Abstract
Infection with HIV-1 results in pronounced immune suppression and susceptibility to opportunistic infections (OI). Reciprocally, OI augment HIV-1 replication. As we have shown for Mycobacterium avium complex (MAC) and Pneumocystis carinii, macrophages infected with opportunistic pathogens and within lymphoid tissues containing OI, exhibit striking levels of viral replication. To explore potential underlying mechanisms for increased HIV-1 replication associated with coinfection, blood monocytes were exposed to MAC antigens (MAg) or viable MAC and their levels of tumor necrosis factor α (TNFα) and HIV-1 coreceptors monitored. MAC enhanced TNFα production in vitro, consistent with its expression in coinfected lymph nodes. Using a polyclonal antibody to the CCR5 coreceptor that mediates viral entry of macrophage tropic HIV-1, a subset of unstimulated monocytes was shown to be CCR5-positive by fluorescence-activated cell sorter analysis. After stimulation with MAg or infection with MAC, CCR5 expression was increased at both the mRNA level and on the cell surface. Up-regulation of CCR5 by MAC was not paralleled by an increase in the T cell tropic coreceptor, CXCR4. Increases in NF-κB, TNFα, and CCR5 were consistent with the enhanced production of HIV-1 in MAg-treated adherent macrophage cultures as measured by HIV-1 p24 levels. Increased CCR5 was also detected in coinfected lymph nodes as compared with tissues with only HIV-1. The increased production of TNFα, together with elevated expression of CCR5, provide potential mechanisms for enhanced infection and replication of HIV-1 by macrophages in OI-infected cells and tissues. Consequently, treating OI may inhibit not only the OI-induced pathology, but also limit the viral burden.
HIV-1 viremia occurs during acute infection when the virus is replicating unchecked and again, late in disease when opportunistic infections (OI) are a universal problem. Common pathogens such as human herpesvirus type 1 (HSV-1) and Mycobacterium tuberculosis reportedly up-regulate HIV-1 production in vitro (1, 2) and in vivo (3, 4). In HIV-1-infected patients, clinical infections with M. tuberculosis, Giardia, Mycobacterium avium complex (MAC), herpesvirus type 1, and Pneumocystis carinii have all been shown to correlate with reversible increases in levels of circulating HIV-1 (5–7). The extensive viral replication identified in the tissues of such patients provides compelling evidence that the OI influence up-regulation of HIV-1 expression (8).
A potential source of the circulating virus, when only a limited number of productively infected CD4+ T cells can be detected in the host, was recently revealed to involve cells of myeloid lineage. Multinucleated giant cells (MNGC) and their mononuclear (MNC) counterpart become a rich source of HIV-1 in tissues coinfected with opportunistic pathogens (5, 8, 9). The productively infected cells, which were HIV-1 core protein (p24) positive by immunohistochemistry and HIV-1-specific RNA-positive by in situ hybridization (ISH), were identified as of monocyte/macrophage origin on the basis of their light and ultrastructural morphology and pattern of immunophenotypic markers. The presence of MAC within HIV-1 expressing MNC and MNGC indicates their phagocytic capacity. In light of the recent classification of similar HIV-1+ MNGC and MNC in hyperplastic tonsils and adenoids as dendritic/Langerhans’ cells (DC/LC) (10, 11), we have focused on characterization of these key viral host cells in OI-infected tissues. By multiple parameters, the predominant productively infected cells in these coinfected tissues are of monocyte/macrophage lineage, albeit distinct in that these cells also express S100 protein and actin bundling protein p55 (fascin), commonly considered to be associated with DC/LC (12). Moreover, we explored the cellular and molecular mechanisms whereby MAC influences viral replication, identifying HIV-1 coreceptors and cytokines as candidate molecules. Recent evidence has documented that, in addition to CD4, cellular viral entry depends on the presence of G protein-coupled, seven transmembrane spanning receptors belonging to the chemokine receptor family (13–15). In what may be a key mechanism for OI-enhanced susceptibility to HIV-1 infection, we provide evidence that MAC augments monocyte/macrophage expression of CCR5, a requisite coreceptor for HIV-1. Increased CCR5 expression together with up-regulation of tumor necrosis factor α (TNFα), a reported promoter of HIV-1 replication (9, 16, 17), by OI such as MAC may facilitate entry of HIV-1 and its replication in target macrophages, contributing to the viremia that occurs in late HIV-1 disease.
MATERIALS AND METHODS
Tissues, ISH, and Immunohistochemistry.
Lymphoid tissue biopsies obtained with consent from patients with AIDS-defining OI and from HIV-1 seropositive subjects without evidence of OI were fixed in 10% neutral buffered formalin, embedded in paraffin and sectioned. Tissue sections were processed for ISH by using 35S-labeled sense and antisense probes for HIV-1 RNA (8) and TNFα (Molecular Histology, Gaithersburg, MD). Deparaffinized sections were washed in Tris buffered saline, preincubated for 10 min with blocking buffer, and incubated with primary antibodies: HAM56 (macrophage-specific), CD68 (KP-1, macrophage-associated), S100 protein, p55 (actin-bundling protein), p24 UCHL/CD45RO (T cells) (Dako), and CCR5 (18). Antibodies for α-1-antitrypsin, α-1-antichymotrypsin and lysozyme were obtained from Dako. The secondary antibodies were visualized with New Fuchsin or peroxidase (8). For colocalization studies, ISH was performed subsequent to immunohistochemical (IHC) staining. For acid fast identification of M. avium, the tissues were first hybridized and developed and Kinyoun’s stain was applied to the emulsion-coated slides (8).
HIV-1 Infection of Monocytes.
Human peripheral blood cells, obtained by leukapheresis of normal volunteers (Department of Transfusion Medicine, National Institutes of Health), were density sedimented, and the monocytes in the mononuclear cell layer were purified by elutriation (19, 20). Monocytes (1.5 × 106/ml) in DMEM (BioWhittaker) with 2 mM l-glutamine and 50 μg/ml gentamicin were plated in 48-well plates (Corning Costar). After adherence (4–6 hr at 37°C), 10% human AB− serum (Department of Transfusion Medicine) was added to the cultures. Cells were cultured 7–10 days before being infected with HIV-1BaL (Advanced Biotechnologies, Columbia, MD) for 60 min at 37°C (21–23). Unbound virus was removed by washing the cells with PBS and refeeding with 0.5 ml DMEM containing 10% fetal calf serum, gentamicin, and l-glutamine (complete medium). Every 3 to 4 days, 0.25 ml medium was removed for virus assay and replaced with fresh complete medium. Supernatant p24 antigen was assayed by using the p24 core profile ELISA kit from DuPont (23).
Assay of Virus Binding to Monocyte Surface.
Approximately 5 × 106 monocytes in suspension or as adherent monolayers were pretreated or not with MAg (10 μg/ml, overnight) or TNFα (1 ng/ml, 4 hr). HIV-1BaL [1 × 103 or 4 × 103 tissue culture 50% infective dose (TCID50)/ml] was added for 30 min at 37°C in 1 ml DMEM (23). Cells were pelleted, washed twice in PBS, and resuspended in 0.5% Triton X-100. Virus in the lysate was quantified by p24 ELISA.
MAC and MAC Antigen (MAg) Preparation.
M. avium was grown under standard conditions (24) and added to adherent macrophage monolayers at a ratio of 5:1. Mycobacterial antigen (MAg) was made by freeze-thaw lysis of M. avium at 108 colony-forming unit/ml. After three cycles, the lysate was disrupted by three 10-sec pulses of a probe sonicator (Heat Systems, Farmingdale, NY). The protein concentration was determined by bicinchoninic acid assay (25), adjusted to 2.5 mg/ml, and added to the cultures as indicated. M. avium cell wall glycolipid lipoarabinomannan (LAM) was a generous gift from D. Chatterjee (Colorado State University, Fort Collins) (26).
Extraction of RNA and Northern Hybridization.
Total cellular RNA was extracted from nonadherent control or stimulated monocytes by using the RNeasy minikit (Qiagen, Chatsworth, CA). The RNA samples (5 μg) were subjected to formaldehyde-agarose gel electrophoresis and transferred in 10× standard saline citrate to Nytran membranes (Schleicher & Schuell). After 80°C for 1 hr, the membranes were prehybridized (4 hr at 42°C) and hybridized with DNA probes: TNFα, CCR5, CXCR4 (14, 18, 27) (National Institutes of Health AIDS Research and Reference Reagent Program), and glyceraldehyde-3-phosphate dehydrogenase (GIBCO/BRL). After washing, the hybridized filters were exposed to phosphor screens and analyzed with a PhosphorImager (Molecular Dynamics).
Electrophoretic Mobility-Shift Assay.
Adherent macrophages were treated with MAg (1–25 μg/ml) for 30 min and nuclear extracts prepared (28). The NF-κB consensus oligonucleotide probe (Promega) was end-labeled with [γ32P] ATP by using T4 polynucleotide kinase. The binding reactions (25 μl) consisted of incubating 3 μg of nuclear extract with reaction buffer (10 mM Tris, pH 7.5/1 mM DTT/1 mM EDTA/0.08 mg/ml salmon sperm DNA/4% glycerol) adjusted to 0.1 M NaCl, with 0.05 pmol of DNA probe for 30 min. The binding reactions were run on nondenaturing 6% polyacrylamide gels (NOVEX, San Diego) in .25× Tris/borate/EDTA buffer (25 mM Tris/25 mM boric acid/0.5 mM EDTA, pH 8.3) that were dried and analyzed with a PhosphorImager using imagequant software (Molecular Dynamics).
Analysis of CCR5 Expression.
Monocytes were incubated in suspension with or without MAg, LAM, or lipopolysaccharide (Escherichia coli 055:B5, Difco) at 37°C overnight and stained with polyclonal anti-CCR5 generated against a synthetic peptide derived from the N-terminal region of CCR5 at 10 μg/ml (18) or with anti-CXCR4 (PharMingen). Goat or donkey anti-rabbit or goat anti-mouse IgG-conjugated with fluorescein isothiocyanate (Jackson ImmunoResearch) was used at 1:100 as secondary antibody for flow cytometry analysis using a FACScan (Becton Dickinson). CD4+ T lymphocytes prepared by elutriation were stimulated with MAg (0.1–10 μg/ml) in parallel, stained for CCR5 and analyzed by fluorescence-activated cell sorter (FACS).
RESULTS
Enhanced HIV-1 Replication in Tissues and Cells Coinfected with MAC.
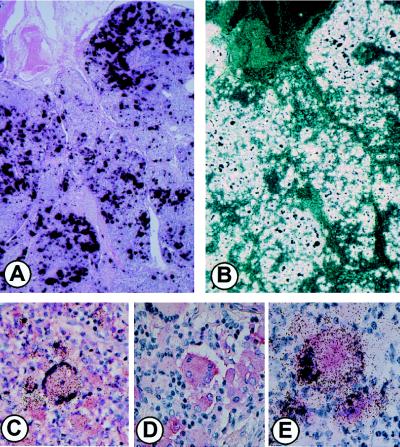
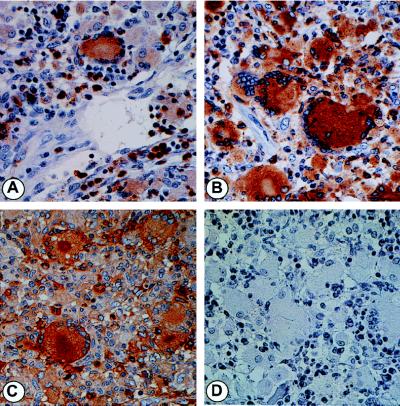
MAC-infected lymph nodes from patients with disseminated disease express large amounts of HIV-1-specific RNA as detected by parallel bright- and dark-field epifluorescence microscopy (Fig. 1 A and B) of ISH preparations (8). The cells producing the large quantities of HIV-1 were shown by ISH, combined with IHC, to be consistent with Langhans’-type MNGC and their MNC counterpart and to be coinfected with MAC (8). Scattered T lymphocytes, detected by CD45RO staining, were also positive for HIV-1 by ISH. To further characterize the population(s) of cells producing the copious amounts of virus in these tissues, we assessed additional phenotypic markers. Unexpectedly, MAC-infected and uninfected HIV-1+ MNC and MNGC variably expressed both S100 and p55, markers traditionally associated with DC/LC (Fig. 1 C and D) (29). Colocalization of p24 in cells expressing HIV-1 RNA by ISH confirmed their productive viral status (Fig. 1E). Although expressing DC/LC markers, these HIV-1+, MAC-infected MNGC and MNC stained positively for the myeloid, Golgi-associated markers, CD68, HAM56, lysozyme, α-1-antitrypsin and α-1-antichymotrypsin (Fig. 2).
Figure 1.
Characterization of HIV-1+ cells. Tissues from patients coinfected with MAC and HIV were hybridized with the antisense probe for HIV RNA together with IHC as described (8). (A) Brightfield and (B) dark-field images of the same field after ISH. (C) Combined IHC for S100 and ISH for HIV RNA. (D) IHC staining for p55. (E) Colocalization of p24 antigen by IHC and HIV RNA by ISH.
Figure 2.
Phenotypic identification of MNC and MNGC in coinfected LN. IHC characterization of cells for lysozyme (A), CD68 (B) and α1-antichymotrypsin (C). (D) Staining without the primary antibodies was negative.
In addition to phenotypic markers consistent with macrophages, the HIV-1 host cells were highly phagocytic and lytic, containing MAC within vacuoles and at various stages of digestion within secondary lysosomes as detected by transmission electron microscopy (ref. 8, not shown). Also typical of monocyte/macrophages, the cells had eccentric, moderately pleomorphic nuclei, abundant Golgi, and complex plasma membranes with stretches of subplasmalemmal linear densities and many pinocytic vesicles. Neither Birbeck granules nor long delicate “dendrite”-like processes associated with DC/LC were evident. Thus, by multiple morphologic and immunophenotypic parameters, the cells expressing HIV-1 in the OI tissues reflect a myeloid lineage.
Up-Regulation of CCR5 Expression in Monocytes by MAC.
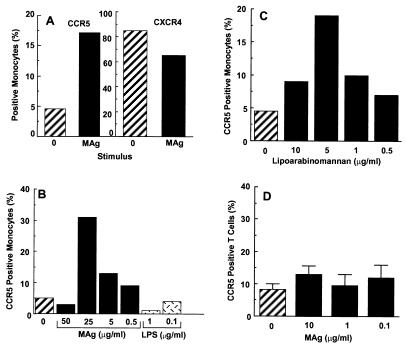
Documentation of the impact of OI on viral replication, particularly in macrophages, prompted exploration of a potential mechanistic pathway that may contribute to the enhanced production of HIV-1. Because CCR5 expression is a requisite for HIV-1 infection of macrophages, we explored a potential link between MAC and CCR5. In response to MAg, a 2- to 6-fold increase in CCR5 positive monocytes occurred as determined by FACS analysis (Fig. 3), whereas MAg did not enhance CXCR4, a coreceptor for T-tropic HIV-1 (Fig. 3A). Based on the MAg dose response (Fig. 3B), 5–25 μg/ml provided optimal induction of cell surface CCR5, whereas higher concentrations were ineffective and/or inhibited CCR5 expression. By comparison, lipopolysaccharide that activates many monocyte/macrophage functions did not enhance CCR5 expression, as reported (30). To establish whether purified M. avium LAM could independently trigger increased CCR5, LAM at concentrations from 0.5–10 μg/ml was added to monocyte cultures for 18 hr and CCR5 expression monitored. As evident in Fig. 3C, LAM at 5 μg/ml induced a 3- to 4-fold increase in FACS-detected monocyte CCR5. In contrast to monocytes, T lymphocytes treated with MAg did not up-regulate cell surface CCR5 expression (Fig. 3D).
Figure 3.
M. avium enhances monocyte CCR5 expression. (A) Nonadherent monocytes were exposed to MAg for 18–24 hr, and analyzed for CCR5 or CXCR4 by FACS. B, C) Monocytes were stimulated with increasing concentrations of (B) MAg (0.5–50 μg/ml), lipopolysaccharide (0.1–1 μg/ml) or (C) LAM (0.5–10 μg/ml) for 18 hr before assessment of CCR5 expression. (D) CD4+ T cells were exposed to MAg (0.1–10 μg/ml) for 18 hr before FACS analysis for CCR5. Data represent mean ± SE for three separate experiments.
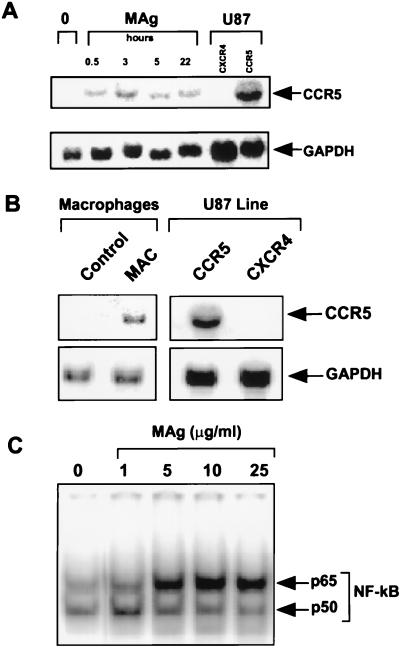
By 30 min after exposure of the cells to MAg, mRNA encoding CCR5 was significantly elevated, increased further at 3–5 hr, and remained above baseline at 22 hr (Fig. 4A). CCR5-specific mRNA was also augmented in adherent macrophages after actual infection with the mycobacteria (Fig. 4B). The rapidity of the MAg response and the recent evidence for a NF-κB binding site in the CCR5 promoter (31) prompted assessment of whether MAg influenced NF-κB activation. Monocytes were cultured in the presence or absence of MAg and NF-κB activation was monitored by electrophoretic mobility-shift assay. NF-κB activation occurred within 30 min after exposure to MAg in vitro (Fig. 4C), consistent with the early increase in CCR5 mRNA.
Figure 4.
M. avium augments CCR5 mRNA and NF-κB in monocytes. (A) RNA isolated from control and MAg-stimulated monocytes was probed for CCR5 and glyceraldehyde-3-phosphate dehydrogenase. RNA from U87 cells transfected with CCR5 or CXCR4 (provided by Carl Wild, BBI-Biotech Research Laboratories, Rockville, MD), were positive and negative controls. (B) Adherent macrophages were infected with viable M. avium (5:1) for 14 days and RNA probed for CCR5 mRNA. (C) By electrophoretic mobility-shift assay, nuclear extracts from MAg-stimulated (30 min) adherent macrophages were analyzed by using a NF-κB consensus probe. Representative of three experiments.
MAg Augments HIV-1 Binding and Infection.
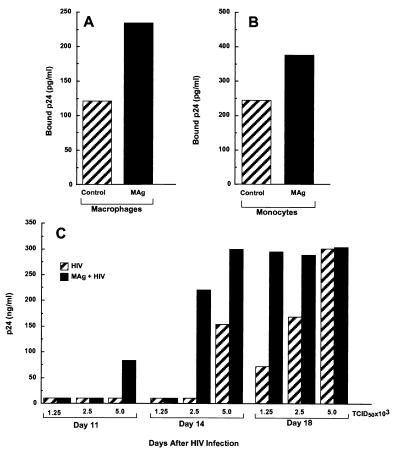
Because MAg augmented CCR5 expression, subsequent studies addressed the impact of this receptor modulation on HIV-1 binding and/or infection. Adherent macrophages or monocytes in suspension were treated with MAg, exposed to HIV-1 for 30 min, washed repetitively, and lysed for quantification of cell bound HIV-1 determined by p24 in the lysate (23). A nearly 2-fold increase in bound p24 was observed for adherent macrophages that had been exposed to MAg (TCID50 = 1 × 103) (Fig. 5A). For unstimulated monocytes in suspension, no detectable binding occurred at TCID50 = 1 × 103, although p24 levels were detected in the MAg-treated cells (<50 pg/ml, limit of detection vs. 64 pg/ml). When the concentration of HIV-1 was increased 4-fold (TCID50 = 4 × 103), p24 binding was detectable in control monocytes and elevated in MAg-treated cells (Fig. 5B). These data are consistent with the evidence that M. avium increases CCR5 to facilitate HIV-1 binding, and supports a role for MAC in increasing monocyte/macrophage vulnerability to HIV-1.
Figure 5.
M. avium increases HIV-1 binding and replication. Adherent macrophages (A) or monocytes in suspension (B) were stimulated with 25 μg/ml MAg overnight, washed, and exposed to HIV-1 at TCID50 = 1 × 103 (A) or TCID50 = 4 × 103 (B) for 30 min. The cells were washed, lysed, and p24-monitored by ELISA. (C) Adherent macrophages were pretreated with MAg (25 μg/ml) and then infected with increasing concentrations (1.25–5 × 103 TCID50) of HIV-1. After 1 hr, the unbound virus was washed away and the cells cultured for 18 days. Supernatants were collected at 3–5-day intervals and tested for p24 by ELISA.
In the next series of experiments, macrophages were exposed to MAg, HIV-1 was added to the cultures at increasing TCID50, and HIV-1 production was monitored over time. Data are shown beginning at day 11 when initial evidence of replication occurs through day 18 when optimal p24 levels are routinely observed (Fig. 5C). At the lowest viral inoculum, no p24 was detected on day 11, whereas at the highest viral concentration, significant p24 was detected only in cultures previously exposed to MAg. This pattern persisted on day 14, when at the intermediate TCID50, HIV-1 replication occurred only in MAg-treated cultures. At the highest viral inoculum, HIV-1 production was evident in the absence of MAg, but the levels were markedly increased with MAg. By day 18, viral replication becomes maximal at TCID50 = 5 × 103, irrespective of MAg, and masking MAg-mediated differences, although at the lower viral levels, MAg effectively augmented p24 levels. The ability of MAg to increase CCR5 expression, thereby augmenting infection, especially at the lower doses of viral challenge, appears to promote earlier and elevated levels of HIV-1 expression.
Augmented TNFα Production in Vitro and in Coinfected Tissues.
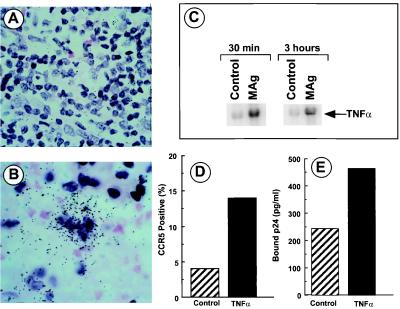
The sustained ability of MAg to augment viral production in macrophage cultures implicated a mechanism(s) beyond increased binding/internalization via CCR5 receptors. One potential mechanism considered was the ability of M. avium to induce production of a regulatory cytokine(s) such as TNFα (26). Based on the in vitro evidence for a role of TNFα in regulating HIV-1 replication (16, 32), the tissues were assayed for TNFα mRNA expression by ISH. As is evident in Fig. 6, TNFα mRNA was detected in coinfected lymph node (LN), in contrast to HIV-1-only infected tissues. Moreover, exposure of monocytes in vitro to M. avium triggered a rapid and transient increase in both TNFα mRNA (Fig. 6C) and protein consistent with the presence of this cytokine in the MAC-infected tissues of AIDS patients (Fig. 6B) and with enhanced viral replication. Based on these data, we exposed monocytes to TNFα, and determined that TNFα could also augment CCR5 mRNA (not shown) and cell surface CCR5 expression (Fig. 6D) to increase HIV-1 binding as monitored by p24 levels (Fig. 6E).
Figure 6.
MAg stimulates TNFα expression. LN from HIV-1-infected (A) and HIV-1 plus MAC-infected (B) subjects were processed for ISH with a TNFα antisense probe. Sense probes were negative. (C) Monocytes were stimulated in vitro with MAg (25 μg/ml) and the isolated RNA probed for TNFα by Northern hybridization. (D) Monocytes were exposed to TNFα (10 ng/ml) overnight before FACS analysis for CCR5. (E) Monocytes were treated with TNFα for 4 hrs and exposed to HIV-1 at TCID50 = 4 × 103 for 30 min. Cells were washed, lysed, and assayed for p24 levels by ELISA.
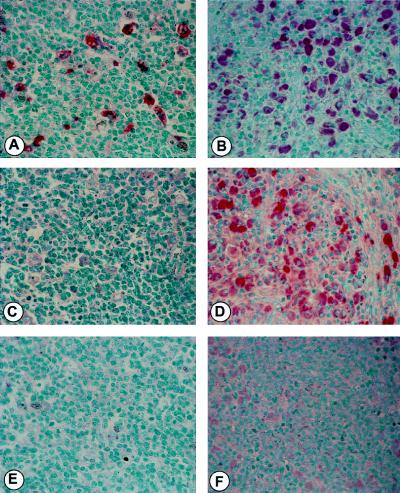
Increased CCR5 Expression in LN.
Based on the augmentation of TNFα and CCR5 HIV-1 coreceptor expression induced by MAC in vitro, we reevaluated LN biopsies from HIV-1+ subjects with and without MAC infection for CD68 (macrophage specific) and CCR5. A marked increase in CD68+ macrophages was observed in the coinfected tissues as compared with those only infected with HIV-1 (Fig. 7 A and B), suggesting active recruitment. Parallel staining revealed large numbers of intensely stained CCR5 positive cells in coinfected tissues, in contrast to the virally-infected nodes lacking MAC, in which paler staining of the lesser numbers of macrophages made them difficult to detect (Fig. 7 C and D). Background staining also appeared to be associated with the intracellular microorganisms. Taken together, these observations reflect both an increase in the number of macrophages and of CCR5+ cells with apparent enhanced receptor density in the coinfected tissues, and support our in vitro evidence linking infection with MAC, up-regulation of CCR5, and enhanced viral replication.
Figure 7.
Increased CCR5 expression in coinfected LN. LN from HIV-1-infected (A, C, and E) or HIV-1 plus MAC-infected (B, D, and F) subjects were assessed by IHC for CD68 (macrophage marker) (A and B) and CCR5 (C and D) expression. E and F represent parallel staining without the primary antibodies.
DISCUSSION
Activation of the immune system, whether by antigen, vaccination, or infection has been associated with increased HIV-1 expression that is detectable both in the peripheral blood and in tissues (reviewed in refs. 5, 8, and 33). Late in HIV-1 disease, when the lymphoid tissues and peripheral blood are markedly depleted of CD4 lymphocytes, not only is the level of circulating HIV-1 maintained, but it is characteristically elevated (17, 33). Our observations suggest that the source of the HIV-1 at this stage includes tissue macrophages. In our studies, macrophages productively-infected with HIV-1 were present in fungal, protozoan, bacterial, and virus-infected tissues (5, 8). Striking production of HIV-1 was observed by tissue macrophages that were in mononuclear, and especially, multinucleated forms. Because the HIV-1-producing macrophages, as well as lymphocytes, need not be themselves infected by pathogens, the up-regulation may occur via paracrine as well as autocrine routes mediated by cytokines. And in fact, we demonstrate that MAC up-regulates TNFα expression, which reportedly can enhance HIV-1 replication (9, 16, 17, 32).
In systems where it has been analyzed, the mechanism of TNFα up-regulation of HIV-1 appears to involve NF-κB (32, 34), and OI-induced TNFα may promote increased transcription of viral long terminal repeat via this pathway (35, 36). Moreover, we now demonstrate that, in addition to TNFα, M. avium activates NF-κB to foster viral replication. Not only may NF-κB promote viral replication, but the recent evidence for NF-κB binding sites in the CCR5 promoter (31), provides an intracellular pathway by which both TNFα and MAg influence coreceptor expression and viral entry. In this regard, MAg augmented NF-κB and CCR5 mRNA within minutes followed by cell surface CCR5 expression.
MAg does not appear to influence T cell CCR5 expression, nor is the response in macrophages triggered by all stimuli. For example, lipopolysaccharide does not enhance, and may even inhibit CCR5 on macrophages (Fig. 3B, ref. 30). As CCR5 is an initial determinant of virus susceptibility (37, 38), these M. avium mediated events likely promote HIV-1 entry as evidenced by increased HIV-1 binding. Associated with the increases in CCR5 and TNFα, macrophages exposed to MAg in culture exhibit a time- and dose-dependent enhancement of HIV-1 infection/replication. By IHC, we also observed striking increases in CD68+ macrophage numbers in HIV-1/MAC-infected tissues, implicating active recruitment mechanisms, including chemokine production (H.H.-D., unpublished observations), with amplification of the potential viral host population. The increased numbers of CCR5+ cells, also expressing more CCR5, in the lymph nodes of patients coinfected with HIV-1 and MAC provide compelling evidence for a parallel process in vivo. Although cellular activation and cytokines such as TNFα may not play such an important role in the absence of OI (Fig. 6, ref. 40), during coinfection, HIV-1 may be up-regulated by multiple mechanisms including both cytokine and coreceptor levels.
Concerning the cellular origin of the virus in vivo, the MAC-infected MNC and MNGC, regardless of HIV-1 p24 or RNA levels, displayed a complex immunophenotype. In the immunodeficient milieu of AIDS, these cells can phagocytize, but do not effectively kill OI such as MAC, M. tuberculosis, Cryptococcus neoformans, Coccidiodes immitis, and Histoplasma capsulatum (5). Morphologically, these cells display features typical of mononuclear phagocytic lineage as opposed to DC/LC. Lacking Birbeck granules, the most specific morphologic marker for DC/LC (41), the MNC and MNGC in the MAC-infected specimens expressed an array of myeloid Golgi-associated markers, as well as S100 protein and p55, generally considered DC/LC markers (29, 41). As p55 is found on DC and is up-regulated by Epstein–Barr virus in B cells (42), perhaps infection with HIV-1 confounded by MAC provides adequate impetus for its expression on myeloid cells. In that both cell types have the same CD34+, bone marrow-derived progenitor cell (43), it is perhaps not surprising that they share phenotypic markers under certain circumstances. Nonetheless, although DC may be susceptible to infection by HIV-1, recent studies conclude that active viral replication does not occur efficiently in these cells (44, 45), thereby redirecting our focus to the macrophages as the viral factories in the coinfected tissues. Characterization of the relevant viral host cell populations is of importance in the development of targeted therapies (9, 21, 22).
MAC represents the most common bacterial opportunist, being diagnosed in up to 40% of AIDS patients (46), with autopsy evidence indicating that the prevalence may be even higher. Our observations are consistent with the evidence and concern over the deleterious side effects of immune activation or vaccination of HIV-1-infected individuals, which can trigger nonspecific stimulation of HIV-1 expression by cells otherwise producing limited amounts of HIV-1 or which are latently infected (5, 8, 48–52). Productively-infected macrophages are infrequently found in LN in the absence of OI; however, greater numbers of cells with replication-competent integrated provirus are present and are likely to be susceptible to immune stimulation-driven viral replication provided by OI (8, 53). Long-lived HIV-1-infected cells in the tissues are also considered major contributors to the second-phase delayed decay of plasma viremia brought on by antiretroviral treatment and may persist for at least 2–3 years after treatment (53, 54). Thus, preventing and controlling immune stimulation and OI, regardless of whether asymptomatic or symptomatic, localized or disseminated, may continue to be an important therapeutic strategy.
Acknowledgments
We thank C. Dye II and S. Honig for technical assistance; Ms. K. Angelis and Ms. C. Bennett for manuscript preparation; and Dr. Cecil Fox (Molecular Histology, Inc.) for ISH. The p.CCR chemokine receptor expression vectors were from the AIDS Research and Reference Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health from Dr. Nathaniel Landau, the transfected U87 cells were generously provided by Dr. Carl Wild (BBI-Biotech Research Laboratories, Rockville, MD) and the M. avium LAM by Dr. D. Chatterjee (Colorado State University). This work was supported in part by National Institute of Dental Research Contract DE 12585.
ABBREVIATIONS
- OI
opportunistic infections
- MAC
Mycobacterium avium complex
- MAg
MAC antigens
- TNFα
tumor necrosis factor α ISH, in situ hybridization
- MNC
mononuclear cells
- MNGC
multinucleated giant cells
- DC/LC
dendritic/Langerhans’ cells
- IHC
immunohistochemical
- LAM
lipoarabinomannan
- FACS
fluorescence-activated cell sorter
- TCID50
tissue culture 50% infective dose
- LN
lymph node
References
- 1. Mole L, Ripich S, Margolis D, Holodniy M. J Infect Dis. 1997;176:766–770. doi: 10.1086/517297. [DOI] [PubMed] [Google Scholar]
- 2.Heng M C, Heng S Y, Allen S G. Lancet. 1994;343:255–258. doi: 10.1016/s0140-6736(94)91110-x. [DOI] [PubMed] [Google Scholar]
- 3.Goletti D, Weissman D, Jackson R W, Graham N M H, Vlahov D, Klein R S, Munsiff S S, Ortona L, Cauda R, Fauci A S. J Immunol. 1996;157:1271–1278. [PubMed] [Google Scholar]
- 4.Whalen C, Horsburgh C R, Horn D, Lahart C, Simberkoff M, Ellner J. Am J Respir Crit Care Med. 1995;151:129–135. doi: 10.1164/ajrccm.151.1.7812542. [DOI] [PubMed] [Google Scholar]
- 5.Wahl S M, Orenstein J M. J Leukocyte Biol. 1997;62:67–71. doi: 10.1002/jlb.62.1.67. [DOI] [PubMed] [Google Scholar]
- 6.Donovan R M, Bush C E, Markowitz N P, Baxa D M, Saravolatz L D. J Infect Dis. 1996;174:401–403. doi: 10.1093/infdis/174.2.401. [DOI] [PubMed] [Google Scholar]
- 7.Denis M, Ghadirian E. Clin Exp Immunol. 1994;97:76–82. doi: 10.1111/j.1365-2249.1994.tb06582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orenstein J M, Fox C, Wahl S M. Science. 1997;276:1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 9.Wahl S M, Orenstein J M, Smith P D. In: Immunology of HIV Infection. Gupta S, editor. New York: Plenum; 1996. pp. 303–336. [Google Scholar]
- 10.Frankel S S, Tennar-Racz K, Racz P, Wenig B M, Hansen C H, Heffner D, Nelson A M, Pope M, Steinman R M. Am J Pathol. 1997;151:89–96. [PMC free article] [PubMed] [Google Scholar]
- 11.Frankel S S, Wenig B M, Burke A P, Mannan P, Thompson L D R, Abbondanzo S L, Nelson A M, Pope M, Steinman R M. Science. 1996;272:115–117. doi: 10.1126/science.272.5258.115. [DOI] [PubMed] [Google Scholar]
- 12.Mosialos G, Birkenbach M, Ayehunie S, Matsumura F, Pinkus G S, Kieff E, Langhoff E. Am J Pathol. 1996;148:593–600. [PMC free article] [PubMed] [Google Scholar]
- 13.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 14.Deng H K, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, DiMarzio P, Marmon S, Sutton R E, Hill C M, et al. Nature (London) 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 15.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, et al. Nature (London) 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 16.Mellors J W, Griffith B P, Ortiz M A, Landry M L, Ryan J L. J Infect Dis. 1991;163:78–82. doi: 10.1093/infdis/163.1.78. [DOI] [PubMed] [Google Scholar]
- 17.Fauci A S. Nature (London) 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 18.Peng, G., Hale-Donze, H. Wild, T., Orenstein, J. M. & Wahl, S. M. (1998) 5th Conference on Retrovirus and Opportunistic Infections, February 1–5, 1998, Chicago, IL, p. 86.
- 19.Wahl L M, Katona I M, Wilder R L, Winter C C, Haraoui B, Sher I, Wahl S M. Cell Immunol. 1984;85:373–383. doi: 10.1016/0008-8749(84)90251-x. [DOI] [PubMed] [Google Scholar]
- 20.Wahl S M, McCartney-Francis N, Hunt D A, Smith P D, Wahl L M, Katona I M. J Immunol. 1987;139:1342–1347. [PubMed] [Google Scholar]
- 21.Finberg R W, Wahl S M, Allen J B, Soman G, Strom T B, Murphy J R, Nichols J C. Science. 1991;252:1703–1705. doi: 10.1126/science.1904628. [DOI] [PubMed] [Google Scholar]
- 22.McNeely T B, Dealy M, Dripps D J, Orenstein J M, Eisenberg S P, Wahl S M. J Clin Invest. 1995;96:456–464. doi: 10.1172/JCI118056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McNeely T B, Shugars D C, Rosendahl M, Tucker C, Eisenberg S P, Wahl S M. Blood. 1997;90:1141–1149. [PubMed] [Google Scholar]
- 24.Doherty T M, Sher A. J Immunol. 1997;158:4822–4831. [PubMed] [Google Scholar]
- 25.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Anal Biochem. 1985;150:76–80. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 26.Chatterjee D, Khoo K H. Glycobiology. 1998;8:113–120. doi: 10.1093/glycob/8.2.113. [DOI] [PubMed] [Google Scholar]
- 27.Morgenstern J P, Land H. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dickensheets H L, Donnelly R P. J Immunol. 1997;159:6226–33. [PubMed] [Google Scholar]
- 29.Wright-Browne V, McClain K L, Ordonez T M, Estrov Z. Hum Pathol. 1997;28:563–579. doi: 10.1016/s0046-8177(97)90079-4. [DOI] [PubMed] [Google Scholar]
- 30.Sica A, Saccani A, Borsatti A, Power C A, Wells T N C, Luini W, Polentarutti N, Sozzani S, Mantovani A. J Exp Med. 1997;185:969–974. doi: 10.1084/jem.185.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guignard F, Combadiere C, Tiffany H L, Murphy P M. J Immunol. 1998;160:985–992. [PubMed] [Google Scholar]
- 32.Osborn L, Kunkel S, Nabel G J. Proc Natl Acad Sci USA. 1989;36:2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feinberg M B. Lancet. 1996;348:239–246. doi: 10.1016/s0140-6736(96)06231-9. [DOI] [PubMed] [Google Scholar]
- 34.Gazzinelli R T, Sher A, Cheever A, Gerstberger S, Martin M A, Dickie P. J Exp Med. 1996;183:1645–1655. doi: 10.1084/jem.183.4.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alcami J, de Lera T L, Folgueira L, Pedraza M A, Jacque J M, Bachelerie F, Noriega A R, Hay R T, Harrich D, Gaynor G R, et al. EMBO J. 1995;14:1552. doi: 10.1002/j.1460-2075.1995.tb07141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toossi Z, Hamilton B D, Phillips M H, Averill L E, Ellner J J, Salvekar A. J Immunol. 1997;159:4109–4116. [PubMed] [Google Scholar]
- 37.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, MacKay C R, LaRosa G, Newman W, et al. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 38.Doranz B, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 39.Garrait V, Cadranel J, Esvant H, Herry I, Morinet P, Mayaud C, Israel-Biet D. J Immunol. 1997;159:2824–2830. [PubMed] [Google Scholar]
- 40.Li Q, Gebhard K, Schacker T, Henry K, Haase A T. J Virol. 1997;71:7080–7082. doi: 10.1128/jvi.71.9.7080-7082.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chu T, Jaffe R. Br J Cancer Suppl. 1994;23:S4–S10. [PMC free article] [PubMed] [Google Scholar]
- 42.Pinkus G S, Pinkus J L, Langhoff E, Matsumura F, Yamashiro S, Mosialos G, Said J W. Am J Pathol. 1997;150:543–562. [PMC free article] [PubMed] [Google Scholar]
- 43.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. Nature (London) 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 44.Granelli-Piperno A, Moser B, Pope M, Chen D, Wei Y, Isdell F, O’Doherty U, Paxton W, Koup R, Mojsov S, et al. J Exp Med. 1996;184:2433–2438. doi: 10.1084/jem.184.6.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reinhart T A, Rogan M J, Viglianti G A, Rausch D M, Eiden L E, Hasse A T. Nat Med. 1997;3:218–221. doi: 10.1038/nm0297-218. [DOI] [PubMed] [Google Scholar]
- 46.Horsburgh C R, Metchock B, Gordon S M, Havlik J A, McGowan J E, Thompson S E. N Engl J Med. 1994;170:573–577. doi: 10.1093/infdis/170.3.573. [DOI] [PubMed] [Google Scholar]
- 47.Pape J W, Jean S S, Ho J L, Hafner A, Johnson W D. Lancet. 1993;342:268–272. doi: 10.1016/0140-6736(93)91817-6. [DOI] [PubMed] [Google Scholar]
- 48.O’Brien W A, Grovit-Ferbas K, Namizi A, Zhao J Q, Diagne A, Idler K, Zack J A, Chen I S Y. Blood. 1995;86:1082–1089. [PubMed] [Google Scholar]
- 49.Staprans S I, Hamilton B L, Follansbee S E, Elbeik T, Barbosa P, Grant R M, Feinberg M B. J Exp Med. 1995;182:1727–1737. doi: 10.1084/jem.182.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stanley S K, Ostrowski M A, Justement J S, Gantt K, Hedayati S, Mannix M, Roche K, Schwartzentruber D J, Fox C H, Fauci A S. N Engl J Med. 1996;334:1222–1230. doi: 10.1056/NEJM199605093341903. [DOI] [PubMed] [Google Scholar]
- 51.Brichacek B, Swindells S, Janoff E N, Pirruccelo S, Stevenson M. J Infect Dis. 1996;174:1191–1199. doi: 10.1093/infdis/174.6.1191. [DOI] [PubMed] [Google Scholar]
- 52.Cohen M S, Hoffman I F, Royce R A, Kazembe P, Dyer J R, Daly C C, Zimba D, Vernazza P L, Maida M, Fiscus S A, et al. Lancet. 1997;349:1868–1873. doi: 10.1016/s0140-6736(97)02190-9. [DOI] [PubMed] [Google Scholar]
- 53.Chun T W, Carruth L, Finzi D, Shen X, DiGiuseppe J A, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn T C, et al. Nature (London) 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 54.Perelson A S, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho D D. Nature (London) 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]