Abstract
Objective To determine the effectiveness of chemoprophylaxis using a single dose of rifampicin to prevent leprosy in close contacts.
Design Single centre, double blind, cluster randomised, placebo controlled trial.
SettingLeprosy control programme in two districts of northwest Bangladesh with a population of more than four million.
Participants28 092 close contacts of 1037 patients with newly diagnosed leprosy. 21 711 contacts fulfilled the study requirements.
Interventions A single dose of rifampicin or placebo given to close contacts in the second month of starting the index patient’s treatment, with follow-up for four years.
Main outcome measure Development of clinical leprosy.
Results 18 869 of the 21 711 contacts (86.9%) were followed-up at four years. Ninety one of 9452 contacts in the placebo group and 59 of 9417 in the rifampicin group had developed leprosy. The overall reduction in incidence of leprosy using a single dose of rifampicin in the first two years was 57% (95% confidence interval 33% to 72%). The groups did not differ between two and four years. The overall number needed to treat (NNT) to prevent a single case of leprosy among contacts was 297 (95% confidence interval 176 to 537). Differences were found between subgroups at two years, both in reduction of incidence and in NNT.
ConclusionA single dose of rifampicin given to contacts of patients with newly diagnosed leprosy is effective at preventing the development of clinical leprosy at two years. The effect was maintained, but no difference was seen between the placebo and rifampicin groups beyond two years.
Trial registration Current Controlled Trials ISRCTN61223447.
Introduction
For more than 60 years it has been known that close contacts of patients with leprosy have an increased risk of contracting the disease.1 The risk of a contact developing clinical leprosy is related to the physical and genetic distance to the index patient, the age of the contact, and the classification of the index patient’s disease.2
Since the early 1940s dapsone was the treatment of choice for leprosy but was replaced in the early ’80s by multidrug therapy—a combination of dapsone, clofazimine, and rifampicin. Before multidrug therapy became the standard treatment for leprosy, randomised controlled trials using dapsone or acedapsone investigated whether these drugs could prevent leprosy among contacts.3 4 A meta-analysis of the studies on chemoprophylaxis with prolonged administration of these drugs estimated an overall efficacy of about 60%.5 The efficacy in household contacts ranged from 34-54%,3 6 whereas in the community intervention trial it was 91%.7 The disadvantages of dapsone as a chemoprophylactic agent are the development of drug resistance and the lack of patient compliance because of the need for administration over a long period. Therefore newer drugs were considered and rifampicin was a logical choice because of its strong bactericidal effect against Mycobacterium leprae, the micro-organism causing leprosy. This drug was expected to have at least a similar prophylactic effect to dapsone but with fewer doses and shorter duration of administration. A protective efficacy of 40-50% with rifampicin was reported in an uncontrolled trial.8 9
Recently an unblinded study on five Indonesian islands used two doses of rifampicin, given with an interval of about 3.5 months.10 Three populations were compared: a “blanket group” consisting of the population of three small islands on which prophylaxis was given to all eligible people, a “contact group” consisting of the population of one island on which prophylaxis was given only to contacts living in the same household or less than 50 m away, and a “control group” consisting of the population of another island on which no prophylaxis was given. In the blanket group, chemoprophylaxis was associated with a 74.6% reduction in incidence of leprosy, at least during the three years after implementation. In the population where only household members and neighbours received prophylaxis no reduction was observed.
A large scale, double blind, placebo controlled trial—the prospective (sero-) epidemiological study on contact transmission and chemoprophylaxis in leprosy (COLEP)—was started in northwest Bangladesh in 2002, using a single dose of rifampicin as chemoprophylaxis. The methodology of this trial and the analysis of the intake data have been reported.2 11 We present the results of the analysis after two and four years of follow-up.
Methods
The COLEP study was carried out in the districts of Rangpur and Nilphamari, northwest Bangladesh, with a population of more than four million people. We excluded two subdistricts where the leprosy control services were provided by organisations other than the Danish Bangladesh Leprosy Mission. Eligible participants (patients and contacts) were informed verbally about the study and invited to participate. Written consent was obtained from all participants at recruitment or from the parent or guardian of under 18s. The study population consisted of close contacts of 1037 patients with newly diagnosed leprosy who were willing to participate. Leprosy was diagnosed when at least one of the cardinal signs was present—one or more skin lesions consistent with leprosy and with definite sensory loss, thickened peripheral nerves, and a positive skin smear result for acid-fast bacilli.
We grouped patients with negative smear results at all sites and who had no more than five skin lesions as having paucibacillary leprosy, and those showing positive smear results at any site or who had more than five skin lesions as having multibacillary leprosy. Within the paucibacillary group we classified those with only one lesion as having single lesion paucibacillary disease. As single lesion disease is the most common form of leprosy in this area and because we preferred to include a sufficient number of patients in all categories, we limited the number of patients with single lesion disease to 400. Of these we later reclassified 11 on the basis of the skin smear results at intake or the initially recorded clinical symptoms, totalling 389 patients with single lesion paucibacillary disease. Three hundred and fifty three patients had paucibacillary leprosy with two to five lesions and 295 had multibacillary leprosy.
The intake of contacts started in June 2002 and was completed by the end of December 2003. We categorised contacts according to their physical and genetic distance to the index patient. For physical distance we defined six categories on the basis of the local housing situation: shares a house and kitchen, shares a kitchen only, shares a house but not kitchen, next door neighbours, neighbours of the neighbours, and social contacts (business contacts and colleagues staying in the same room for at least four hours a day, five days a week).
During the intake phase it seemed that only a small proportion of the social contacts satisfied the criteria for physical distance, and most were in fact neighbours of the contacts who were classed as neighbours of the neighbours. We therefore pooled these two groups in the analysis. As only a small proportion of contacts were sharing a house but not a kitchen we pooled these with contacts who were next door neighbours.
For genetic distance we initially defined seven categories,2 11 but for analysis we combined these categories to form two groups—closely related (parent, child, or sibling) and not closely related (all others).
We excluded contacts when they refused informed consent, were pregnant, were receiving treatment for tuberculosis or leprosy during intake, had leprosy (previously undiagnosed) at intake, were younger than 5 years, were known to have liver disease or jaundice, or were residing only temporarily in the area. Contacts could only be included in the contact group of one patient.
We took finger prick blood samples from index cases during intake and from contacts during intake and follow-up to test for antibodies to M leprae specific phenolic glycolipid-I. Samples were collected on Schleicher and Schuell blotting paper GB 002, dried, and stored at −20°C until transport to the Netherlands. An enzyme linked immunosorbent assay (ELISA) for the detection of IgM antibodies to phenolic glycolipid-I was done according to established procedures.12 The antigen used was natural trisaccharide linked to bovine serum albumin via a phenolic ring, a semisynthetic analogue containing the M leprae specific terminal trisaccharide moiety of phenolic glycolipid-I.
Objective
We hypothesised that, firstly, transmission of M leprae from the index patient to contacts would take place before the diagnosis and start of treatment in the index case, and, secondly, that a single dose of rifampicin would be effective in eradicating small numbers of M leprae that were possibly present in the contacts. In this way rifampicin could be effective as a measure to prevent clinical leprosy among close contacts of patients with leprosy. Our objective was to determine the effectiveness of chemoprophylaxis using a single dose of rifampicin to prevent leprosy in close contacts.
We formed subgroups of contacts according to their contact status, age, genetic relation to the index patient, sex, disease classification of the index patient, presence of a BCG scar, and serological status.
Outcome
The primary outcome was the development of clinical leprosy. A leprosy control officer and a medical officer, who also made a digital photograph of the lesions for future reference, confirmed the disease of every patient with newly found leprosy. The health professionals confirming the diagnosis had a minimum of five years’ experience in the diagnosis of leprosy at referral centre level.
Intervention
At intake—that is, after the index patient had received the second supervised dose of multidrug therapy—all contacts of one patient received treatment from the same numbered container, which included either capsules with 150 mg rifampicin or identical placebo capsules without an active (antibiotic) ingredient. The number on the container was the same as the central registration number of the index case. According to bodyweight and age, each contact took two to four capsules under direct supervision of a staff member. The dosage schedules were 600 mg for adults weighing 35 kg and over, 450 mg for adults weighing less than 35 kg and for children older than 9 years, and 300 mg for children aged 5 to 9 years.
Randomisation
Randomisation of the rifampicin or placebo containers was done by computerised methods by the database designer (RF) in Rotterdam, the Netherlands. In this way the randomisation was at contact group (cluster) level. The codes were kept locked away in Rotterdam and could only be accessed by the database designer. The number on the container was identical to the registration number of the index patient, and the numbering followed the order of inclusion.
Follow-up
Two follow-up investigations took place. The first started two years after intake, in June 2004, and was completed in February 2006; the second started four years after intake, in June 2006, and was completed at the end of October 2007. The follow-ups followed the sequence of recruitment to achieve a uniform follow-up period of 48 months. During the visits we examined as many contacts as possible and if we could not see them all we made an appointment for a second visit to assess the missed contacts. If we could still not get in touch with the contacts we asked relatives to notify them about coming to the clinic for examination. If contacts had moved within reasonable distance, the field staff tried to trace them at their new address.
If leprosy was diagnosed we recorded the date of official registration. So as not to miss any new cases emerging from the contact groups we scanned the main central registry and listed per clinic all patients found during the two years between intake and follow-up visits. We sent these lists to the clinics and asked if they could check if any of the patients was a study contact.
Blinding
As only the database designer in Rotterdam had access to the treatment codes, the participants, the field and hospital staff, and the primary researchers were blinded. The total follow-up period of the trial was 48 months. To avoid compromising the double blind design because of the mid-term analysis after 24 months’ follow-up, we gave the file with the data of all contacts in the trial to the database designer. He was asked to merge this file with his file of the treatment codes. The combined file was given to the statistician (GJJMB) who did the analyses. He was asked not to give the results of a particular analysis to the primary researchers in case one of the numbers would be zero, as this would compromise the blinding of the study. After completion of the 48 months’ follow-up, the codes were broken and the analysis of the trial carried out unblinded.
Statistical analysis
We initially planned analyses at two and four years. If there had been no effect at two years we could have discontinued the trial. This was firstly because an effect beginning after two years was unlikely and, secondly, because an overwhelming effect would call for earlier recommendations for implementation of the intervention in routine leprosy control. This meant that, for the sake of blinding, analyses such as a survival analysis could not be done after the first follow-up period.
The power calculations were based on the total follow-up period of four years. We did not make a separate power calculation for the follow-up after two years. When the proposal and protocol were prepared, we stated that “appropriate analytical procedures” would be used. The protocol version of September 2002 is the final version that the institutional review board approved, and there were no protocol deviations.
We determined that to detect reliably an expected efficacy of intervention of 50%, even taking into account an expected 10-20% loss to follow-up of contacts, we needed 20 000 contacts, 10 000 in each treatment arm. For the power calculation we assumed an incidence rate of 2 per 1000 per year with an expected 50% reduction through intervention, α=0.05 two sided, and 0.80 power. We enrolled 21 711 contacts in the trial, divided over 1037 clusters.
Statistical analyses were done using SAS software, version 9.1. We used techniques for the analysis of survey samples to account for the clustering at the level of the index patient in the sample. Bivariate associations were investigated using “proc surveyfreq” and the Rao Scott χ2 instead of the Pearson χ2. Also we used “proc surveylogistic” instead of the ordinary logistic regression procedure. We report odds ratios, but because of the low prevalence of the outcome these are comparable with relative risks. The number needed to treat (NNT) was calculated per subgroup of contacts. A significance level of 5% was used in all tests. We converted the probabilities of having developed leprosy during the follow-up period of two years to incidence rates at one year assuming a constant hazard during the period (rate=log (1-leprosy/total)/2). To obtain confidence intervals we applied standard errors for the probability (sqrt (1/leprosy+1/no leprosy) around the log (rate).
Results
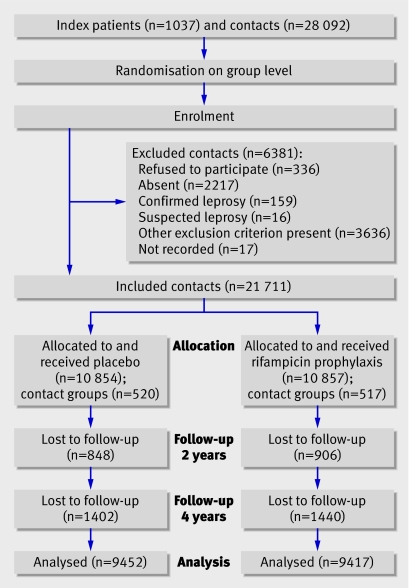
The figure shows the numbers of participants in each group. Overall, 28 092 eligible contacts were identified. Reasons for exclusion were absenteeism (n=2217), refusal to participate (n=336), being a temporary resident (n=131), age under 5 years (n=2970), pregnancy (n=438), liver disease or jaundice (n=51), current treatment for tuberculosis or leprosy (n=42), contact of another patient in the trial (n=4), suspected leprosy (n=16), confirmed leprosy (n=159), and not recorded (n=17). This left 21 711 contacts included in the trial.
Flow of participants through trial
The groups were well balanced for characteristics (table 1). Of the 21 711 contacts included, 20 032 (92.3%) were seen during the first follow-up (over years 1 and 2) and 18 869 (86.9%) during the second follow-up (over years 3 and 4). Among these, 96 new patients with leprosy were found during the first follow-up and another 54 during the second follow-up, totalling 150 new patients with leprosy (table 2). During the first follow-up leprosy had developed in 67 of 10 006 contacts in the placebo group (incidence rate 33.59 per 10 000 person years at risk) and 29 of 9951 contacts in the rifampicin group (14.59 per 10 000 person years at risk). During the second follow-up leprosy had developed in 24 of 9385 contacts in the placebo group (12.80 per 10 000 person years at risk) and 30 of 9388 in the rifampicin group (16.00 per 10 000 person years at risk). Over the four years of observation 91 participants developed leprosy in the placebo group and 59 in the rifampicin group. The reduction in incidence in the rifampicin group was 56.5% (95% confidence interval 32.9% to 71.9%; P=0.0002) in the two years and 34.9% (9.8% to 53.0%; P=0.02) during the four years. The great reduction of new cases in the rifampicin group occurred in the two years after treatment; in years 3 and 4 no statistically significant difference was found between the number of new cases in the groups, although the number was slightly higher in the rifampicin group (30 v 24), which is predominantly determined by more cases of single lesion paucibacillary disease. The overall NNT to prevent one new case of leprosy was 265 (95% confidence interval 176 to 537) after two years and 297 (170 to 1206) after four years.
Table 1.
Characteristics of contacts of patients with newly diagnosed leprosy (n=18 869, 87% of those included) after four years’ follow-up, by treatment allocation. Values are numbers (percentages of total numbers) of contacts
| Variable | Placebo group (n=9452) | Rifampicin group (n=9417) |
|---|---|---|
| Age at intake (years): | ||
| 5-9 | 1497 (7.9) | 1555 (8.2) |
| 10-14 | 1500 (7.9) | 1488 (7.9) |
| 15-19 | 992 (5.3) | 935 (5.0) |
| 20-29 | 1667 (8.8) | 1587 (8.4) |
| ≥30 | 3795 (20.2) | 3852 (20.5) |
| Genetic distance to index patient: | ||
| Closely related* | 1475 (7.8) | 1423 (7.5) |
| Not closely related† | 7977 (42.3) | 7994 (42.4) |
| Male | 4343 (23.0) | 4354 (23.1) |
| Female | 5109 (27.1) | 5063 (26.8) |
| Type of disease in index patient: | ||
| Multibacillary | 2758 (14.6) | 2486 (13.2) |
| Paucibacillary with 2-5 lesions | 2954 (15.7) | 3254 (17.2) |
| Single lesion paucibacillary | 3740 (19.8) | 3677 (19.5) |
| BCG scar absent | 5552 (29.7) | 5576 (29.8) |
| BCG scar present | 3831 (20.5) | 3760 (20.1) |
| Physical distance: | ||
| Shares kitchen and house | 863 (4.6) | 880 (4.7) |
| Shares kitchen only | 687 (3.6) | 684 (3.6) |
| Shares house only plus is next door neighbour | 2645 (14.0) | 2425 (12.9) |
| Neighbour of neighbour plus social contact‡ | 5257 (27.9) | 5428 (28.8) |
| ELISA result§: | ||
| Negative¶ | 8106 (47.0) | 8137 (47.2) |
| Positive** | 515 (3.0) | 494 (2.8) |
ELISA=enzyme linked immunosorbent assay.
*Parent, child, or sibling.
†Other than parent, child, or sibling.
‡Business contacts and colleagues staying in same room at least four hours a day, five days a week.
§Contact population after two years’ follow-up.
¶Optical density <0.2.
**Optical density ≥0.2.
Table 2.
Cases of leprosy in contacts of patients with newly diagnosed leprosy by treatment during four years’ follow-up, with incidence rates per 10<thin>000 person years at risk
| Treatment | Leprosy | No leprosy | Total investigated | Incidence rate per 10<thin> 000 person years at risk (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Single lesion paucibacillary | Paucibacillary with 2-5 lesions | Multibacillary | Total | ||||
| Placebo | |||||||
| Follow-up (years): | |||||||
| 1-2 | 28 | 30 | 9 | 67 | 9939 | 10 006 | 33.59 (26.42 to 42.72) |
| 3-4 | 8 | 11 | 5 | 24 | 9361 | 9385 | 12.80 (8.58 to 19.11) |
| 1-4 | 36 | 41 | 14 | 91 | — | — | |
| Rifampicin | |||||||
| Follow-up (years): | |||||||
| 1-2 | 15 | 10 | 4 | 29 | 9922 | 9951 | 14.59 (10.14 to 21.01) |
| 3-4 | 16 | 8 | 6 | 30 | 9358 | 9388 | 16.00 (11.18 to 22.90) |
| 1-4 | 31 | 18 | 10 | 59 | — | — | |
Overall reduction in incidence in rifampicin group:
During years 1-2, 56.5% (95% confidence interval 32.9% to 71.9%); Rao Scott χ2=13.476 (df=1), P=0.0002; overall number needed to treat 265 (95% confidence interval 176 to 537).
During years 1-4, 34.9% (9.8% to 53.0%); Rao Scott χ2=5.4019 (df=1), P=0.02; overall number needed to treat 297 (170 to 1206).
Table 3 shows the effect of rifampicin prophylaxis by variable category. This analysis is presented for the first two years only because no statistically significant (P<0.05) effect of the intervention was seen in the third and fourth years, either overall or in the individual variable categories (data not shown). Rifampicin seems to be especially effective (odds ratio <0.5, P<0.05) in contacts not closely related to the index patient, in contacts of patients with paucibacillary disease, in contacts with the largest physical distance from the index patient, in females, in seronegative contacts, in those without a BCG like scar, and in the age groups 10-14 and 20-29. The odds ratio and the NNT in the different age groups show a trend that broadly mirrors the trend in incidence over age in the placebo group—that is, the higher the incidence, the less effective prophylaxis seems to be.
Table 3.
Effect of rifampicin prophylaxis in contacts of patients with newly diagnosed leprosy by variable category at two years’ follow-up
| Variable | Follow-up at two years | |||||||
|---|---|---|---|---|---|---|---|---|
| Placebo | Rifampicin | Odds ratio (95% CI)* | P value | NNT† | ||||
| No leprosy | Leprosy | No leprosy | Leprosy | |||||
| Age (years): | ||||||||
| 5-9 | 1552 | 5 | 1608 | 4 | 0.77 (0.21 to 2.87) | 0.6992 | 1370 | |
| 10-14 | 1645 | 12 | 1616 | 2 | 0.17 (0.04 to 0.76) | 0.0205 | 167 | |
| 15-19 | 1107 | 7 | 1042 | 5 | 0.76 (0.24 to 2.39) | 0.6372 | 663 | |
| 20-29 | 1705 | 10 | 1688 | 1 | 0.10 (0.01 to 0.79) | 0.0285 | 191 | |
| ≥30 | 3962 | 33 | 4011 | 17 | 0.51 (0.28 to 0.91) | 0.0237 | 248 | |
| Genetic distance to index patient: | ||||||||
| Closely related‡ | 1585 | 18 | 1507 | 13 | 0.76 (0.35 to 1.65) | 0.4862 | 374 | |
| Not closely related§ | 8386 | 49 | 8458 | 16 | 0.32 (0.18 to 0.57) | <0.0001 | 251 | |
| Male | 4658 | 37 | 4660 | 17 | 0.46 (0.26 to 0.82) | 0.0088 | 247 | |
| Female | 5313 | 30 | 5305 | 12 | 0.40 (0.20 to 0.82) | 0.0120 | 298 | |
| Type of disease in index patient: | ||||||||
| Multibacillary | 2846 | 21 | 2626 | 10 | 0.52 (0.22 to 1.19) | 0.1201 | 283 | |
| Paucibacillary with 2-5 lesions | 3133 | 22 | 3408 | 9 | 0.38 (0.16 to 0.87) | 0.0218 | 230 | |
| Single lesion paucibacillary | 3992 | 24 | 3931 | 10 | 0.42 (0.20 to 0.89) | 0.0233 | 291 | |
| BCG scar absent | 5878 | 52 | 5917 | 22 | 0.42 (0.26 to 0.69) | 0.0007 | 197 | |
| BCG scar present | 4023 | 15 | 3962 | 7 | 0.47 (0.18 to 1.26) | 0.1342 | 513 | |
| Physical distance: | ||||||||
| Shares house and kitchen | 912 | 13 | 924 | 6 | 0.46 (0.15 to 1.38) | 0.1652 | 132 | |
| Shares kitchen only | 730 | 5 | 705 | 7 | 1.45 (0.38 to 5.52) | 0.5863 | NA | |
| Shares house plus is next door neighbour | 2770 | 17 | 2544 | 8 | 0.51 (0.22 to 1.19) | 0.1208 | 337 | |
| Neighbour of neighbour plus social contact | 5559 | 32 | 5792 | 8 | 0.24 (0.11 to 0.52) | 0.0003 | 230 | |
| Negative ELISA result¶ | 8054 | 52 | 8115 | 22 | 0.42 (0.25 to 0.70) | 0.0009 | 269 | |
| Positive ELISA result** | 511 | 4 | 492 | 2 | 0.52 (0.08 to 3.25) | 0.4834 | 269 | |
ELISA=enzyme linked immunosorbent assay.
*Odds ratio for leprosy in rifampicin group versus placebo group.
†Number needed to treat to prevent one case of leprosy.
‡Parent, child, or sibling.
§Other than parent, child, or sibling.
¶Optical density <0.2.
**Optical density ≥0.2.
Discussion
The overall incidence of leprosy among contacts of patients with newly diagnosed disease can be reduced by a single dose of rifampicin. A reduction of 57% was achieved in the two years after treatment but no statistically significant difference was observed between the rifampicin and placebo groups in the third and fourth years after treatment.
The prospective (sero-) epidemiological study on contact transmission and chemoprophylaxis in leprosy (COLEP) study was designed as a single centre, prospective, cluster randomised, double blind, placebo controlled trial to verify results of earlier studies that did not have all of these methodological qualities. The strength of the trial is its robust design and the large number of participants who could be included within a fairly short time because of the relatively high incidence of leprosy in the study area. We cannot be certain that the results are equally applicable to situations where leprosy is less highly endemic, however, although we see no reason to assume otherwise. It must also be kept in mind that this is an analysis after four years of follow-up. A longer observation time is necessary to show whether the effect of rifampicin prophylaxis will be sustained over longer periods.
The results of our study confirm those of previous studies on the efficacy of rifampicin prophylaxis. It seems, however, that this effect is not the same for all subgroups of contacts. Contacts who were not closely related to the index patient or lived further away, and who on the basis of the intake data were expected to be at a lower risk,2 benefited more from prophylaxis. This inverse relation between efficacy and expected risk also seems to exist for classification of disease in the index patient. By contrast, a direct relation with age of the contact is suggested, higher efficacy being recorded in those groups with a higher incidence of leprosy.
Female contacts seemed to benefit slightly more from prophylaxis than male contacts, but this was not statistically significant as the confidence intervals overlapped to a great extent. Although males are generally regarded as being more at risk for leprosy,13 14 neither our intake data nor the presently discussed values could confirm a significantly higher risk. A reason for the difference in efficacy between the sexes, if present, could be that females, who are generally lighter, had a relatively higher dose of rifampicin and more often developed paucibacillary disease rather than multibacillary disease. This assumption would need further investigation.
Prophylaxis seemed somewhat more effective in those contacts who were seronegative for antibodies to M leprae specific phenolic glycolipid-I at intake. Studies on the prognostic value of serology have shown contradictory findings,15 16 17 but research has indicated that contacts who are seropositive for antibodies to M leprae specific phenolic glycolipid-I are at an increased risk of developing leprosy, especially multibacillary disease.18
The incidence rate of new cases of leprosy in the placebo group between two and four years of follow-up showed a downward trend, whereas the rate remained similar in the rifampicin group over the two observation periods (see table 2). The downward trend in the placebo group can be understood from the perspective that regular surveys of contacts with treatment of newly detected cases is in itself an intervention by removing potential sources of infection and thereby reducing transmission to the contacts, which in turn can be expected to lead to a decreasing incidence rate in the contact groups over time. The difference between the placebo and rifampicin groups is determined primarily by a reduction of paucibacillary (single lesions and 2-5 lesions) leprosy in the rifampicin group in the first two years. After four years’ follow-up we cannot yet establish to what extent there is a true prevention of new cases of leprosy by intervention with rifampicin. There may be merely a delay in the occurrence of disease, which can only be confirmed through a longer observation period.
Finally, the presence of a BCG scar did not affect the response to prophylaxis, as measured by odds ratios. As BCG has a complementary effect to rifampicin in preventing leprosy, the absolute numbers of new patients with leprosy among those vaccinated is smaller and therefore the NNT is higher.
The findings of our trial are consistent with those of a study from Indonesia.10 That study found no effect of rifampicin in communities where only household contacts and direct neighbours were given prophylaxis, but showeda significant effect in those communities where everybody was given prophylaxis. But even in those communities, rifampicin prophylaxis seemed to be more effective in non-contacts than in household contacts. Studies on dapsone prophylaxis also showed that this was more effective when given as a blanket treatment rather than only to household contacts.5 A possible explanation of these findings could be that by the time the prophylaxis is given the potential bacillary load in physically close contacts, closely related contacts, seropositive contacts, and contacts of patients with multibacillary disease is on average already too high to be eliminated by a single (or double, in Indonesia two doses were given) dose of rifampicin. This possibly higher average bacterial load could be caused either by a higher exposure (household contacts, contacts of patients with multibacillary disease) or by a higher vulnerability (genetic make-up, partly reflected in seropositivity, male sex). If a higher bacterial load is indeed a reason for failure of prophylaxis with a single dose of rifampicin, more extended chemoprophylaxis schedules may be needed in those groups of contacts, but this requires further research.
In summary, a single dose of rifampicin given to contacts of new patients with leprosy was 57% effective in preventing the development of clinical leprosy after two years but a further effect could not be shown between two and four years. This efficacy at two years is similar to that found in a meta-analysis of dapsone trials, although in those trials dapsone was given for 1-5 years.5 The finding of single dose rifampicin as a cheap and practical preventive intervention for contacts of patients with leprosy in leprosy control programmes is a promising. The effect is not consistent in all subgroups, requiring further study before recommendations can be made for routine implementation.
What is already known on this topic
Chemoprophylaxis with prolonged administration of dapsone had an overall efficacy in preventing leprosy in contacts of about 60%
Two doses of rifampicin had a protective efficacy of 40-50% in an uncontrolled trial
What this study adds
A single dose of rifampicin in contacts of new patients with leprosy was 57% effective at preventing the development of leprosy after two years
No further effect was found between two and four years
We thank the staff of the leprosy control unit and the statistical department of the Rural Health Programme (formerly Danish Bangladesh Leprosy Mission) in Nilphamari and Rangpur for their dedicated work, often under difficult conditions.
Contributors: FJM, LO, and JHR were responsible for planning, carrying out, and reporting the study. DP was responsible for planning and carrying out the study. JHR is guarantor. Scientific advice was provided by the following members of the COLEP Scientific Advisory Group: W Cairns S Smith, Wim H van Brakel, Paul R Klatser, Paul R Saunderson, and Steve G Withington. Ron P Schuring provided laboratory support. Roel Faber developed and maintained the database. Gerard J J M Borsboom provided statistical support.
Funding: American Leprosy Missions and the Leprosy Mission International.
Competing interests: None declared.
Ethical approval: This study was approved by the ethical review committee of the Bangladesh Medical Research Council in Dhaka (BMRC/ERC/2001-2004/799).
The COLEP Study Group consists of Wim H van Brakel, Paul R Klatser, Paul R Saunderson, W Cairns S Smith, Steve G Withington (scientific advisers), F Johannes Moet, Linda Oskam, David Pahan, Jan Hendrik Richardus (project director), Ron P Schuring, Roel Faber, and Gerard J J M Borsboom.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Doull JA, Guinto RS, Rodriguez JN, Bancroft H. The incidence of leprosy in Cordova and Talisay, Cebu, Philippines. Int J Lepr 1942;10:107-31. [Google Scholar]
- 2.Moet FJ, Pahan D, Schuring RP, Oskam L, Richardus JH. Physical distance, genetic relationship, age, and leprosy classification are independent risk factors for leprosy in contacts of patients with leprosy. J Infect Dis 2006;193:346-53. [DOI] [PubMed] [Google Scholar]
- 3.Noordeen SK, Neelan PN. Chemoprophylaxis among contacts of non-lepromatous leprosy. Lepr India 1976;48(4 suppl):635-42. [PubMed] [Google Scholar]
- 4.Noordeen SK. Long term effects of chemoprophylaxis among contacts of lepromatous cases. Results of 8 1/2 years follow-up. Lepr India 1977;49:504-9. [PubMed] [Google Scholar]
- 5.Smith CM, Smith WC. Chemoprophylaxis is effective in the prevention of leprosy in endemic countries: a systematic review and meta-analysis. MILEP2 Study Group. Mucosal immunology of leprosy. J Infect 2000;41:137-42. [DOI] [PubMed] [Google Scholar]
- 6.Noordeen SK. Chemoprophylaxis in leprosy. Lepr India 1969;41:247-54. [Google Scholar]
- 7.Wardekar RV. DDS prophylaxis against leprosy. Lepr India 1967;39:155-9. [Google Scholar]
- 8.Cartel JL, Chanteau S, Boutin JP, Taylor R, Plichart R, Roux J, et al. Implementation of chemoprophylaxis of leprosy in the Southern Marquesas with a single dose of 25 mg per kg rifampin. Int J Lepr Other Mycobact Dis 1989;57(4):810-6. [PubMed] [Google Scholar]
- 9.Cartel JL, Chanteau S, Moulia-Pelat JP, Plichart R, Glaziou P, Boutin JP, et al. Chemoprophylaxis of leprosy with a single dose of 25 mg per kg rifampin in the southern Marquesas; results after four years. Int J Lepr Other Mycobact Dis 1992;60:416-20. [PubMed] [Google Scholar]
- 10.Bakker MI, Hatta M, Kwenang A, Van Benthem BH, Van Beers SM, Klatser PR, et al. Prevention of leprosy using rifampicin as chemoprophylaxis. Am J Trop Med Hyg 2005;72:443-8. [PubMed] [Google Scholar]
- 11.Moet FJ, Oskam L, Faber R, Pahan D, Richardus JH. A study on transmission and a trial of chemoprophylaxis in contacts of leprosy patients: design, methodology and recruitment findings of COLEP. Lepr Rev 2004;75:376-88. [PubMed] [Google Scholar]
- 12.Bührer-Sékula S, Sarno EN, Oskam L, Koop S, Wichers I, Nery JA, et al. Use of ML dipstick as a tool to classify leprosy patients. Int J Lepr Other Mycobact Dis 2000;68:456-63. [PubMed] [Google Scholar]
- 13.Fine PE, Sterne JA, Ponnighaus JM, Bliss L, Saui J, Chihana A, et al. Household and dwelling contact as risk factors for leprosy in northern Malawi. Am J Epidemiol 1997;146:91-102. [DOI] [PubMed] [Google Scholar]
- 14.Ranade MG, Joshi GY. Long-term follow-up of families in an endemic area. Ind J Lepr 1995;67:411-25. [PubMed] [Google Scholar]
- 15.Chanteau S, Glaziou P, Plichart C, Luquiaud P, Plichart R, Faucher JF, et al. Low predictive value of PGL-I serology for the early diagnosis of leprosy in family contacts: results of a 10-year prospective field study in French Polynesia. Int J Lepr Other Mycobact Dis 1993;61:533-41. [PubMed] [Google Scholar]
- 16.Ulrich M, Smith PG, Sampson C, Zuniga M, Centeno M, Garcia V, et al. IgM antibodies to native phenolic glycolipid-I in contacts of leprosy patients in Venezuela: epidemiological observations and a prospective study of the risk of leprosy. Int J Lepr Other Mycobact Dis 1991;59:405-15. [PubMed] [Google Scholar]
- 17.Douglas JT, Celona RV, Abalos RM, Madarang MG, Fajardo T. Serological reactivity and early detection of leprosy among contacts of lepromatous patients in Cebu, the Philippines. Int J Lepr Other Mycobact Dis 1987;55(4):718-21. [PubMed] [Google Scholar]
- 18.Oskam L, Slim E, Buhrer-Sekula S. Serology: recent developments, strengths, limitations and prospects: a state of the art overview. Lepr Rev 2003;74:196-205. [PubMed] [Google Scholar]