Abstract
Study objectives
To compare adherence and clinical outcomes between flexible positive airway pressure (PAP) [C-Flex; Respironics; Murraysville, PA] and standard PAP therapy (ie, continuous positive airway pressure [CPAP]).
Design and setting
A controlled clinical trial of CPAP therapy vs therapy using the C-Flex device in participants with moderate-to-severe obstructive sleep apnea. Participants were recruited from and followed up through an academic sleep disorders center.
Participants
Eighty-nine participants were recruited into the study after they had undergone complete in-laboratory polysomnography and before initiating therapy. Participants received either therapy with CPAP (n = 41) or with the C-Flex device (n = 48), depending on the available treatment at the time of recruitment, with those recruited earlier receiving CPAP therapy and those recruited later receiving therapy with the C-Flex device. Follow-up assessments were conducted at 3 months.
Measurements and results
The groups were similar demographically. The mean (± SD) treatment adherence over the 3-month follow-up period was higher in the C-Flex group compared to the CPAP group (weeks 2 to 4, 4.2 ± 2.4 vs 3.5 ± 2.8, respectively; weeks 9 to 12, 4.8 ± 2.4 vs 3.1 ± 2.8, respectively). Clinical outcomes and attitudes toward treatment (self-efficacy) were also measured. Change in subjective sleepiness and functional outcomes associated with sleep did not improve more in one group over the other. Self-efficacy showed a trend toward being higher at the follow-up in those patients who had been treated with the C-Flex device compared to CPAP treatment.
Conclusions
Therapy with the C-Flex device may improve overall adherence over 3 months compared to standard therapy with CPAP. Clinical outcomes do not improve consistently, but C-Flex users may be more confident about their ability to adhere to treatment. Randomized clinical trials are needed to replicate these findings.
Keywords: continuous positive airway pressure, outcomes, sleep apnea, treatment adherence
Obstructive sleep apnea (OSA) is a common disorder1 that is associated with important neuropsychological and cardiovascular sequelae.2–10 Positive airway pressure (PAP) is acknowledged as the treatment of choice for OSA. The most common form of PAP, continuous PAP (CPAP), works through the application of positive pressure to the upper airway at a constant level, acting as a pneumatic splint to keep the pharyngeal airway open during sleep. CPAP also acts to maintain lung volume, which may help to maintain the patency of the pharyngeal airway via tethering forces.11,12 Treatment with CPAP improves daytime functioning and quality of life, and reduces BP.2,13–16 Despite its reported efficacy, nightly adherence to CPAP therapy is suboptimal.17 Considerable investigation has been directed at defining the factors affecting CPAP adherence. Variables such as age, gender, degree of daytime sleepiness, and attitudes toward use have been shown to be effective predictors of adherence, but few studies17–21 have made substantial improvements in adherence with related interventions. CPAP use may also be influenced by side effects of the device, which are commonly reported as pressure intolerance, difficulty exhaling, mask leak, or air leak through the mouth.22
Technological advances in the delivery of airflow have occurred that have aimed to improve patient comfort, treatment adherence, and effectiveness for reducing apneas and inspiratory flow limitation. Many of these advances, such as autotitrating CPAP (APAP), have been designed to adjust PAP throughout the night, keeping the pressure elevated when upper airway collapsing forces are high and lowering pressure when the likelihood of airway collapse is low.23 Other machines, including bilevel PAP devices, deliver different set pressures during exhalation (lower pressure) and inhalation (higher pressure) in an attempt to improve comfort.24 Despite these advances, previous studies24,25 have suggested that these different flow delivery devices do not substantially improve treatment adherence over traditional CPAP. Investigators agree, however, that improvements in the comfort of the method of flow delivery might serve as one important target in attempting to improve patient adherence.
A recent technological advance (C-Flex; Respironics; Murraysville, PA) alternates airway pressure between exhalation and inhalation on a breath-by-breath basis (Fig 1). Airway pressure is reduced during early exhalation in proportion to the patient’s expiratory flow rate. Pressure is then increased again toward the end of exhalation when airway collapse is most likely.26 In theory, the C-Flex technology could be associated with increased comfort during exhalation, and may improve treatment adherence and clinical outcomes, although these have not been studied. The present study is a controlled clinical trial to compare the effect of therapy with C-Flex and traditional CPAP therapy on treatment adherence, treatment outcomes, and attitudes toward treatment.
FIGURE 1.
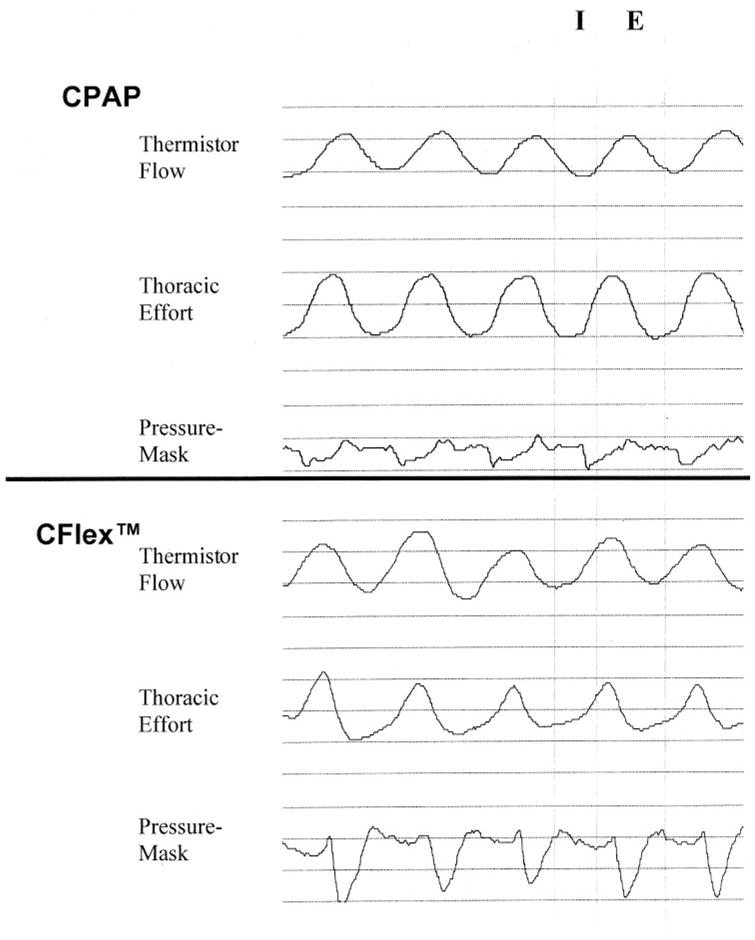
Raw data from one patient receiving conventional CPAP therapy at 6 cm H2O (top) and therapy using the C-Flex technology (bottom). I = inspiration; E = expiration; Thermistor Flow = oronasal thermistor; Thoracic Effort = qualitative signal obtained from a piezobelt; Pressure Mask = mask pressure obtained using standard pressure transducer (qualitative signal). Note the drop in mask pressure during expiration during therapy using the C-Flex technology compared to that with conventional CPAP therapy.
Materials And Methods
Participants
Eighty-nine participants (64 men and 25 women) between the ages of 25 and 80 years with OSA were recruited consecutively from the Sleep Disorders Center of Lifespan Hospitals (a Brown University-affiliated hospital in Providence, RI) as part of a larger study of the efficacy of CPAP therapy. OSA was diagnosed in all participants by full in-laboratory overnight polysomnography monitoring. All participants were CPAP-naïve. Adequate CPAP settings, defined as the absence of detectable apneas/hypopneas and snoring, were determined by a separate full-night titration study. Titration utilized traditional CPAP therapy. Participants with evidence of previously diagnosed neurologic or psychiatric illness, other major intrinsic sleep disorders, congestive heart failure, or end-stage renal disease were excluded from the study. All participants provided informed consent prior to participation in the study. This study was approved by the Rhode Island Hospital and Brown University institutional review boards.
Procedures
Participants were enrolled in the study after their CPAP titration night but before initiating PAP treatment. All participants were referred to the same home health-care company to maintain consistent approaches to PAP setup and clinical management. We realize that utilizing a single home health-care company can limit recruitment and result in a selection bias. We attempted to protect against this by utilizing the company that was most widely used by insurance companies and the physicians in the referring practice. This resulted in the exclusion of 11 potential participants (11.8%) whose insurers would not pay for the use of the chosen company. We are aware of no reason, however, that this selection criterion would systematically bias the results of our study. Participants were supplied with PAP machines (REMstar Pro; Respironics). These machines are equipped with objective monitors of PAP use at the prescribed pressure. Consistent with previous studies of PAP adherence,27–29 study participants were not told that their CPAP treatment adherence was being measured. Instead, informed consent stated, “During the course of the study, we will occasionally access your records from the PAP machine to assess efficacy of use.” This procedure was approved by both institutional review boards (Rhode Island Hospital and Brown University) as well as by an external Data Safety and Monitoring Board approved by the National Heart, Lung, and Blood Institute. Participants were told that their treatment adherence was being monitored only if they asked directly. This query was made a total of six times (three occurrences in each group) during the course of this study. Heated humidification was supplied for each of the machines to minimize the impact of upper airway dryness on treatment adherence.30 Participants enrolled from April 2002 to April 2003 were provided with conventional CPAP machines (n = 41), and those recruited after this date (ie, April 2003 to March 2004) received PAP machines with the C-Flex technology (n = 48). Although not planned at the initiation of the study, this change in technology allowed for an examination of the relative benefits of this new device. The same technicians were used to set up both types of devices. Although technicians were aware of the type of device provided to the patient, there was no difference in setup directions between the two devices, and technicians were naïve to the goals of the study and to patient involvement in the study. Technicians were allowed to decide the C-Flex setting at the time of setup in a manner that was consistent with standard clinical care. We chose to do this rather than to dictate the C-Flex setting so that our findings would generalize to the majority of patients who will have this setting determined by their home health-care therapist. Participants’ adherence data were collected during a follow-up visit at 3 months following the initiation of therapy. Measures of sleep-related quality of life, self-reported excessive daytime sleepiness, and attitudes toward CPAP use were assessed both before treatment initiation, but after titration (baseline), and at the 3-month follow-up. Assessors of outcome variables were trained research assistants who were not directly involved in patient care and who were blinded to group membership. All participants were followed up regardless of whether or not they continued to use the treatment.
Blinding
All research assistants who conducted clinical assessments and downloaded adherence data were blinded to group membership. Physicians and home health-care therapists were blinded to whether or not their patients were enrolled in this study. All patients had the potential to be study participants, but neither physicians nor therapists were ever informed of the roster of those patients who had agreed to take part in the study. Participants were informed about their machines by the home health-care therapists, potentially unblinding them as to the machine type they were using. This prompted us to sample the C-Flex group to find out how many of them knew that they were receiving therapy with the C-Flex machine vs standard CPAP therapy. We randomly contacted 21 participants from the C-Flex group to assess their knowledge of group membership. Seven patients correctly stated that they were receiving therapy with the C-Flex machine, while the other 14 patients claimed to be receiving standard CPAP therapy or to be uncertain which therapy they were receiving. It is possible that simply knowing that they were prescribed therapy using the C-Flex machine would enhance the adherence of these seven individuals, resulting in a placebo effect. We have, however, compared these 7 patients to the remaining 14 patients to address this concern. There was no difference in 3-month treatment adherence between these two subgroups of patients (t(19) = −0.075; p > 0.05).
Measures
Adherence was monitored using the technology (Smart Card; Respironics) that was provided with the PAP machines (REMstar Pro; Respironics). Treatment adherence was reported as the total number of hours the devices were used at the prescribed pressure per 24-h period. Average treatment adherence data are presented in this study for week 1, weeks 2 to 4, weeks 5 to 8, and weeks 9 to 12. We grouped the treatment adherence data to maximize power by limiting the levels of the dependent variable. We chose our time periods to reflect early use (week 1) and monthly follow-ups. The apnea-hypopnea index (AHI), an index of the number of apneas plus hypopneas per hour of sleep, provided a measure of OSA severity. An AHI of 15 events per hour served as the lower limit for entry into the study. The amount of time spent below 90% blood oxygenation saturation was also recorded during the overnight polysomnography monitoring. Measurements of height and weight were recorded, and body mass index (BMI) was calculated by dividing weight (in kilograms) over height (in square meters).
Subjective sleepiness was measured using the Epworth sleepiness scale.31 This self-report scale requires respondents to rate their likelihood of dozing off under various circumstances on a scale of 0 (no chance) to 3 (high chance). Scores ranged from 0 to 24, with higher scores indicative of greater subjective daytime sleepiness, using a score of 10 as a clinical cutoff. This measure has demonstrated adequate reliability and validity. Sleep-related functional outcomes were measured using the functional outcomes associated with sleepiness questionnaire (FOSQ). The reliability and validity of this measure is adequate.32 The measure assesses the impact of excessive sleepiness on multiple activities of daily living. The FOSQ provides a total score between 0 and 20, representing the overall impact of sleepiness on the participant’s life. The measure can also be divided into the following five separate subscales: activity level; vigilance; intimacy and sexual relationships; general productivity; and social outcome. A higher score on this measure indicates lesser impairment in daytime functioning. Attitudes toward CPAP use were measured using a self-efficacy scale developed by Stepnowsky and Marler.29 This scale is based on psychological theories of behavior change and has been shown to be a reliable predictor of CPAP adherence in previous studies.29,33 The five-item self-efficacy scale asks participants to rate, on a scale of 1 (very low confidence) to 5 (highly confident), how confident they are in their ability to continue to use CPAP even under difficult circumstances (eg, discomfort or travel). A higher score on this scale represents greater confidence for use.
Statistical Analysis
Data analyses were conducted using a statistical software program (SPSS, version 11.5; SPSS Inc; Chicago, IL). The significance level was set at 0.05 for all analyses. The data were reported as the mean ± SD, unless otherwise noted. Demographic data were compared using t tests to determine the degree to which certain variables were different between the groups, thus serving as potential threats to the internal validity of the study.
Adherence data were analyzed using a repeated-measures analysis of variance (ANOVA) with time (ie, week 1, weeks 2 to 4, weeks 5 to 8, and weeks 9 to 12) as the within-subjects independent variable and group (ie, C-Flex and CPAP) as the between-subjects variable. Post hoc comparisons were adjusted for multiple comparisons using the Bonferroni adjustment.
All clinical outcome data were compared using repeated-measures ANOVA with time (baseline and 3 months) as the within-subjects independent variable and group (C-Flex and CPAP) as the between-subjects variable. No covariates were used for these analyses. In an attempt to replicate previously reported data,29 Spearman rank order correlations were performed to examine the predictive utility of self-efficacy on adherence regardless of group membership.
Results
Baseline Assessments
Table 1 shows the demographic data for the two study groups. There was an equal proportion of men and women in each group (CPAP group: men, 28 patients; women, 13 patients; C-Flex group: men, 36 patients; women, 12 patients). In addition, the two groups were not different in their apnea severity, major demographic variables, or self-efficacy at baseline. In general, participants were highly educated, middle-aged men with severe sleep apnea, substantial subjective sleepiness, and a high BMI.
Table 1.
Demographic and OSAS Severity Measures for the CPAP Group (n = 41) and the C-Flex Group (n = 48)*
| Variable | CPAP | C-Flex | t Test | p Value |
|---|---|---|---|---|
| Age, yr | 51.4 (10.8) | 53.7 (11.0) | −0.97 | NS |
| Education, yr | 14.4 (2.4) | 14.7 (3.4) | −0.50 | NS |
| AHI | 39.4 (25.9) | 43.2 (22.7) | −0.74 | NS |
| Sa90 | 79.3 (99.2) | 60.4 (79.6) | 1.31 | NS |
| CPAP pressure | 9.8 (2.9) | 10.1 (2.0) | −0.61 | NS |
| BMI | 31.7 (5.2) | 32.0 (3.7) | −0.32 | NS |
| Epworth sleepiness scale score | 11.2 (4.8) | 10.7 (4.7) | 0.50 | NS |
| Self-efficacy | 19.9 (3.3) | 20.4 (3.1) | −0.724 | NS |
Values given as mean (SD), unless otherwise indicated. Sa90 = the number of minutes spent below 90% oxygen saturation on the diagnostic overnight polysomnography; NS = not significant.
Treatment Adherence
Only one participant in this study (in the CPAP group) failed to ever use PAP treatment during the course of this study. All other participants at least attempted to use PAP at some point in the study. There was a main effect for time (F = 3.54; p = 0.02), with patients in both of the groups generally using CPAP therapy less over time. The mean length of use for the two groups combined at week 1 was 4.5 ± 2.5 h per night, which decreased to 4.2 ± 2.6, 4.1 ± 2.7, and 4.0 ± 2.7 h per night at the respective follow-up periods. There was also a main effect for group (F = 6.26; p < .01) on adherence, with the C-Flex group outperforming the CPAP group (4.8 vs 3.5 h per night overall, respectively). There was also a significant interaction between time and group, with the C-Flex group showing relatively greater use over time compared to the CPAP group (F = 5.76; p = 0.02). As shown in Figure 2, the CPAP group showed a mean decrease in use within the first month of treatment, from 4.2 ± 2.4 h of nightly use at week 1 to 3.5 ± 2.8 h per night over weeks 2 to 4. The C-Flex group, however, maintained their mean use within the first month (week 1, 4.7 ± 2.5 h per night; weeks 2 to 4, 4.7 ± 2.2 h per night). Even greater differences were noted between the groups by the end of 2 months, when the CPAP group decreased its mean use to 3.2 ± 2.9 h per night (weeks 5 to 8) and the C-Flex group maintained it mean use at 4.8 ± 2.4 h per night. Little had changed by the third month, with the CPAP group using PAP for 3.1 ± 2.8 h per night on average between weeks 9 and 12, and the C-Flex group using PAP for 4.8 ± 2.4 h per night. The end result was greater use by the C-Flex group by an average of 1.7 h per night.
Figure 2.
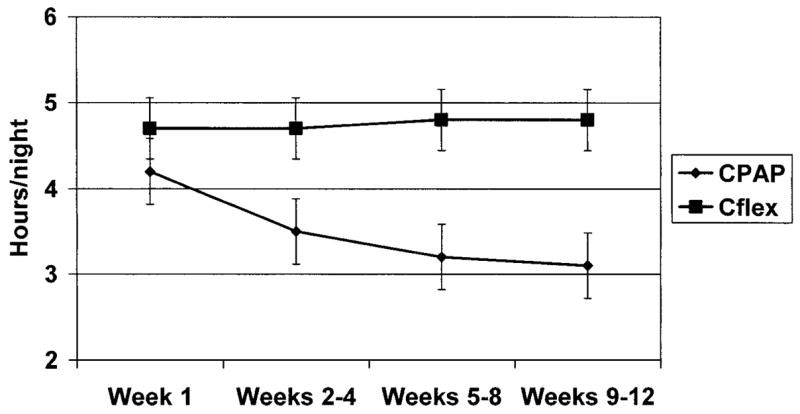
Average nightly use over time by group. Error bars = SEM.
Clinical Outcomes
Means and SDs for the clinical outcome measures are shown in Table 2. There was a significant effect of time (F = 19.0; p < .001) on Epworth sleepiness scale scores. Scores decreased for both groups from baseline to 3 months (CPAP use: baseline, 11.2 ± 4.8 h per night; at 3 months, 9.4 ± 4.6 h per night; C-Flex use: baseline, 10.7 ± 4.7 h per night; at 3 months, 8.3 ± 3.9 h per night). There was no significant difference between groups (F = 0.93; p > 0.05) [Table 2] and no differences in group-by-time interaction (F = 0.42; p > 0.05).
Table 2.
Outcome Measures for the CPAP and C-Flex Groups at Baseline and at the 3-Month Follow-up*
| CPAP
|
C-Flex
|
||||
|---|---|---|---|---|---|
| Variable | Baseline | 3 mo | Baseline | 3 mo | p Value |
| Epworth sleepiness scale score | 11.2 (4.8) | 9.4 (4.6) | 10.7 (4.7) | 8.3 (3.9) | 0.56 |
| FOSQ total | 8.0 (2.0) | 9.9 (2.8) | 7.7 (2.2) | 9.6 (2.7) | 0.99 |
| Self-efficacy | 19.9 (3.3) | 19.6 (5.4) | 20.4 (3.1) | 21.9 (3.4) | 0.06 |
Values given as mean (SD), unless otherwise indicated.
Functional outcomes associated with sleepiness, as measured by the FOSQ total score, also showed a significant time effect (F = 50.67; p < .001), with all participants decreasing in the degree to which self-reported functional abilities were affected by their sleep disorder. There was again no significant group effect (F = 0.39; p > 0.05) or group-by-time interaction (F = 0.01; p > 0.05). To assess the degree to which specific subscales of the FOSQ were affected differently in the two groups, we conducted separate repeated measures ANOVAs for each subscale but found no significant effects (all p > 0.10).
Finally, there was no significant effect of time (F = 1.57; p > 0.05) on confidence in using the treatment. There was an overall main effect of group on this measure (F = 4.70, p = 0.03), with the C-Flex group reporting greater confidence overall compared with the CPAP group. There was, however, only a trend toward a significant interaction between group and time (F = 3.61; p = 0.06), with patients in the C-Flex group gaining in their confidence to use the therapy with time and patients in the CPAP group maintaining their level of confidence (Table 2). The CPAP group had a mean baseline self-efficacy score of 19.9 ± 3.3 (of a possible 25 points) and a mean 3-month score of 19.6 ± 5.4. The C-Flex group, on the other hand, increased from a mean baseline score of 20.4 ± 3.1 to a mean score of 21.9 ± 3.4 at 3 months. The self-efficacy score at 3 months was significantly related to average use at the same time in both groups (CPAP group: r= 0.78; p < 0.001; C-Flex group: r = 0.55; p < 0.001), replicating its efficacy as a predictor of adherence regardless of the method of flow delivery.
Discussion
PAP has been shown to be an effective treatment for OSA, but treatment adherence has been notably poor.17 Previous technological advances in the flow-delivery method of positive pressure have met with variable improvements in treatment adherence. This study reports the relative benefits of a new flow-delivery method (C-Flex) on treatment adherence and clinical outcomes in a sample of individuals with moderate-to-severe OSA. Although this was not a randomized clinical trial, the study offers the first published findings of the relative benefits of therapy with the C-Flex device compared with traditional CPAP therapy.
We have shown that the flow-delivery method offered by C-Flex is associated with better adherence at 3 months than is traditional CPAP therapy. This improvement in treatment adherence equates to an average increase of 1.7 h of use per night. Few previous studies investigating flow-delivery patterns have met this level of improvement in adherence. Most of these studies have been conducted with APAP. These devices are capable of increasing or decreasing CPAP level based on the presence/absence of inspiratory flow limitation, snoring, or apnea detection. Meurice et al34 observed the largest improvement in use (1.4 h per night at 3 weeks) with APAP therapy. The sample size of this study, however, was quite small (eight patients per group), and the follow-up was limited to 3 weeks. In addition, Konermann et al35 showed a trend toward improvement in patient adherence (ie, a difference of 0. 8 h using an APAP device compared with standard CPAP therapy). This study recruited approximately 25 participants per group, but treatment adherence was highly variable within each group, resulting in a lack of statistical significance. One recent study by Massie and colleagues22 showed an increase in use of 35 min (p < 0.05) for therapy using an APAP device compared with conventional CPAP therapy. Although this too was a small trial, it suggested that flow delivery devices such as APAP devices that are aimed at lowering fixed CPAP pressures across the night may affect patient adherence. Despite these promising studies, a recent meta-analysis25 including 282 patients reported that adherence was not significantly greater with autotitrating compared to conventional CPAP therapy (pooled estimate of the APAP-CPAP difference, 0.2; range, −0.16 to 0.57; p = 0.28). Thus, there is no existing device technology that is currently accepted to improve CPAP adherence.
Previous efforts to partition inspiratory and expiratory pressures to improve patient adherence with OSA therapy also have been reported.24 The use of bilevel PAP therapy often enables a reduction of the expiratory positive-pressure level required to maintain airway patency during sleep in OSA patients. Bilevel PAP therapy has not been shown to have an effect on treatment adherence; however, the few studies undertaken have notably been small.24,36 Bilevel PAP is currently used for specific OSA patients with requirements for high levels of pressure who complain of expiratory discomfort. However, the data supporting this practice are currently lacking.
There are similarities between the C-Flex device and other modes of flow delivery (such as APAP and bilevel PAP) in that expiratory pressures are reduced. In C-Flex mode, the device monitors both inhalation and exhalation pressures. The prescribed PAP level is maintained throughout inhalation, but pressure is varied during exhalation. The C-Flex device begins to decrease pressure when exhalation is sensed and reaches its lowest pressure after midexhalation. Pressure is then ramped up at the end of exhalation when the airway is most susceptible to collapse and continues to rise to the prescribed pressure for inhalation (Fig 1).
The patency of the pharyngeal airway is a function of a balance of forces that promote pharyngeal patency and those that promote collapse. For example, increased lung volume, increased intraluminal positive pressure,37 and pharyngeal dilator muscle activation all promote patency. The major pharyngeal dilator muscles such as the genioglossus are phasic muscles, implying an inspiratory burst in activity but a lower level of tonic activity during exhalation. During early exhalation, PAP generated via lung recoil serves to preserve pharyngeal patency. However, at end-exhalation, when the lung is at functional residual capacity, the pharyngeal airway is most prone to collapse due to a lack of intraluminal positive pressure and longitudinal tethering forces.38 Thus, the need for applied positive-pressure therapy is most critical at the end of exhalation.
Similar to therapy using the C-Flex device, bilevel PAP delivers a lower expiratory pressure (compared to inspiration); however, the pressure is not variable within the exhalation phase. Instead, pressure drops suddenly to a lower prescribed pressure throughout all of exhalation and rises abruptly to a higher prescribed pressure during inhalation. As noted above, APAP machines alter PAP levels throughout the night (inspiration and expiration) in response to apnea detection, airflow limitation, snoring, or changes in airway resistance. APAP devices differ greatly in their detection algorithms, limiting comparison between machines. However, many studies have suggested that APAP therapy does reduce the mean PAP requirements, which, again, are thought to impact treatment adherence. C-Flex is the only technology designed to flexibly deliver pressure on a breath-by-breath basis by adjusting pressure within exhalation, which may be a more important variable for treatment adherence than the overall PAP level. Of note, the magnitude of the improvement in adherence observed in the present study is greater than that reported for any previous technological intervention.
Although clinical outcome variables (ie, Epworth sleepiness scale score and FOSQ) generally improved with time in both groups, there appeared to be no major advantage to using C-Flex technology to attain these improvements despite greater treatment adherence. These subjective measures, however, are susceptible to social desirability. For example, participants can report less sleepiness simply because they are aware that they should be less sleepy when treated, resulting in improvements in all participants. It is also possible that the C-Flex group did not met the threshold for showing functional improvement on these variables, averaging only 4.8 h of use per night. Evidence for this is provided by Weaver,39 who suggested that 6 h of use per night may be needed to normalize FOSQ scores. The clinical outcome findings of this study suggest that the flow-delivery method may not differentially affect these measures, despite having an overall effect on adherence. Based on studies in the sleep deprivation literature,40,41 we would speculate that 1.7 h of additional consolidated sleep may have both metabolic and neurocognitive benefits. There are currently no available data, however, showing a dose-response relationship between PAP use and cardiovascular risk reduction in patients with sleep apnea. In theory, however, clinical differences between flow-delivery methods may be seen with different outcome measures (eg, BP or objective sleepiness), at longer follow-up periods, or with an increased sample size.
This is the first study to examine the effects of a technological change in positive-pressure devices on attitudes toward its use. We think that the inclusion of a measure of self-efficacy is important to outcome studies using PAP. These measures may arguably be the most efficacious predictors of adherence.29,33,42 Participants’ self-efficacy, or their confidence that they can use treatment device in difficult situations, showed a trend toward being higher at follow-up in those using the C-Flex device compared to those using conventional CPAP devices. Self-efficacy was indeed related to use at follow-up in both groups. Given that correlations between use and confidence were high, it is possible that improved confidence in using the C-Flex device can represent one potential mechanism for increasing adherence in this study. In other words, using a device that is easier to tolerate may increase confidence for future use early in treatment, securing future use.
There are limitations to our study. First, as stated earlier, this was not a clinical trial with random group assignments. Although we showed that the groups were matched on several key variables, random assignment may affect the results of the study. We recognize this limitation but think that the study contributes significantly to the existing literature, as it is the first study of the C-Flex technology and incorporates one of the larger sample sizes of all the longitudinal studies of PAP treatment adherence. We attempted to rigorously maintain blinding to reduce the susceptibility of a spurious finding due to the lack of randomization. A second limitation is that we did not include objective measures of daytime sleepiness as an outcome measure of the efficacy of treatment. The existing literature43 suggests that there is a greater effect size for subjective measurements of sleepiness than for objective measurements. Thus, objective and subjective measurements of sleepiness are both important but likely measure somewhat different phenomena. Another limitation is that we did not use the C-Flex technology to titrate our C-Flex group. This limits our ability to say with certainty that this group was treated effectively with the new device. Early studies have shown that the C-Flex device reduces flow-limited breathing as effectively as do standard CPAP devices.44,45 We cannot, however, know that this was the case for our experimental group. With regard to our primary outcome, treatment adherence, this only poses a problem if ineffective treatment were likely to result in better adherence. There are now several studies15,16,46 utilizing sham or subtherapeutic CPAP that have shown no greater adherence with low or ineffective pressure. Given these findings, and the early efficacy studies of the C-Flex technology, we think that our findings represent a true potential benefit for C-Flex technology. We recognize the importance of randomized clinical trials to compare these treatments and to replicate these initial findings. A final limitation is that this study only addressed treatment adherence and outcomes in individuals with moderate-to-severe OSA. The same study in patients with mild OSA may yield different results. We chose to include only participants with moderate-to-severe OSA because they represent those patients who, most consistently, are prescribed PAP therapy.
In conclusion, use of the C-Flex mode of expiratory pressure release was associated with improved nightly adherence compared to that with conventional CPAP therapy. Further randomized controlled trials will be necessary to compare this to other flow-delivery patterns to assess the impact on both short-term and long-term outcomes.
Acknowledgments
The authors thank Leisha Smith, Jaime Skrekas, and Charleen Pysz for assistance with manuscript preparation.
This research was supported by a grant to the first author (M.S.A.) from the National Institutes of Health (No. R01 HL67209). Dr. Malhotra has received an unrestricted research grant from Respironics to study the cardiovascular effects of sleep apnea. Drs. Millman, Stanchina, and Aloia also received an unrestricted research grant from Respironics after the collection of the data provided in this article.
Abbreviations
- AHI
apnea-hypopnea index
- ANOVA
analysis of variance
- APAP
autotitrating positive airway pressure
- BMI
body mass index
- CPAP
continuous positive airway pressure
- FOSQ
functional outcomes of sleepiness questionnaire
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
Footnotes
Respironics is the manufacturer of the C-Flex device, which is discussed in this article.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/misc/reprints.shtml).
References
- 1.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Aloia MS, Ilniczky N, Di Dio P, et al. Neuropsychological changes and treatment compliance in older adults with sleep apnea. J Psychosom Res. 2003;54:71–76. doi: 10.1016/s0022-3999(02)00548-2. [DOI] [PubMed] [Google Scholar]
- 3.Engleman H, Martin SE, Douglas NJ. Cognitive function in the sleep apnea/hypopnea syndrome. Sleep. 2000;23:S102–S107. [PubMed] [Google Scholar]
- 4.Finn L, Young T, Palta M, et al. Sleep-disordered breathing and self-reported general health status in the Wisconsin Sleep Cohort Study. Sleep. 1998;21:701–706. [PubMed] [Google Scholar]
- 5.Flemons WW, Reimer MA. Development of a disease-specific health-related quality of life. Am J Respir Crit Care Med. 1998;158:494–503. doi: 10.1164/ajrccm.158.2.9712036. [DOI] [PubMed] [Google Scholar]
- 6.Kim HC, Young T, Matthews CG, et al. Sleep-disordered breathing and neuropsychological deficits: a population-based study. Am J Respir Crit Care Med. 1997;156:1813–1819. doi: 10.1164/ajrccm.156.6.9610026. [DOI] [PubMed] [Google Scholar]
- 7.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 8.Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 9.Redline S, Strauss ME, Adams N, et al. Neuropsychological function in mild sleep-disordered breathing. Sleep. 1997;20:160–167. doi: 10.1093/sleep/20.2.160. [DOI] [PubMed] [Google Scholar]
- 10.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 11.Stanchina ML, Malhotra A, Fogel R, et al. The influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleep. Sleep. 2003;26:851–856. doi: 10.1093/sleep/26.7.851. [DOI] [PubMed] [Google Scholar]
- 12.Van de Graaf WB. Thoracic influence on upper airway patency. J Appl Physiol. 1998;65:2124–2131. doi: 10.1152/jappl.1988.65.5.2124. [DOI] [PubMed] [Google Scholar]
- 13.Engleman HM, Martin SE, Kingshott RN, et al. Randomised placebo controlled trial of daytime function after continuous positive airway pressure (CPAP) therapy for the sleep apnoea/hypopnoea syndrome. Thorax. 1998;53:341–345. doi: 10.1136/thx.53.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faccenda JF, Mackay TW, Boon NA, et al. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;163:344–348. doi: 10.1164/ajrccm.163.2.2005037. [DOI] [PubMed] [Google Scholar]
- 15.Jenkinson C, Davies RJO, Mullins R, et al. Comparison of therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised prospective parallel trial. Lancet. 1999;353:2100–2105. doi: 10.1016/S0140-6736(98)10532-9. [DOI] [PubMed] [Google Scholar]
- 16.Montserrat JM, Ferrer M, Hernandez L, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med. 2001;164:608–613. doi: 10.1164/ajrccm.164.4.2006034. [DOI] [PubMed] [Google Scholar]
- 17.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887–895. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 18.Hudgel DW, Fung C. A long-term randomized, cross-over comparison of auto-titrating and standard nasal continuous positive airway pressure. Sleep. 2000;23:645–648. [PubMed] [Google Scholar]
- 19.McArdle N, Devereux G, Heidarnejad H, et al. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159:1108–1114. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- 20.Mortimore IL, Whittle AT, Douglas NJ. Comparison of nose and face mask CPAP therapy for sleep apnoea. Thorax. 1998;53:290–292. doi: 10.1136/thx.53.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reeves-Hoche MK, Hudgel DW, Meck R, et al. Continuous versus bilevel positive airway pressure for obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:443–449. doi: 10.1164/ajrccm.151.2.7842204. [DOI] [PubMed] [Google Scholar]
- 22.Massie C, McArdle N, Hart R, et al. Comparison between automatic and fixed positive airway pressure therapy in the home. Am J Respir Crit Care Med. 2003;167:20–23. doi: 10.1164/rccm.200201-022OC. [DOI] [PubMed] [Google Scholar]
- 23.Teschler H, Berthon-Jones M, Thompson AB, et al. Automated continuous positive airway pressure titration for obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 1996;154:734–740. doi: 10.1164/ajrccm.154.3.8810613. [DOI] [PubMed] [Google Scholar]
- 24.Sanders MH, Kern N. Obstructive sleep apnea treated by independently adjusted inspiratory and expiratory positive airway pressure via nasal mask. Chest. 1990;98:317–324. doi: 10.1378/chest.98.2.317. [DOI] [PubMed] [Google Scholar]
- 25.Ayas NT, Patel SR, Malhotra A, et al. Auto-titrating versus standard continuous positive airway pressure for the treatment of obstructive sleep apnea: results of a meta-analysis. Sleep. 2004;27:249–253. doi: 10.1093/sleep/27.2.249. [DOI] [PubMed] [Google Scholar]
- 26.Schwab R, Gefter W, Hoffman F, et al. Dynamic upper airway imaging during awake respiration in normal subjects and patients with sleep disordered breathing. Am Rev Respir Dis. 1993;148:1375–1400. doi: 10.1164/ajrccm/148.5.1385. [DOI] [PubMed] [Google Scholar]
- 27.Hui DSC, Chan JKW, Choy DKL, et al. Effects of augmented continuous positive airway pressure education and support on compliance and outcome in a Chinese population. Chest. 2000;117:1410–1416. doi: 10.1378/chest.117.5.1410. [DOI] [PubMed] [Google Scholar]
- 28.Hui DSC, Choy DKL, Li TST, et al. Determinants of continuous positive airway pressure compliance in a group of Chinese patients with obstructive sleep apnea. Chest. 2001;120:170–176. doi: 10.1378/chest.120.1.170. [DOI] [PubMed] [Google Scholar]
- 29.Stepnowsky CJ, Jr, Marler MR, Ancoli-Israel S. Determinants of nasal CPAP compliance. Sleep Med. 2002;3:239–247. doi: 10.1016/s1389-9457(01)00162-9. [DOI] [PubMed] [Google Scholar]
- 30.Massie C, Hart R, Peralez K, et al. Effects of humidification on nasal symptoms and compliance in sleep apnea patients using continuous positive airway pressure. Chest. 1999;116:403–408. doi: 10.1378/chest.116.2.403. [DOI] [PubMed] [Google Scholar]
- 31.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–381. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 32.Weaver TE, Laziner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–838. [PubMed] [Google Scholar]
- 33.Stepnowsky CJ, Jr, Ancoli-Israel S. CPAP adherence is associated with the decisional balance index [abstract] Sleep. 2000;23:A287–A288. [Google Scholar]
- 34.Meurice JC, Marc I, Series F. Efficacy of auto-CPAP in the treatment of obstructive sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1996;153:794–798. doi: 10.1164/ajrccm.153.2.8564134. [DOI] [PubMed] [Google Scholar]
- 35.Konermann M, Sanner BM, Vyleta M, et al. Use of conventional and self-adjusting nasal continuous positive airway pressure for treatment of severe obstructive sleep apnea syndrome. Chest. 1998;113:714–718. doi: 10.1378/chest.113.3.714. [DOI] [PubMed] [Google Scholar]
- 36.Engleman H, Wild MR. Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS) Sleep Med Rev. 2003;7:81–99. doi: 10.1053/smrv.2001.0197. [DOI] [PubMed] [Google Scholar]
- 37.Remmers JE, Bartlett D. Reflex control of expiratory airflow and duration. J Appl Physiol. 1977;42:80–87. doi: 10.1152/jappl.1977.42.1.80. [DOI] [PubMed] [Google Scholar]
- 38.Malhotra A, White DP. Obstructive sleep apnoea. Lancet. 2002;360:237–245. doi: 10.1016/S0140-6736(02)09464-3. [DOI] [PubMed] [Google Scholar]
- 39.Weaver TE. How much is enough CPAP? Sleep Med. 2003;4:S52. [Google Scholar]
- 40.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 41.Van Dongen HPA, Maislin G, Mullington JM, et al. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 42.Weaver TE, Maislin G, Dinges DF, et al. Self-efficacy in sleep apnea: instrument development and patient perceptions of obstructive sleep apnea risk, treatment benefit, and volition to use continuous positive airway pressure. Sleep. 2003;26:727–732. doi: 10.1093/sleep/26.6.727. [DOI] [PubMed] [Google Scholar]
- 43.Patel SR, White DP, Malhotra A, et al. Continuous positive airway pressure therapy for treating sleepiness in a diverse population with obstructive sleep apnea: results of a meta-analysis. Arch Intern Med. 2003;163:565–571. doi: 10.1001/archinte.163.5.565. [DOI] [PubMed] [Google Scholar]
- 44.Loube DI, Ball NJ. Comparison of compliance with proportional positive airway pressure (C-Flex) to continuous positive airway pressure treatment in obstructive sleep apnea. Sleep. 2004;27:A228. [Google Scholar]
- 45.Penzel T, Kesper K, Ploch T, et al. Inspiratory flow limitation during NREM and REM sleep investigated under CPAP and C-Flex conditions. Sleep. 2004;27:A191. [Google Scholar]
- 46.Barbé F, Mayoralas LR, Duran J, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness: a randomized, controlled trial. Ann Intern Med. 2001;134:1015–1023. doi: 10.7326/0003-4819-134-11-200106050-00007. [DOI] [PubMed] [Google Scholar]


