVEF (22 ± 1.7%) was present in our study by design. Therefore, improvements in cardiac function might be seen in both studies, but for different reasons (ie, with either pacing or CRT)14; both would likely improve AHI. However, it is not clear that cardiac output increased in the studies by Garrigue et al,10 Simantirakis et al,11 or Luthje et al12 as no hemodynamic data were provided. The study by Simantirakas et al11 included measures of BNP. However, the utility of BNP for measuring temporal changes in cardiac function and filling pressures has been challenged.21 In the current study, the improvement in AHI strongly correlated with this change in circulatory time, which is an indirect measure of cardiac output, and suggests that if cardiac output can be increased during the night, then apneas possibly may be reduced. Based on this logic, it was somewhat surprising that the overdrive CRT, utilizing a higher heart rate, did not further reduce the AHI. However, as noted above, the LFCT also did not change with use of the combined modality, suggesting that circulatory delay/cardiac output was not further improved. In addition, mechanisms other than circulatory delay are also important for apnea generation in CHF patients.2
The etiology of the strong relationship between LFCT and sleep-disordered breathing in this study remains unclear. As suggested by others,22,23 improvements in cardiac output may improve both CSA and OSA events by improving respiratory system control loop gain. Loop gain is the ratio of a response to the perturbation in systems controlled by negative feedback loops. When the response of the system is greater than the initial stimulus, the system becomes unstable and oscillates. Reduced cardiac output increases ventilatory loop gain by prolonging lung-to-chemoreceptor delay, thus promoting respiratory system instability (ie, apneas). Mixing gain, which is a component of overall loop gain, is influenced by circulatory delay.24,25 This lung-to-chemoreceptor delay can be estimated by the LECT, which is a measure validated against cardiac output.18 We observed that our surrogate, LFCT, was closely associated with an improved change in AHI. There was also a relationship between LVEF and our LFCT, albeit a weak relationship that was likely limited by the study sample size. Although circulation time is only one component of the respiratory system loop gain measure,8 fluctuations in output from the central pattern generator in the brainstem are likely to lead to peaks and troughs in the output to the upper airway dilator muscles in these patients. Presumably, during the nadirs in upper airway dilator activity the susceptible airway is vulnerable to collapse, leading to obstructive apneas. This mechanism is also important in the development of CSR, a situation in which ventilatory loop gain is known to be increased. Sinha and colleagues6 observed in patients with heart failure that CRT devices led to the elimination of CSA/CSR. Increases in cardiac output were suggested by a significant decrease in the ventilatory response to exercise measured by the minute ventilation/carbon dioxide output ratio, which is a surrogate for circulation time. Thus, circulatory delay and cardiac output appear to be important in both OSA and CSA.
The lack of baseline sleepiness and the limited changes in both Epworth sleepiness scale and FOSQ data are not entirely surprising for the following reasons. First, previous studies have reported low (normal) mean Epworth sleepiness scale scores in their CHF patients with OSA,3 confirming the results of other studies26 reporting a low prevalence of sleepiness (24%) in CHF patients with sleep-disordered breathing. The mechanisms underlying this lack of sleepiness are unclear but may include a lack of sensitivity for Epworth sleepiness scale score in sedentary CHF patients, a gradual induction and tolerance to somnogenic cytokines (eg, interleukin-6 and tumor necrosis factor-α), or possibly an alerting influence of counterregulatory hormones (eg, cat-echolamines). Second, although there was a small but insignificant change in the FOSQ and Epworth sleepiness scale scores with CRT, the residual OSAs may have impeded further improvement in the sleepiness scores. Although the change in sleepiness does correlate with the change in AHI, this is typically observed in studies in which the AHI is more normalized, which was not the case in most individuals in this study. Because residual AHI persisted, we do not advocate CRT as a “cure” for sleep apnea; rather, we believe that our findings point to interesting and potentially robust underlying physiologic mechanisms.
The impact of overdrive CRT on sleep architecture, arousals, and SaO2 should be noted. First, no significant change was seen in total sleep time, or in the percentages of stage 1/2, stage 3/4, or REM sleep between conditions. However, the amount of stage 3/4 sleep did trend upward from the CRT-P to the overdrive CRT-P nights. The etiology or significance of this finding is unclear, but it was not associated with a decline in the AHI between the CRT-P and overdrive CRT-P nights. We speculate that with habituation to the polysomnography equipment, by the third night the stage 3/4 sleep increased (analogous to the “first-night effect” in healthy control subjects). It is also possible that patients were mildly sleep deprived prior to the final study, potentially explaining the subtle changes in sleep stage distribution. Second, the spontaneous arousal index decreased from baseline to CRT polysomnography, but was not statistically significant and thus did not likely contribute to the changes in apnea severity noted. Last, the lack of improvement in SaO2 nadir was somewhat unexpected. With improvements in LV function and declines in AHI, a higher nadir SaO2 might be expected. However, a moderate degree of OSA remained in many of these individuals, which likely explains the persistence of hypoxemia.
There are some limitations to this study. First, the order of our CRT and overdrive CRT PSGs were consecutive because of the nature of the CRT defibrillator being placed. The bias introduced by order effects was likely small, as the impact on sleep architecture, arousals, and functional outcomes of sleep did not substantially change between studies. Second, the lung-to-finger measure was a modification of the previously validated LECT. However, this measure was obtained using the same finger in each person for each study and was repeated in each patient during each study. Thus, we believe that the change in values over time within individuals is not biased and reflects a true change in circulation time. We have also compared values derived from ear oximetry with those from finger oximetry, and have found only minor and predictable changes in circulatory time with the site of measurement. Third, repeat sleep studies were not performed on the 11 patients who underwent an initial screening polysomnography but did not meet the study inclusion criteria. The lack of an untreated control group possibly introduced regression to the mean bias on the patients studied. However, we feel that this is not a substantial problem for three reasons. First, the cut point for exclusion from the study was an AHI of 5 events per hour of sleep, and the observed drop in AHI was 11 events per hour. Thus, our findings cannot be completely explained by regression to the mean bias. Second, the correlation with circulation time supports the presence of a biological mechanism for the decrease in AHI, rather than a statistical artifact. Third, patients served as their own control subjects, and the trend in AHI reduction was quite consistent rather than a function of night-to-night variability.
A final limitation that should be addressed is our limited sample size. Because we regard this as a pilot study, we strongly believe that larger controlled studies are necessary in the future. However, because we believe our findings are novel, we hope that they will encourage further research in this area, particularly with regard to potential new therapeutic targets (eg, mixing gain) in patients with OSA. Regardless, because our findings are significant for all of our primary outcome measures, we do not believe that our studies are underpowered for our prespecified hypotheses. Indeed, we have calculated > 80% power with α = 0.05 to show a difference in AHI based on the observed sample size, mean values, and variance. However, we acknowledge that some of our exploratory analyses such as effects on sleep architecture and influences of ejection fraction may well yield different results in subsequent larger studies.
In conclusion, we found that CRT improved cardiac function and reduced AHI in patients with CHF and ventricular desynchrony, but yielded limited improvements in daytime sleepiness. The combination of CRT and increased-rate pacing had no further impact on the severity of sleep-disordered breathing. These findings add to our understanding of OSA pathogenesis in CHF patients and suggest potential new therapeutic targets in OSA patients.
Acknowledgments
All authors had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Drs. Stanchina and Ellison have received grant support from St. Jude Medical for this work. Dr. Malhotra is funded by a National Institute of Aging Beeson Award (AG024837–01), National Institutes of Health grant RO1-HL73146–01, and SCOR Project 1; and serves as a consultant to Restore Medical, Inspiration Medical, Respironics, Inc, NMT Medical, and Pfizer. All other authors have reported to the ACCP that no significant conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Abbreviations
- AHI
apnea-hypopnea index
- ANOVA
analysis of variance
- BMI
body mass index
- BNP
brain natriuretic peptide
- CHF
congestive heart failure
- CRT
cardiac resynchronization therapy
- CRT-P
cardiac resynchronization therapy with repeat polysomnography
- CSA
central sleep apnea
- CSR
Cheyne-Stokes respiration
- FOSQ
functional outcomes of sleep questionnaire
- LECT
lung-to-ear circulation time
- LFCT
lung-to-finger circulation time
- LV
left ventricular
- LVEF
left ventricular ejection fraction
- OSA
obstructive sleep apnea
- PSG
polysomnogram
- SaO2
arterial oxygen saturation
Footnotes
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/misc/reprints.shtml).
References
- 1.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 2.Leung RST, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med. 2001;164:2147–2165. doi: 10.1164/ajrccm.164.12.2107045. [DOI] [PubMed] [Google Scholar]
- 3.Kaneko Y, Floras J, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233–1241. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 4.Bradley T, Logan A, Kimoff R, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–2033. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 5.Solin P, Bergin P, Richardson M, et al. Influence of pulmonary capillary wedge pressure on central apnea in heart failure. Circulation. 1999;99:1574–1579. doi: 10.1161/01.cir.99.12.1574. [DOI] [PubMed] [Google Scholar]
- 6.Sinha AM, Skobel EC, Breithardt OA, et al. Cardiac resynchronization therapy improves central sleep apnea and Cheyne-Stokes respiration in patients with chronic heart failure. J Am Coll Cardiol. 2004;44:68–71. doi: 10.1016/j.jacc.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 7.Wellman A, Jordan AS, Malhotra A, et al. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:1225–1232. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med. 2003;168:645–658. doi: 10.1164/rccm.200302-201OC. [DOI] [PubMed] [Google Scholar]
- 9.Younes M, Ostrowski M, Thompson W, et al. Chemical control stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1181–1190. doi: 10.1164/ajrccm.163.5.2007013. [DOI] [PubMed] [Google Scholar]
- 10.Garrigue S, Bordier P, Jais P, et al. Benefit of atrial pacing in sleep apnea syndrome. N Engl J Med. 2002;346:404–412. doi: 10.1056/NEJMoa011919. [DOI] [PubMed] [Google Scholar]
- 11.Simantirakis EN, Schiza SE, Chrysostomakis SI, et al. Atrial overdrive pacing for the obstructive sleep apnea-hypopnea syndrome. N Engl J Med. 2005;353:2568–2577. doi: 10.1056/NEJMoa050610. [DOI] [PubMed] [Google Scholar]
- 12.Luthje L, Unterberg-Buchwald C, Dajani D, et al. Atrial overdrive pacing in patients with sleep apnea and implanted pacemaker. Am J Respir Crit Care Med. 2005;172:118–122. doi: 10.1164/rccm.200409-1258OC. [DOI] [PubMed] [Google Scholar]
- 13.Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 14.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 15.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles, CA: Brain Information Service/ Brain Research Institute, UCLA; 1968. [Google Scholar]
- 16.American Sleep Disorders Association. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–184. [PubMed] [Google Scholar]
- 17.American Academy of Sleep Medicine. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in adults. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 18.Hall MJ, Xie A, Rutherford R, et al. Cycle length of periodic breathing in patients with and without heart failure. Am J Respir Crit Care Med. 1996;154:376–381. doi: 10.1164/ajrccm.154.2.8756809. [DOI] [PubMed] [Google Scholar]
- 19.Ryan CM, Bradley TD. Periodicity of obstructive sleep apnea in patients with and without heart failure. Chest. 2005;127:536–542. doi: 10.1378/chest.127.2.536. [DOI] [PubMed] [Google Scholar]
- 20.Stein E, Damato AN, Kosowsky BD, et al. The relation of heart rate to cardiovascular dynamics: pacing by atrial electrodes. Circulation. 1966;33:925–932. doi: 10.1161/01.cir.33.6.925. [DOI] [PubMed] [Google Scholar]
- 21.O’Neill JO, Bott-Silverman CE, McRae AT, et al. B-type natriuretic peptide levels are not a surrogate marker for invasive hemodynamics during management of patients with severe heart failure. Am Heart J. 2005;149:363–369. doi: 10.1016/j.ahj.2004.05.051. [DOI] [PubMed] [Google Scholar]
- 22.Wellman A, Malhotra A, White DP. Atrial pacing in sleep apnea syndrome. N Engl J Med. 2002;347:445–446. doi: 10.1056/NEJM200208083470613. [DOI] [PubMed] [Google Scholar]
- 23.Floras J, Bradley TD. Atrial overdrive pacing for sleep apnea: a door now closed? Am J Respir Crit Care Med. 2005;172:1–3. doi: 10.1164/rccm.2503007. [DOI] [PubMed] [Google Scholar]
- 24.Malhotra A, Jordan AS. Did fat boy Joe need hormone replacement therapy? Sleep. 2006;29:16–18. [PubMed] [Google Scholar]
- 25.Eckert D, Jordan AS, Merchia P, et al. Central sleep apnea: pathophysiology and treatment. Chest. 2007;131:595–607. doi: 10.1378/chest.06.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Circulation. 1998;97:2154–2159. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]


