Abstract
Specific mutations in the human gene encoding lamin A or in the lamin A–processing enzyme, Zmpste24, cause premature aging. New data on mice and humans suggest that these mutations affect adult stem cells by interfering with the Notch and Wnt signaling pathways.
Many premature aging diseases are caused by mutations in the lamin A gene, resulting in severe nuclear abnormalities, but, curiously, although some tissues show severe phenotypes, others are hardly affected. Two new studies, Scaffidi and Misteli (2008) and Espada et al. (see p. 27 of this issue), show that somatic stem cells are misregulated in premature aging, explaining some of the pathological defects observed in these situations.
Structure and function of nuclear lamins
The nuclear lamina underlies the inner membrane of the nuclear envelope (inner nuclear membrane [INM]). It is composed of lamins and lamin-associated proteins, including integral proteins of the INM. Lamins interact directly or indirectly with many known INM proteins, several nucleoplasm proteins, and proteins that bridge the inner and outer nuclear membranes and interact with cytoskeleton elements. These lamin-based complexes are involved in most nuclear activities, including determining nuclear structure, spacing of nuclear pores, replication of DNA, regulation of gene expression, transcription by RNA Pol II, nuclear positioning, segregation of chromosomes, meiosis, and apoptosis (Gruenbaum et al., 2005).
Lamins are type V intermediate filament proteins. They are found in all metazoans, and, like all intermediate filament proteins, they are composed of a short globular N-terminal (head) domain, an α-helical rod domain, and a globular C (tail) domain. The tail domain of lamins contains an Ig fold flanked by unstructured regions. Lamins form stable, fibrous structures both at the nuclear periphery and in the nucleoplasm (Moir et al., 2000; Wiesel et al., 2008).
Lamins are divided into A and B types based on their expression patterns and protein structure. A-type lamins are found only in more complex metazoans and are expressed in differentiated tissues and in some adult stem cells, including mesenchymal and hair stem cells (Melcer et al., 2007). They are absent in other types of stem cells, including embryonic stem cells (Constantinescu et al., 2006). B-type lamins are generally more ubiquitously expressed, and at least one of the B-type lamins is expressed in all cell types throughout development (Stuurman et al., 1998). In mammals, three lamin genes are present, encoding four major proteins. Lamin A and lamin C are the products of the LMNA gene, whereas lamin B1 and lamin B2 are the products of LMNB1 and LMNB2, respectively (Stuurman et al., 1998).
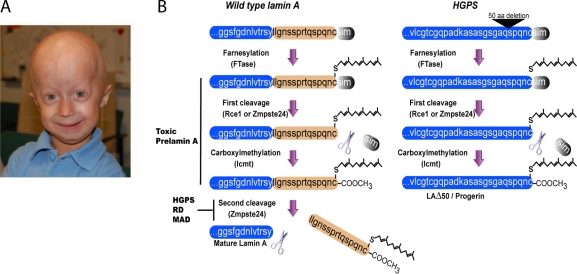
All B-type lamins and lamin A (but not lamin C) have a CAAX box at their C terminus that is subjected to several posttranslational modifications. First, the cysteine is farnesylated by a farnesyl transferase. Then, the last three residues (AAX) are cleaved off by either Ras-converting enzyme (Rce1) or Zinc metalloproteases related to Ste24p (Zmpste24). Subsequently, the cysteine undergoes methylation by the isoprenylcysteine carboxyl methyltransferase. Although B-type lamins remain farnesylated, pre–lamin A undergoes a fourth maturation step in which the 15 C-terminal amino acids, including the farnesyl group, are cleaved off by Zmpste24 (Fig. 1 B; Rusiñol and Sinensky, 2006).
Figure 1.
Processing of lamin A in normal and HGPS cells. (A) A photograph of a 3.5-yr-old HGPS patient with a classic HGPS mutation (photo courtesy of The Progeria Research Foundation). (B, left) The process of maturation of pre–lamin A. The first three steps are common to all CAAX proteins, including all B-type lamins. Inhibition of the second or third steps results in toxic lamin A accumulation, causing HGPS, restricted dermopathy (RD), or mandibuloacral dysplasia (MAD). The fourth step involves cleavage of 15 amino acids away from the terminal cysteine by Zmpste24. (right) The processing of pre–lamin A in the most common HGPS mutation, which deletes amino acids 607–656 (progerin/LAΔ50), including the second cleavage site of lamin A by Zmpste24. The scheme in B was modified from Mattout et al. (2006).
Mutations in lamin A or in Zmpste24 cause heritable diseases, including Hutchison-Gilford progeria syndrome
Mutations in the human LMNA gene cause at least 11 different heritable diseases, which are collectively termed laminopathies, ranging from muscular dystrophies to premature aging (Broers et al., 2006; Worman and Bonne, 2007). Much of the attention to laminopathies is the result of its involvement in the premature aging disease Hutchison-Gilford progeria syndrome (HGPS; Fig. 1 A). Most HGPS patients have a single-nucleotide substitution, 1824 C>T, in the LMNA gene, which activates a cryptic splice donor, resulting in a mutant mRNA that is translated into a lamin A lacking 50 amino acids. The deleted region includes the second cleavage site in pre–lamin A, which is usually cleaved by the endoprotease Zmpste24, but in patients, no cleavage occurs, and the lamin A is permanently carboxyfarnesylated and methylated (De Sandre-Giovannoli et al., 2003; Eriksson et al., 2003; Dechat et al., 2007). The mutant protein, which is termed progerin/LAΔ50, incorporates abnormally into the nuclear lamina, leading to mechanical defects (Dahl et al., 2006), thickening of the nuclear lamina, nuclear lobulation, and loss of peripheral heterochromatin (Goldman et al., 2004). It also causes changes in histone modifications and increased DNA damage (Scaffidi and Misteli, 2005) as well as a delay in nuclear reassembly, abnormal chromosome segregation, and binucleated cells (Cao et al., 2007; Dechat et al., 2007). The cellular phenotypes can be reversed either by inhibition of the expression of the mutant allele or by administration of farnesyl transferase inhibitors (Scaffidi and Misteli, 2005; Rusiñol and Sinensky, 2006). The latter demonstrates that permanent farnesylation of progerin/LAΔ50 is toxic and causes the diseases. In agreement with this hypothesis, mutations in Zmpste24 cause the accumulation of farnesylated and methylated pre–lamin A, leading to premature aging diseases similar to mutant lamin A (Young et al., 2006; Stewart et al., 2007).
Interestingly, mice that are homozygous for Zmpste24-null mutation and heterozygous for a deletion in LMNA have reduced levels of pre–lamin A and are apparently normal (Varela et al., 2005), suggesting that the toxicity of the permanent carboxyfarnesylated and methylated pre–lamin A depends on its expression levels.
Somatic stem cells
Unlike the pluripotent and highly proliferative embryonic stem cells, somatic (or adult) stem cells are tissue-specific residents that are usually self-renewing slowly or quiescent and are restricted in their differentiation potential, often giving rise to specific lineages of usually one germ layer origin. They are normally maintained as a small reservoir and provide a source of tissue replenishment and maintenance, especially during damage. They are present in a wide variety of tissues, including intestine, muscle, skin, brain, hair, blood, bone marrow, and more, and they seem to be highly dependent on their niche, from which they receive signals influencing their fate (Jones and Wagers, 2008). Interestingly, the signaling pathways that operate in the different stem cell niches converge to four main pathways (Notch, Wnt, TGFβ, and Sonic hedgehog; Lowry and Richter, 2007), suggesting common mechanisms for adult stem cell regulation and maintenance. Most notable of these pathways are the Notch and Wnt signaling pathways, which are now shown to be implicated in premature aging (Espada et al., 2008; Scaffidi and Misteli, 2008).
Stem cells and lamins
Favreau et al. (2004) provided the first evidence that mutations in lamin A can interfere with muscle progenitor differentiation. Mouse C2C12 cells are capable of differentiation from myoblasts to myotubes, and expression of a mutant lamin A containing the Emery-Dreifuss muscular dystrophy–causing mutation R453W strongly delayed the ability of these cells to differentiate. Interestingly, overexpression of wild-type lamin A also caused a partial delay in the ability of C2C12 cells to undergo myotube differentiation. The effects of lamin A on muscle progenitor differentiation was verified in mice lines that are heterozygous or homozygous for a deletion in the Lmna gene. These mice displayed delayed muscle stem cell differentiation kinetics (Frock et al., 2006). Ectopic expression of either desmin or MyoD in the mutant lines partially restored the differentiation defects. The effects of mutations in lamin A were also tested in the mouse 3T3-L1 cells that are capable of differentiation from fibroblasts to adipocytes (Boguslavsky et al., 2006). Lipid accumulation was inhibited by expression of the familiar partial lipodystrophy mutations R482Q or R482W as well as overexpression of wild-type lamin A. The role of lamin A in adipocyte differentiation was also observed in fibroblasts derived from mice with a lamin A deletion, as these cells differentiated more readily to fat-containing cells. Therefore, lamin A seems to be an effective inhibitor of adipocyte differentiation. Collectively, these results highlight the connection between lamins, especially lamin A and somatic stem cells, and demonstrate that mutations in lamin A cause somatic stem cell dysfunction.
The progeria–stem cell connection
The first hint that progeria might be connected with stem cell functioning was proposed by Hofer et al. (2005), who suggested a link between aberrant telomere functioning and accelerated aging in progeroid symptoms. They hypothesized that the fingernail atrophy and gray hair observed in progeria patients as well as in normal aging are the result of stem cell depletion caused by telomere shortening, which is enhanced in several progeroid syndromes. This idea was further developed by Gotzmann and Foisner (2006), who proposed the regeneration model, which argues that tissue degeneration in progeria is caused by mesenchymal stem cell self-renewal defects. This model explains the specificity of tissue degeneration, as most affected tissues in progeroid syndromes are of mesenchymal origin (Hennekam, 2006). Halaschek-Wiener and Brooks-Wilson (2007) also subscribed to this view, suggesting that lamin A deficiency, which renders the nucleus fragile, results in increased cell death. Tissue-specific stem cells that are required to replenish the damaged tissues cannot meet the needs, and the result is an accelerated process of tissue degeneration. Similar to Gotzmann and Foisner (2006), they further argue that the differential effect observed for different tissues is a result of their regenerative potential and/or rate of cell death, and, therefore, HGPS patients do not suffer brain damage, for example. However attractive, these ideas were not experimentally tested or validated until now.
Further insight into the connection between stem cells and progeria now come from two studies published by Scaffidi and Misteli (2008) and Espada et al. (2008). Although the two groups studied different systems (i.e., cell lines vs. mice), induced different mutations (i.e., truncated lamin A vs. Zmpste24−/−), examined different niches (mesenchyme vs. hair follicle), and converged on different signaling pathways (i.e., Notch vs. Wnt), both studies demonstrate, for the first time, that progeria and age-related nuclear defects are directly linked with stem cell dysfunction.
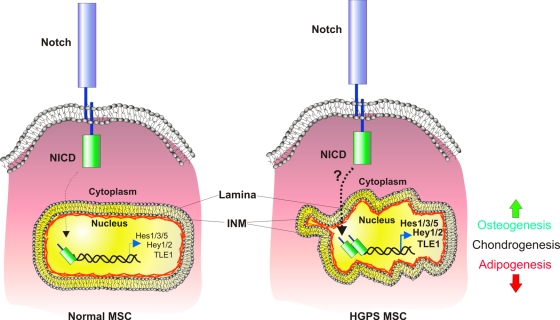
To study the molecular mechanisms by which the truncated form of lamin A (progerin/LAΔ50) induces progeroid phenotypes, Scaffidi and Misteli (2008) prepared stable cell lines carrying inducible forms of either GFP-progerin/LAΔ50 or GFP–wild-type lamin A and compared gene expression profiles after the transgenes' induction. They found conspicuous activation of Notch signaling in progerin/LAΔ50-expressing cells. Curiously, this effect was limited to downstream targets of the Notch pathway and seemed to be induced by the Notch coactivator Ski-interacting protein, which lost its grip from the progerinated nuclear lamina, freeing it to exert up-regulation of its target genes Hes1/3 and 5, Hey1 and 2, and TLE1 inside the nucleus (Fig. 2). Because the Notch pathway is a major regulator of stem cell maintenance and fate and because the main tissues affected in progeria are of mesenchymal origin, Scaffidi and Misteli (2008) turned to human mesenchymal stem cells, into which they introduced their previously prepared GFP-progerin/LAΔ50 or GFP–wild-type lamin A transgenes. In addition to induction of the Notch pathway, the mesenchymal stem cells responded to progerin/LAΔ50 by sporadic, undirected differentiation along all three germ layers. Directed differentiation of progerin/LAΔ50-expressing mesenchymal stem cells demonstrated enhanced osteogenesis, inhibition of adipogenesis, and unperturbed chondrogenesis, similar to what the authors observed when they activated the Notch pathway using the constitutively active Notch intracellular domain. Collectively, these results demonstrate the involvement of mesenchymal stem cell regulation in the pathology of HGPS through aberrant Notch signaling (Fig. 2).
Figure 2.
A model for mesenchymal stem cell dysfunction in HGPS. In wild-type mesenchymal stem cells (MSC; left), Notch signaling, which operates through the cleavable and nuclear-penetrating Notch intracellular domain (NICD), is active at basal levels, resulting in basal expression of Notch downstream targets, including Hes1, Hes3, Hes5, Hey1, Hey2, and TLE1. In Hutchison-Gilford progeria syndrome (HGPS), Notch signaling is increased in mesenchymal stem cells (right), leading to an increase in the expression of Notch pathway genes and to perturbed differentiation, including increased osteogenesis and decreased adipogenesis. INM, inner nuclear membrane.
Espada et al. (2008) addressed the question of the involvement of stem cell regulation in progeria by using Zmpste24−/− mice, which display age-related nuclear lamina defects and progeroid-like symptoms (Pendas et al., 2002). They focused on a well-characterized stem cell niche, the bulge cells of the hair follicle, where they found increased numbers of resident stem cells with decreased proliferative potential accompanied by accumulation of the unprocessed pre–lamin A and altered nuclear architecture.
Espada et al. (2008) next investigated the effects of Zmpste24 depletion on the differentiation capacity of hair follicle stem cells. They used either tetradecanoylphorbol 13-acetate, a tumor-promoting agent, which is known to induce both proliferation and differentiation of hair follicle stem cells, or calcium shock. Unlike the differentiation defects observed in mesenchymal stem cells expressing progerin/LAΔ50, bulge stem cell differentiation in the absence of Zmpste24 appeared normal after Ca2+ or tetradecanoylphorbol 13-acetate treatments. Finally, the authors examined possible defects in signaling pathways that are known to regulate stem cell proliferation. They found an almost complete absence of both the transcriptionally active form of β-catenin, the well-characterized regulator of the Wnt pathway, and Mitf, which is known to bind β-catenin and to regulate melanocyte stem cells. The Wnt pathway was found to be a master regulator of stem cell self-renewal and cancer (Reya and Clevers, 2005) and has been studied extensively in well-characterized stem cell niches, including the intestine, blood, brain, and epidermis, where it normally promotes stem cell proliferation (Lowry and Richter, 2007). Therefore, the decreased epidermal stem cell proliferation observed in the Zmpste24−/− mice is likely a result of the absence of Wnt signaling in these cells. In Zmpste24−/− Lmna+/− mice, no accumulation of pre–lamin A was observed, and all of the aberrant phenotypes at the cellular and organism levels were rescued, suggesting that the anomalous signaling pathways and stem cell dysfunction are mediated by pre–lamin A and/or defects in the nuclear lamina, directly linking disease genetics and phenotypes with stem cell function.
These two complementary studies (Espada et al., 2008; Scaffidi and Misteli, 2008) show that both intrinsic pathways operating in mesenchymal stem cells and niche-derived signaling in the hair follicle can affect stem cell function and contribute to the aging phenotype. Even more than providing a direct link between progeria and stem cells, however, these studies also imply that the same deterioration in stem cell regulation occurs in normal aging, although to a lesser extent. Support for this idea comes from the fact that progerin/LAΔ50 is present in healthy individuals at low levels and accumulates in old age (Scaffidi and Misteli, 2006; Cao et al., 2007) and that lamin A is significantly reduced in aged hematopoietic stem cells (Chambers et al., 2007), which also display the age-related accumulation of DNA damage (Rossi et al., 2007). In addition, recent studies demonstrated impairment in Wnt signaling during aging, affecting the regulation and fate of stem cells in the muscle (Brack et al., 2007), skin, and intestine (Liu et al., 2007).
It remains to be seen why various lamin A mutations have such diverse effects on mesenchymal stem cells and hair follicle stem cells, whether the differentiation capacity of other, nonmesenchymal stem cells is altered in progeria, the role that Notch, Wnt, and other signaling pathways play in other stem cell niches, and whether the observed phenotypes in HGPS are indeed caused by lack of tissue replenishment as a result of stem cell dysfunction. Based on the established link between progeria and stem cell regulation, such questions can now be asked and expectantly answered.
Acknowledgments
E. Meshorer is supported by the Israel Science Foundation (grant 215/07), the European Union (grant IRG-206872), and an Alon Fellowship. Y. Gruenbaum is supported by the Muscular Dystrophy Association (grant MDA4329) and a EURO-Laminopathies research project of the European Commission (contract LSHM-CT-2005-018690).
References
- Boguslavsky, R.L., C.L. Stewart, and H.J. Worman. 2006. Nuclear lamin A inhibits adipocyte differentiation: implications for Dunnigan-type familial partial lipodystrophy. Hum. Mol. Genet. 15:653–663. [DOI] [PubMed] [Google Scholar]
- Brack, A.S., M.J. Conboy, S. Roy, M. Lee, C.J. Kuo, C. Keller, and T.A. Rando. 2007. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 317:807–810. [DOI] [PubMed] [Google Scholar]
- Broers, J.L., F.C. Ramaekers, G. Bonne, R.B. Yaou, and C.J. Hutchison. 2006. Nuclear lamins: laminopathies and their role in premature ageing. Physiol. Rev. 86:967–1008. [DOI] [PubMed] [Google Scholar]
- Cao, K., B.C. Capell, M.R. Erdos, K. Djabali, and F.S. Collins. 2007. A lamin A protein isoform overexpressed in Hutchinson-Gilford progeria syndrome interferes with mitosis in progeria and normal cells. Proc. Natl. Acad. Sci. USA. 104:4949–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers, S.M., C.A. Shaw, C. Gatza, C.J. Fisk, L.A. Donehower, and M.A. Goodell. 2007. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 5:e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu, D., H.L. Gray, P.J. Sammak, G.P. Schatten, and A.B. Csoka. 2006. Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells. 24:177–185. [DOI] [PubMed] [Google Scholar]
- Dahl, K.N., P. Scaffidi, M.F. Islam, A.G. Yodh, K.L. Wilson, and T. Misteli. 2006. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA. 103:10271–10276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sandre-Giovannoli, A., R. Bernard, P. Cau, C. Navarro, J. Amiel, I. Boccaccio, S. Lyonnet, C.L. Stewart, A. Munnich, M. Le Merrer, and N. Levy. 2003. Lamin A truncation in Hutchinson-Gilford progeria. Science. 300:2055. [DOI] [PubMed] [Google Scholar]
- Dechat, T., T. Shimi, S.A. Adam, A.E. Rusinol, D.A. Andres, H.P. Spielmann, M.S. Sinensky, and R.D. Goldman. 2007. Alterations in mitosis and cell cycle progression caused by a mutant lamin A known to accelerate human aging. Proc. Natl. Acad. Sci. USA. 104:4955–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson, M., W.T. Brown, L.B. Gordon, M.W. Glynn, J. Singer, L. Scott, M.R. Erdos, C.M. Robbins, T.Y. Moses, P. Berglund, et al. 2003. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 423:293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espada, J., I. Varela, I. Flores, A.P. Ugalde, J. Cadiñanos, A.M. Pendás, C.L. Stewart, K. Tryggvason, M.A. Blasco, J.M.P. Freije, and C. López-Otín. 2008. Nuclear envelope defects cause stem cell dysfunction in premature-aging mice. 181:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favreau, C., D. Higuet, J.C. Courvalin, and B. Buendia. 2004. Expression of a mutant lamin A that causes Emery-Dreifuss muscular dystrophy inhibits in vitro differentiation of C2C12 myoblasts. Mol. Cell. Biol. 24:1481–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frock, R.L., B.A. Kudlow, A.M. Evans, S.A. Jameson, S.D. Hauschka, and B.K. Kennedy. 2006. Lamin A/C and emerin are critical for skeletal muscle satellite cell differentiation. Genes Dev. 20:486–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman, R.D., D.K. Shumaker, M.R. Erdos, M. Eriksson, A.E. Goldman, L.B. Gordon, Y. Gruenbaum, S. Khuon, M. Mendez, R. Varga, and F.S. Collins. 2004. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford Progeria Syndrome. Proc. Natl. Acad. Sci. USA. 101:8963–8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotzmann, J., and R. Foisner. 2006. A-type lamin complexes and regenerative potential: a step towards understanding laminopathic diseases? Histochem. Cell Biol. 125:33–41. [DOI] [PubMed] [Google Scholar]
- Gruenbaum, Y., A. Margalit, R.D. Goldman, D.K. Shumaker, and K.L. Wilson. 2005. The nuclear lamina comes of age. Nat. Rev. Mol. Cell Biol. 6:21–31. [DOI] [PubMed] [Google Scholar]
- Halaschek-Wiener, J., and A. Brooks-Wilson. 2007. Progeria of stem cells: stem cell exhaustion in Hutchinson-Gilford progeria syndrome. J. Gerontol. A Biol. Sci. Med. Sci. 62:3–8. [DOI] [PubMed] [Google Scholar]
- Hennekam, R.C. 2006. Hutchinson-Gilford progeria syndrome: review of the phenotype. Am. J. Med. Genet. A. 140:2603–2624. [DOI] [PubMed] [Google Scholar]
- Hofer, A.C., R.T. Tran, O.Z. Aziz, W. Wright, G. Novelli, J. Shay, and M. Lewis. 2005. Shared phenotypes among segmental progeroid syndromes suggest underlying pathways of aging. J. Gerontol. A Biol. Sci. Med. Sci. 60:10–20. [DOI] [PubMed] [Google Scholar]
- Jones, D.L., and A.J. Wagers. 2008. No place like home: anatomy and function of the stem cell niche. Nat. Rev. Mol. Cell Biol. 9:11–21. [DOI] [PubMed] [Google Scholar]
- Liu, H., M.M. Fergusson, R.M. Castilho, J. Liu, L. Cao, J. Chen, D. Malide, I.I. Rovira, D. Schimel, C.J. Kuo, et al. 2007. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 317:803–806. [DOI] [PubMed] [Google Scholar]
- Lowry, W.E., and L. Richter. 2007. Signaling in adult stem cells. Front. Biosci. 12:3911–3927. [DOI] [PubMed] [Google Scholar]
- Mattout, A., T. Dechat, S.A. Adam, R.D. Goldman, and Y. Gruenbaum. 2006. Nuclear lamins, diseases and aging. Curr. Opin. Cell Biol. 18:335–341. [DOI] [PubMed] [Google Scholar]
- Melcer, S., Y. Gruenbaum, and G. Krohne. 2007. Invertebrate lamins. Exp. Cell Res. 313:2157–2166. [DOI] [PubMed] [Google Scholar]
- Moir, R.D., M. Yoon, S. Khuon, and R.D. Goldman. 2000. Nuclear lamins A and B1. Different pathways of assembly during nuclear envelope formation in living cells. J. Cell Biol. 151:1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendas, A.M., Z. Zhou, J. Cadinanos, J.M. Freije, J. Wang, K. Hultenby, A. Astudillo, A. Wernerson, F. Rodriguez, K. Tryggvason, and C. Lopez-Otin. 2002. Defective prelamin A processing and muscular and adipocyte alterations in Zmpste24 metalloproteinase-deficient mice. Nat. Genet. 31:94–99. [DOI] [PubMed] [Google Scholar]
- Reya, T., and H. Clevers. 2005. Wnt signalling in stem cells and cancer. Nature. 434:843–850. [DOI] [PubMed] [Google Scholar]
- Rossi, D.J., D. Bryder, J. Seita, A. Nussenzweig, J. Hoeijmakers, and I.L. Weissman. 2007. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 447:725–729. [DOI] [PubMed] [Google Scholar]
- Rusiñol, A.E., and M.S. Sinensky. 2006. Farnesylated lamins, progeroid syndromes and farnesyl transferase inhibitors. J. Cell Sci. 119:3265–3272. [DOI] [PubMed] [Google Scholar]
- Scaffidi, P., and T. Misteli. 2005. Reversal of the cellular phenotype in the premature aging disease Hutchinson-Gilford progeria syndrome. Nat. Med. 11:440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi, P., and T. Misteli. 2006. Lamin A-dependent nuclear defects in human aging. Science. 312:1059–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi, P., and T. Misteli. 2008. Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nat. Cell Biol. 10.1038/ncb1708. [DOI] [PMC free article] [PubMed]
- Stewart, C.L., S. Kozlov, L.G. Fong, and S.G. Young. 2007. Mouse models of the laminopathies. Exp. Cell Res. 313:2144–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuurman, N., S. Heins, and U. Aebi. 1998. Nuclear lamins: their structure, assembly, and interactions. J. Struct. Biol. 122:42–66. [DOI] [PubMed] [Google Scholar]
- Varela, I., J. Cadinanos, A.M. Pendas, A. Gutierrez-Fernandez, A.R. Folgueras, L.M. Sanchez, Z. Zhou, F.J. Rodriguez, C.L. Stewart, J.A. Vega, et al. 2005. Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 signalling activation. Nature. 437:564–568. [DOI] [PubMed] [Google Scholar]
- Wiesel, N., A. Mattout, S. Melcer, N. Melamed-Book, H. Herrmann, O. Medalia, U. Aebi, and Y. Gruenbaum. 2008. Laminopathic mutations interfere with the assembly, localization and dynamics of nuclear lamins. Proc. Natl. Acad. Sci. USA. 105:180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worman, H.J., and G. Bonne. 2007. “Laminopathies”: a wide spectrum of human diseases. Exp. Cell Res. 313:2121–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, S.G., M. Meta, S.H. Yang, and L.G. Fong. 2006. Prelamin a farnesylation and progeroid syndromes. J. Biol. Chem. 281:39741–39745. [DOI] [PubMed] [Google Scholar]